Published online Oct 15, 2019. doi: 10.4251/wjgo.v11.i10.842
- This article has been corrected.
- See: World J Gastrointest Oncol. Jun 15, 2022; 14(6): 1216-1217
Peer-review started: May 7, 2019
First decision: June 4, 2019
Revised: June 19, 2019
Accepted: July 26, 2019
Article in press: July 28, 2019
Published online: October 15, 2019
Processing time: 176 Days and 22.2 Hours
Ectopic expression of miRNAs promotes tumor development and progression. miRNA (miR)-320a is downregulated in many cancers, including gastric cancer (GC). However, the mechanism underlying its downregulation and the role of miR-320a in GC are unknown.
To determine expression and biological functions of miR-320a in GC and investigate the underlying molecular mechanisms.
Quantitative real-time polymerase chain reaction (PCR) was used to determine expression of miR-320a in GC cell lines and tissues. TargetScanHuman7.1, miRDB, and microRNA.org were used to predict the possible targets of miR-320a, and a dual luciferase assay was used to confirm the findings. Western blotting was used to detect the protein levels of pre-B-cell leukemia homeobox 3 (PBX3) in GC cells and tissue samples. Cell Counting Kit-8 proliferation, Transwell, wound healing, and apoptosis assays were performed to analyze the biological functions of miR-320a in GC cells. Methylation-specific PCR was used to analyze the methylation level of the miR-320a promoter CpG islands. 5-Aza-2’-deoxycytidine (5-Aza-CdR) and trichostatin A (TSA) were used to treat GC cells.
miR-320a expression was lower in GC cell lines and tissues than in the normal gastric mucosa cell line GES-1 and matched adjacent normal tissues. miR-320a overexpression suppressed GC cell proliferation, invasion and migration, and induced apoptosis. PBX3 was a target of miR-320a in GC. The methylation level of the miR-320a promoter CpG islands was elevated and this was partly reversed by 5-Aza-CdR and TSA.
miR-320a acts as a tumor suppressor and inhibits malignant behavior of GC cells, partly by targeting PBX3. DNA methylation is an important mechanism associated with low expression of miR-320a.
Core tip: miRNA (miR)-320a functioned as a tumor suppressor and was downregulated in gastric cancer (GC). miR-320a overexpression suppressed proliferation, migration and invasion, and induced apoptosis through targeting Pre-B-cell leukemia homeobox 3 in GC cells. miR-320a depletion showed the opposite results. The potential mechanism of miR-320a deficiency in GC was the increased methylation level of the miR-320a promoter CpG islands.
- Citation: Li YS, Zou Y, Dai DQ. MicroRNA-320a suppresses tumor progression by targeting PBX3 in gastric cancer and is downregulated by DNA methylation. World J Gastrointest Oncol 2019; 11(10): 842-856
- URL: https://www.wjgnet.com/1948-5204/full/v11/i10/842.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i10.842
Gastric cancer (GC) is a common malignant tumor. Although the incidence and mortality of GC have substantially decreased over the last decade in most countries worldwide, the prevalence of GC is still ranked fifth and GC mortality is ranked third in the world[1]. The occurrence and development of advanced GC involve a complex process. Efficient methods for early diagnosis and curative treatment for GC are currently lacking. As precision medicine is the future direction in cancer therapy, identifying an early diagnostic or therapeutic biomarker for GC is essential.
MicroRNAs (miRNAs) are short [19–25 nucleotides (nt)] noncoding RNAs that recognize specific target mRNAs and participate in the post-transcriptional regulation of gene expression by promoting degradation and inhibiting translation of mRNAs[2]. It was previously thought that only regulatory proteins encoded by genes could control physiological functions. However, in recent years, researchers have found that miRNAs play an important role in regulating cell differentiation and cell fate decisions[3]. They can act as oncogenes or tumor suppressors according to their target genes[4]. For example, miR-98 is expressed at low levels and acts as a tumor suppressor in hepatocellular carcinoma by targeting Sal-like protein 4, and the highly expressed miR-27a-3p functions as an oncogene in GC by targeting the B-cell translocation gene 2[5,6]. Abnormal expression of miRNAs occurs in many types of cancer, and miRNAs are involved in tumorigenesis and tumor progression[7]. miR-212 is significantly downregulated in GC and is involved in GC development and progression by regulating expression of oncogene Myc[8]. miRNAs also play an important role in regulating cancer cell proliferation, invasion, migration and apoptosis[9,10].
The function of miR-320a, located on chromosome 8p21.3, was investigated previously. miR-320a acts as a tumor-suppressive miRNA in many cancers, including blood malignancies and solid tumors. Xishan et al[11] showed that miR-320a is downregulated in chronic myeloid leukemia (CML) and inhibits CML cell migration, invasion and proliferation, and promotes apoptosis by targeting the BCR/ABL oncogene. miR-320a inhibits multiple myeloma cell proliferation and induces apoptosis by targeting pre-B-cell leukemia homeobox 3 (PBX3)[12]. miR-320a is downregulated in many solid tumors and has important functions. mir-320a plays a tumor-suppressing role in colorectal cancer, nasopharyngeal carcinoma, breast cancer, and bladder carcinoma, and overexpression of miR-320a partly inhibits tumor malignant behavior[13-16]. However, the mechanism underlying the downregulation of these miRNAs is unknown.
Epigenetic regulation plays a crucial role in the development and progression of tumors, and DNA methylation is an important part of this process. The expression of miRNAs is regulated by DNA methylation, and abnormal DNA hypermethylation can lead to cancer suppressor gene silencing and promotion of tumor progression. Ayala-Ortega et al[17] showed that DNA hypermethylation at the miR-181c promoter region results in low miR-181c expression in glioblastoma cell lines compared with normal brain tissues. High methylation levels of the miR-27b promoter region downregulate expression of miR-27b, whereas demethylation restores miR-27b expression in breast cancer[18]. Downregulation of miR-320a is associated with regulation by methylation in breast cancer[19].
miRNAs are expressed in a tissue-specific manner. However, whether miR-320a acts as a tumor suppressor in GC is unknown. Wang et al[20] found that the expression of miR-320a was reduced in GC tissues. However, the biological function and epigenetic regulatory mechanism of miR-320a in GC remains unknown.
In this study, we identified the biological role of miR-320a in GC and clarified the relationship between miR-320a expression and DNA methylation. miR-320a expression was reduced in GC cell lines and tissues. miR-320a interacted with the 3′ untranslated region (UTR) of the oncogene PBX3 in GC. miR-320a overexpression inhibited malignant biological actions in GC cells. These results suggest that miR-320a acted as a tumor suppressor by regulating PBX3 expression in GC. The promoter CpG islands of miR-320a showed abnormal hypermethylation, and the methylation inhibitor 5-aza-2′-deoxycytidine (5-Aza-dC) partially reversed miR-320a expression. These findings demonstrated that methylation-associated silencing of miR-320a suppressed tumor progression by targeting PBX3 in GC.
This study was approved by the Ethics Committee of the Fourth Affiliated Hospital, China Medical University (Shenyang, China). We obtained 84 GC tissues and matched adjacent normal tissues (located > 5 cm from the tumor) from patients who had a diagnosis of GC confirmed by histopathology at the Cancer Research Institute of China Medical University (Shenyang, China) between 2013 and 2014. These patients did not receive chemotherapy before surgical resection, and all tissues were immediately frozen in liquid nitrogen after surgery until DNA or RNA extraction. The basic patient data are listed in Table 1.
| Clinicopathological characteristics | No. of patients | miR-320a low expression | miR-320a high expression | χ2 | P value |
| Age (yr) | |||||
| ≤ 60 | 25 | 15 | 10 | 0.285 | 0.594 |
| > 60 | 59 | 39 | 20 | ||
| Gender | |||||
| Female | 20 | 11 | 9 | 0.986 | 0.321 |
| Male | 64 | 43 | 21 | ||
| Location | |||||
| Gastric body | 20 | 15 | 5 | 1.524 | 0.467 |
| Gastric antrum | 52 | 31 | 21 | ||
| Gastric fundus | 12 | 8 | 4 | ||
| Tumor size (cm) | |||||
| < 5 | 25 | 15 | 10 | 0.285 | 0.594 |
| ≥ 5 | 59 | 39 | 20 | ||
| Differentiation | |||||
| Poor | 55 | 40 | 15 | 4.945 | 0.026 |
| Well and moderate | 29 | 14 | 15 | ||
| TNM stage | |||||
| I+II | 34 | 15 | 19 | 10.120 | 0.001 |
| III+ IV | 50 | 39 | 11 | ||
| Lymph node metastasis | |||||
| No | 18 | 7 | 11 | 6.436 | 0.011 |
| Yes | 66 | 47 | 19 |
We used five cell lines, including one normal gastric mucosa cell line (GES-1) and four GC cell lines (BGC-823, MGC-803, SGC-7901 and MKN-45). All cell lines were purchased from the Institute of Biochemistry and Cell Biology, China Academy of Science (Shanghai, China). Cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco/BRL, Waltham, MA, USA) at 37°C in 5% CO2.
The miR-320a mimics and miR-320a scramble mimics were designed and produced by GenePharma (Shanghai, China) and transfected into MKN-45 cells. The final concentration for transfection was 50 nmol/L. The miR-320a inhibitor and miR-320a scramble inhibitor were designed and produced by GenePharma and transfected into BGC-823 cells. The final concentration for transfection was 100 nmol/L. Transfection was performed using Lipofectamine 3000 Transfection Reagent (Invitrogen) according to the manufacturer’s instructions. Experiments were carried out in triplicate.
TRIzol reagent (Invitrogen) was used to extract total RNA from cell lines, GC tissues, and matched adjacent normal tissues. RNA concentration and purity were determined spectroscopically, and samples were stored at -80°C long term or -20°C short term. The miRNA RT-PCR Quantitation Kit (GenePharma) was used for miR-320a reverse transcription using the following protocol: 30 min at 25°C, 30 min at 42°C, and 5 min at 85°C. The cDNA product was subjected to quantitative real-time PCR using the following program: 3 min at 95°C, 40 cycles of 12 s at 95°C, and 40 s at 62°C. All mRNA quantification data were normalized to U6. The primers were designed and produced by GenePharma and the primer sequences are shown in Table 2.
| Name | Sequence (5’–3’) |
| miR-320a reverse transcription primer | |
| miR-320a | GTCTGTATGGTTGTTCTCGACTCCTTCACATCCCTATCCAACCATACAGACTCGCCCTC |
| U6 snRNA | GGAACGCTTCACGAATTTG |
| Real-time PCR primer sequence | |
| miR-320a | FO:AAGGGATCGCGGGCG |
| RE:TGCGTGTCGTGGAGTC | |
| U6 snRNA | FO:ATTGGAACGATACAGAGAAGATT |
| RE:GGAACGCTTCACGAATTTG | |
| MSP primer sequence | |
| Methylated | FO:ACGTCGTAATGTGAGGATTC |
| RE:CGCAAACAAAACTCGATATAA | |
| Nonmethylated | FO:ATTATGTTGTAATGTGAGGATTT |
| RE:CACAAACAAAACTCAATATAACCC | |
| miR-320a mimics sequence | |
| miR-320a | Sense: AAAAGCUGGGUUGAGAGGGCGA |
| Antisense: GCCCUCUCAACCCAGCUUUUUU | |
| Negative control | Sense: UUCUUCGAACGUGUCACGUTT |
| Antisense: ACGUGACACGUUCGGAGAATT | |
| PBX3 primer sequence | FO:GCCAAATTGACCCAGATCAGAC |
| RE:GAAATGGGACGTGTTCTACTCTGTT | |
GC cells (MKN-45 and BGC-823) were inoculated into 96-well plastic dishes containing 10% CCK-8 (Dojindo, Kumamoto, Japan) diluted in culture medium and incubated at 37°C in 5% CO2 after transfection with miR-320a mimics, scramble mimics, miR-320a inhibitor, scramble inhibitor, or no transfection. The OD value at 0, 12, 24, 48, and 72 h after transfection was measured using an enzyme assay. All experiments were performed three times. GC cells (n = 106) transfected with miR-320a mimics, scramble mimics, miR-320a inhibitor, scramble inhibitor, or no transfection were inoculated into six-well plates after transfection for 48 h, and all cells were harvested at 72 h. The Annexin V-PE/7AAD Apoptosis Detection Kit (KeyGen, Jiangsu, China) was used to determine apoptosis.
GC cells (MKN-45 and BGC-823) were inoculated into six-well plastic dishes and transfected with miR-320a mimics, scramble mimics, miR-320a inhibitor, scramble inhibitor or no transfection for 36 h. Cuts were then made using a 200-μL pipette tip. The wound healing percentage was measured at 0, 24, and 48 h after transfection using ImageJ software to evaluate the migration ability of GC cells. A Transwell assay was used to determine the invasion ability of GC cells (MKN-45 and BGC-823). A Matrigel-coated membrane matrix (UnivBio, Shanghai, China) was used to simulate a physiological matrix membrane between the upper and lower chambers. GC cells (n = 105) transfected with miR-320a mimics, scramble mimics, miR-320a inhibitor, scramble inhibitor, or no transfection were inoculated into the upper chamber filled with serum-free medium. Medium containing 10% FBS was added to the lower chamber. After 24 h, cotton wool was used to remove the noninvading cells in the upper chamber, and crystal violet (Tiangen Biotech, Beijing, China) was used to stain the invasive cells on the lower surface of the chamber. The number of invasive cells was counted under an inverted microscope. All experiments were repeated three times.
MKN-45 cells were inoculated into six-well plates. Cells were treated with 5-Aza-CdR (Sigma–Aldrich, St Louis, MO, USA) at 0.5, 1, or 1.5 μmol/L for 72 h, or with TSA (Beyotime, Beijing, China) at 300 nmol/L for 24 h. TSA was also added to the medium for the final 24 h of the 72 h 5-Aza-CdR (0.5 μmol/L) treatment period. To maintain drug effectiveness, the medium containing the drug was replaced every 24 h. Cells were then harvested, and RNA from MKN-45 cells was purified using the TRIzol reagent. cDNA synthesis was performed as described previously, and 1 mL of diluted cDNA from each sample was amplified by quantitative PCR using a previously described protocol.
Genomic DNA was isolated from primary GC samples (cancer tissues and matched adjacent normal tissues) and GC cells, and harvested after transfection for 48 h with miR-320a mimics, scramble mimics, or no transfection. The MiniBEST Universal Genomic DNA Extraction Kit (TaKaRa, Shiga, Japan) was used for DNA isolation. The genomic DNA underwent bisulfite modification using the EZ DNA Methylation-Gold Kit (TaKaRa). DNA (400 ng) was diluted to 20 μL, and 130 μL of the conversion reagent (900 μL water, 50 μL M-dissolving buffer, 300 μL M-dilution buffer) was added. Bisulfite conversion was carried out as follows: 98°C for 10 min, 64°C for 150 min, and 4°C for 5 min, and DNA was recovered using a Zymo-Spin column (Zymo Research, Irvine, CA, USA). Bisulfite-treated DNA was used for methylation analyses.
The methylation status of the miR-320a promoter CpG islands in cell lines and tissues was analyzed using methylation-specific PCR (MSP). The MSP primers were designed using MethPrimer (http://www.urogene.org/methprimer) and are listed in Table 2. The PCR product length was 149 bp. The amplification reaction volume was 25 μL, and the reaction procedure was as follows: One cycle at 94°C for 5 min, 40 cycles at 94°C for 30 min, 58°C for 30 min, 72°C for 30 min, and one cycle at 72°C for 10 min. Agarose gel electrophoresis was performed to analyze the MSP products.
Luciferase activity was analyzed using a dual luciferase reporter assay system (Promega, Shanghai, China). miR-320a mimics, miR-320a negative control miRNA, wild-type PBX3 (ACAGCUUUA), or mutant PBX3 (ACACCCUUA) was transfected into MKN-45 cells for 48 h using Lipofectamine 3000 (Invitrogen). Experiments were repeated three times.
Total protein was extracted from GC tissues and cells (MKN-45 and BGC-823) after transfection for 48 h with miR-320a mimics, scramble mimics, miR-320a inhibitor, scramble inhibitor, or no transfection using RIPA buffer (Beyotime, Shanghai, China), and protein concentration was determined using a BCA Protein Assay Kit (TaKaRa, Dalian, China). Appropriate protein samples were separated by SDS-PAGE (TaKaRa, Dalian, China), and electrophoresis was carried out for 100 min at 120 V. The proteins were then transferred to polyvinylidene difluoride membranes (Millipore, Shanghai, China), which were blocked in bovine serum albumin (BSA) for 2 h at room temperature. Rabbit anti-PBX3 monoclonal antibody (1:3000; Abcam, Cambridge, UK; #Ab109173) was added overnight at 4°C. Rabbit anti-GAPDH monoclonal antibody (1:3000; ABclonal, Boston, MA, USA) was used as the internal reference. Goat anti-rabbit secondary antibody was added to the polyvinylidene difluoride membranes for 2 h the following day. Then, a chemiluminescence instrument (Tanon, Shanghai, China) was used to determine the MSP product levels.
All samples were incubated overnight in buffered formalin, after which they were embedded in paraffin, cut into 3-μm-thick sections, and stained with hematoxylin–eosin (HE).
The miR-320a target gene was predicted by TargetScanHuman7.1 (http://www.targetscan.org/vert_71/), miRDB (http://www.mirdb.org/miRDB/), and microrna.org (http://www.microrna.org/microrna/home.do). MethPrimer (http://www.urogene.org/methprimer2/index.html) was used to predict the miR-320a promoter CpG islands.
Statistical analysis was performed using SPSS version 17.0 (SPSS, Chicago, IL, USA). X2 test or Student’s t test (two-tailed) were used. P < 0.05 was considered statistically significant.
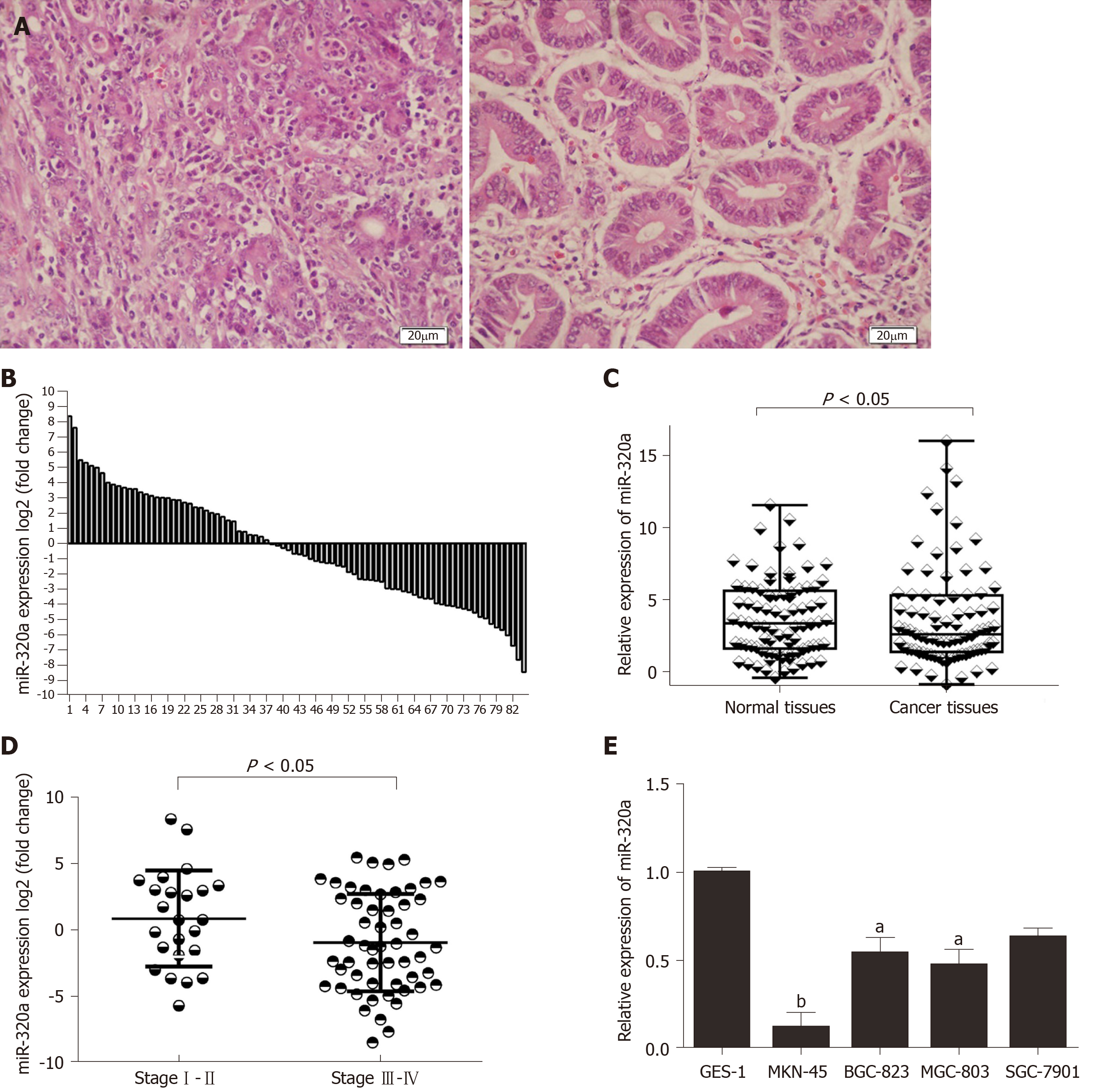
miR-320a expression in GC tissues was measured by quantitative real-time PCR. Assessment of miR-320a expression in 84 GC tissues and matched adjacent normal tissues (Figure 1A) showed that miR-320a was downregulated in 54 samples (54/84, 64%) compared with matched adjacent normal tissues, and the difference was significant (Figure 1B, 1C). To assess the association between miR-320a expression and clinicopathological characteristics, the tissues were separated into two groups: upregulated and downregulated miR-320a groups. miR-320a expression was significantly associated with TNM stage, tumor differentiation, and lymph node metastasis (Table 1 and Figure 1D). These results suggest that miR-320a is involved in GC progression.
miR-320a expression was assessed in four GC cell lines (BGC-823, MGC-803, SGC-7901 and MKN-45) and one normal gastric mucosa cell line (GES-1). miR-320a expression was lower in GC than in GES-1 cells, and particularly downregulated in MKN-45 cells (Figure 1E). These data suggest that miR-320a expression is associated with gastric carcinoma. MKN-45 and BGC-823 cells were used for subsequent miR-320a mimic and inhibitor transfection experiments, respectively.
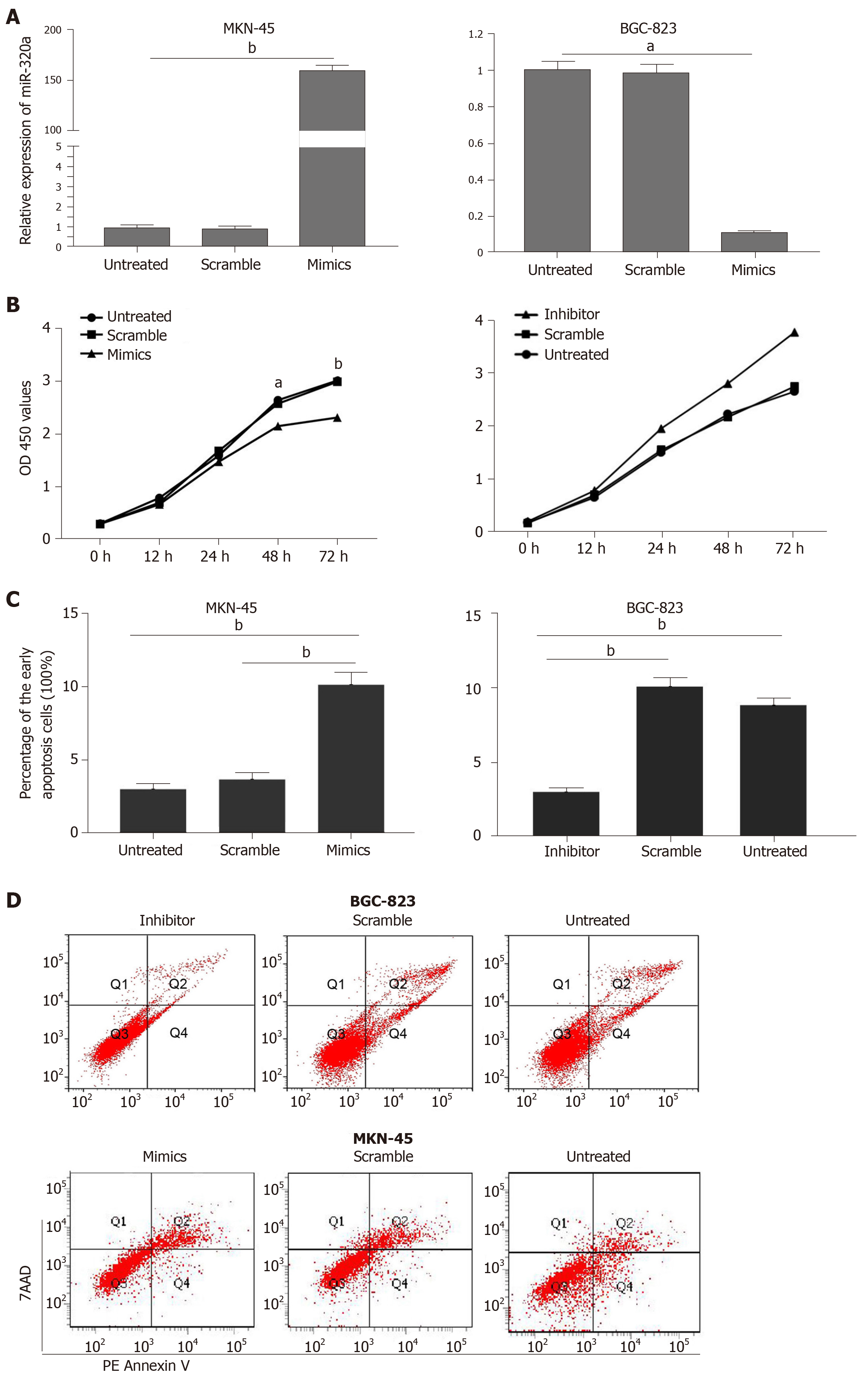
To determine the role and potential biological function of miR-320a in GC, GC cells were transfected with miR-320a mimics and miR-320a inhibitor, and the effects of miR-320a up- and downregulation on cell function were evaluated. miR-320a mimics transfection significantly upregulated, whereas miR-320a inhibitor significantly downregulated miR-320a expression compared with that in the other groups (Figure 2A). The CCK-8 assay was performed to estimate the effect of miR-320a on the viability of GC cells. We found that overexpression of miR-320a inhibited MKN-45 cell viability, whereas miR-320a depletion promoted BGC-823 cell viability (Figure 2B). Because apoptosis is an important part of the cell life cycle, we determined the effect of miR-320a on the early apoptosis rate of GC cells. miR-320a mimics accelerated MKN-45 cell apoptosis, whereas miR-320a inhibitor suppressed BGC-823 cell apoptosis, compared with that in the scramble and untreated groups (Figure 2C, 2D).
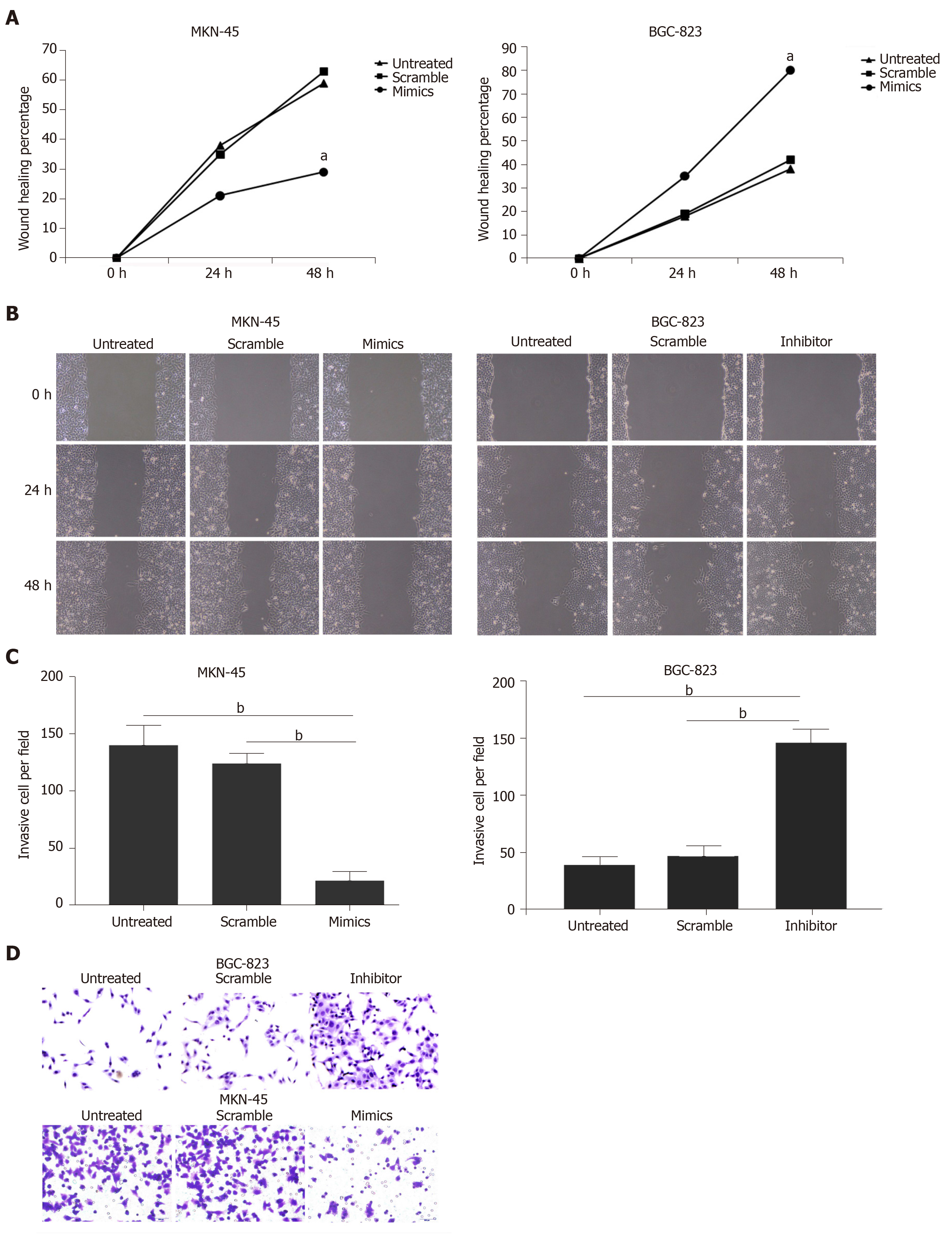
Wound scratch and Transwell invasion assays were used to determine the effects of miR-320a on the metastatic capacity and invasiveness of GC cells, which are important malignant activities of tumor cells. GC cells were divided into three treatment groups: miR-320a mimics/inhibitor, scramble mimics/inhibitor, and untreated. Wound healing was suppressed in cells treated with miR-320a mimics, whereas it was accelerated in cells treated with miR-320a inhibitor compared with that in the other two groups at 24 and 48 h after scratching (Figure 3A and 3B). The number of cells traversing the Matrigel matrix was lower in cells treated with miR-320a mimics, whereas it was higher in groups treated with miR-320a inhibitor than in the other two groups (Figure 3C and 3D). These data suggest that miR-320a overexpression inhibits GC cell migration and invasion to overcome the malignant properties of GC cells.
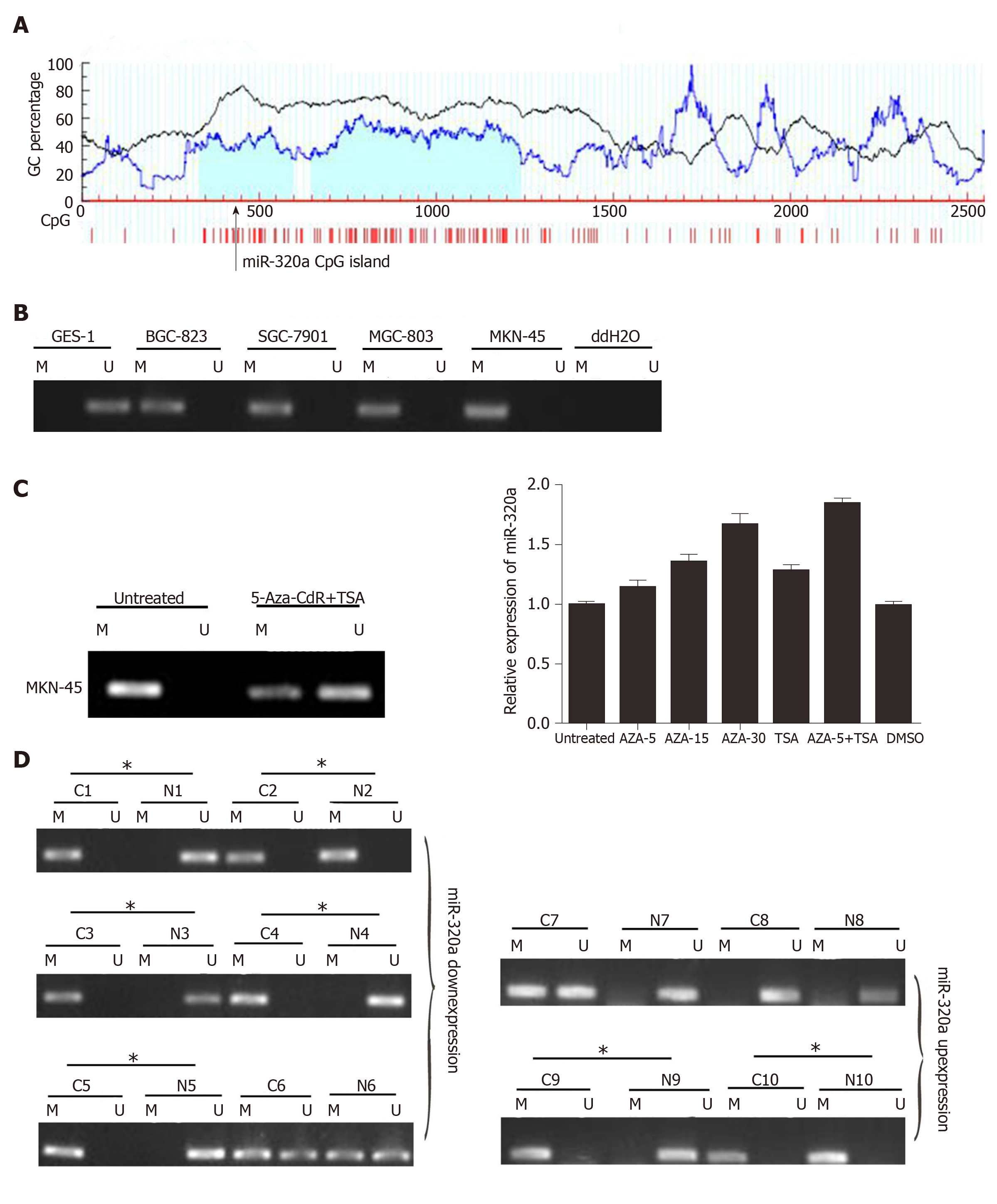
To determine the cause of miR-320a downregulation, we analyzed the relationship between DNA methylation and miR-320a expression. A search of the human genome database identified CpG islands around the miR-320a promoter (Figure 4A). MSP was used to detect the methylation level of miR-320a in the four GC cell lines and normal GES-1 cell line. miR-320a promoter CpG islands were hypermethylated in GC cells, whereas no methylation was observed in GES-1 cells (Figure 4B). To examine further the effect of methylation, MKN-45 cells were treated with 5-Aza-CdR (DNA methylation inhibitor) and TSA (histone deacetylase inhibitor), and the expression and methylation levels of miR-320a were determined by quantitative real-time PCR and MSP, respectively. miR-320a expression was partially reversed by 5-Aza-CdR and TSA, particularly by the combination of 5-Aza-CdR and TSA, and the methylation level of the miR-320a promoter CpG islands was markedly reduced (Figure 4C). To determine the status of miR-320a CpG islands in GC samples, methylation levels were measured in six pairs of GC samples (primary cancer tissues and matched adjacent normal tissues) with low miR-320a expression and four pairs of GC samples with high miR-320a expression levels. miR-320a CpG islands tended to be hypermethylated in the miR-320a downregulated group (83%, 5/6) compared with the miR-320a upregulated group (50%, 2/4) (Figure 4D). These findings indicated that DNA methylation plays an important role in the downregulation of miR-320a expression in GC.
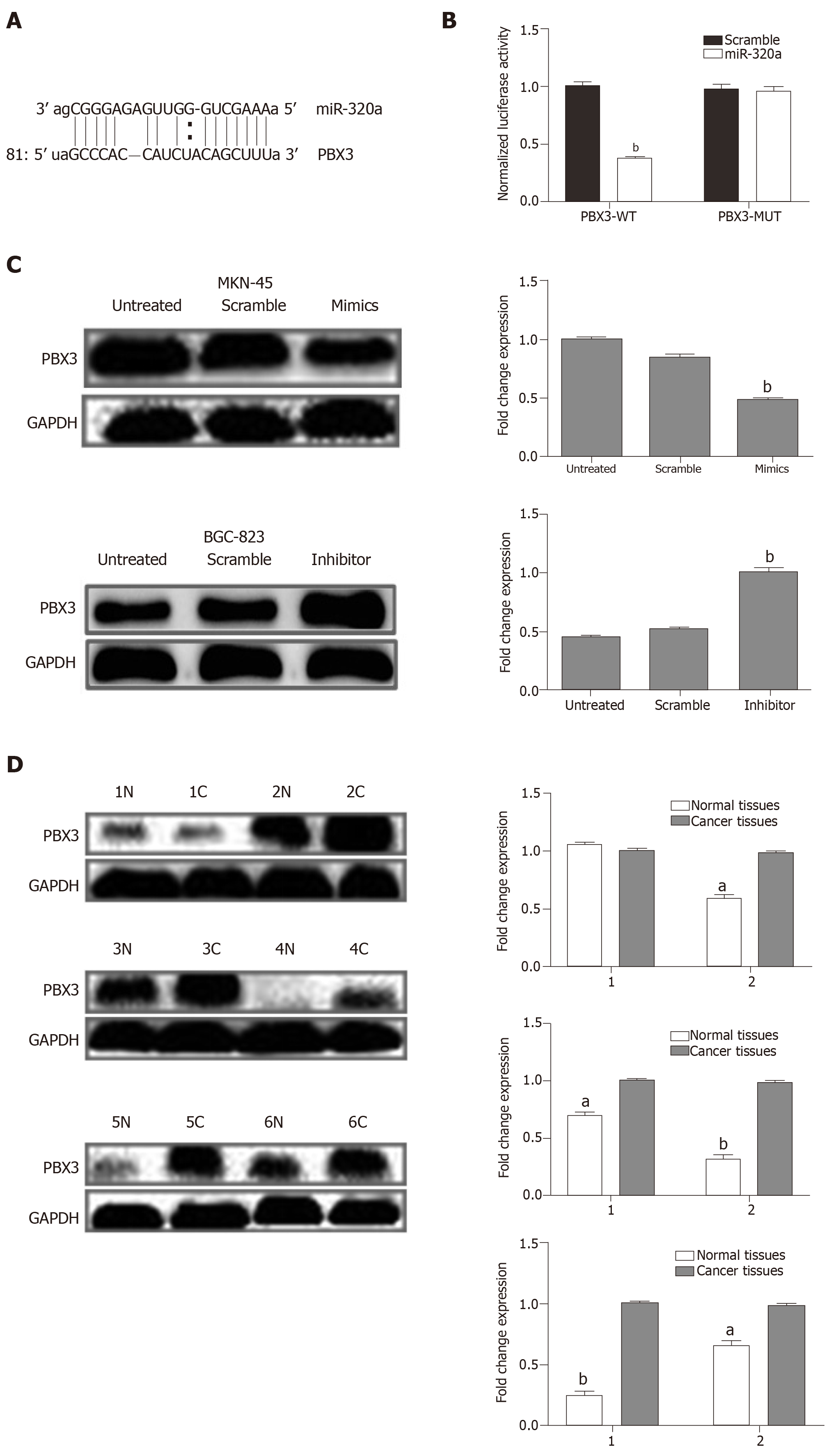
To examine the mechanism underlying the effect of miR-320a in GC, online software programs (TargetScanHuman7.1, miRDB, and microRNA.org) were used to predict the target gene of miR-320a. The three databases predicted the interaction of miR-320a with the 3′-UTR of PBX3 (Figure 5A). The dual luciferase assay confirmed that PBX3 was a direct target gene of miR-320a, and the regulatory activities are shown in Figure 5B. To determine whether miR-320a modulates PBX3 protein expression, six pairs of samples (GC tissues and matched adjacent normal tissues) with low miR-320a expression were analyzed by western blotting using anti-PBX3 antibodies. The results showed that PBX3 was significantly upregulated in five pairs of GC tissues compared with matched adjacent normal tissues (Figure 5D). Assessment of PBX3 protein expression in MKN-45 cells transfected with miR-320a mimics, scramble mimics, or no transfection showed that PBX3 was significantly downregulated in cells transfected with miR-320a mimics compared with the other two groups (Figure 5C).
miRNAs act as oncogenes or tumor suppressor genes and have important functions in the progression of many types of cancers[4]. In the present study, we found that miR-320a was significantly downregulated in GC cell lines and tissues. miR-320a overexpression inhibited GC cell proliferation, invasion and migration, and induced apoptosis in MKN-45 cells. Further studies demonstrated that low miR-320a expression was partly due to hypermethylation of the promoter CpG islands of miR-320a. PBX3 was identified as a direct target gene of miR-320a. Our findings demonstrated that miR-320a plays a tumor-suppressing role in GC, and DNA methylation is a crucial mechanism underlying miR-320a silencing.
Recent studies show that miR-320a is downregulated in many types of cancers, including breast, colorectal and ovarian cancers, and is involved in several biological functions by regulating specific target genes[11-16]. Xie et al[21] showed that miR-320a is markedly decreased in hepatocellular carcinoma, and the lack of miR-320a promotes cell migration and invasion. Decreased expression of miR-320a promotes proliferation and invasion of non-small cell lung cancer by targeting voltage dependent anion channel 1[22]. These data demonstrate the involvement of miR-320a in cancer. In this study, miR-320a expression was significantly lower in GC cells and tissues than in the normal GES-1 cell line and matched adjacent normal tissues. Assessment of the effect of miR-320a on the malignant biological behavior of GC cells showed that miR-320a overexpression prevented GC cell proliferation, invasion and migration, and promoted apoptosis compared with those in control MKN-45 cells. These data demonstrated that miR-320a acted as a tumor suppressor in GC. However, these findings need to be confirmed using a larger data sample.
PBX3, located on chromosome 9q33.3, belongs to the highly conserved PBX family of proteins, which are members of the PBX class of three-amino-acid loop extension superclass of homeodomain proteins[23]. PBX3 is overexpressed in many types of cancers[24-26], and plays a crucial role by activating various signaling pathways including the MAPK/ERK signaling pathway[24,26]. These data showed the powerful function of PBX3. PBX3 can act as an oncogene, promoting cell proliferation and colony formation, and its expression is associated with the clinicopathological characteristics of GC patients[27]. In the present study, Western blot analysis showed that PBX3 was upregulated in GC tissues and cells. miRNAs function by inhibiting translation or promoting degradation of target mRNAs[2]. PBX3 is regulated by multiple miRNAs in cancer, including let-7d, let-7c, miR-200b, miR-222, miR-424, and miR-181[25,26,28]. In multiple myeloma, PBX3 is regulated by miR-320a and involved in disease progression[12]. However, whether PBX3 is regulated by miR-320a in GC remains unknown. Our findings showed that miR-320a directly interacted with the 3′-UTR of PBX3 and downregulated PBX3 protein expression in GC, suggesting a mechanism underlying the post-transcriptional control of PBX3 by miR-320a.
DNA methylation plays an important role in the expression of tumor suppressor miRNAs[29]. Many miRNAs show hypermethylation of promoter CpG islands, including classic miRNAs such as miR-335 in GC, miR-181 in glioblastoma, and miR-342 in colorectal cancer[17,30,31]. However, the mechanism underlying the downregulation of miR-320a in GC is unknown. We found that CpG islands were present around miR-320a by searching the human genome database. He et al[19] reported that miR-320a was hypermethylated in colorectal cancer, which led us to hypothesize that low miR-320a expression is associated with DNA methylation in GC. MSP showed that, compared with normal GES-1 cells and matched adjacent normal tissues, miR-320a promoter CpG islands were hypermethylated in GC cell lines and GC tissues in which miR-320a was downregulated. Furthermore, the level of miR-320a hypermethylation was partially reversed by the demethylating agent 5-Aza-CdR. Overall, these data demonstrate that the expression of miR-320a is regulated by DNA methylation in GC.
There were some limitations in the present study. First, our study was a small, retrospective, single-institution study, and further larger, multicenter studies are required to validate our results. Second, although the expression of miR-320a is regulated by DNA methylation, the specific mechanisms remain largely undefined. Further detailed studies are currently underway to explore the participation of miR-320a in this process, to provide more comprehensive information about the functions of miR-320a in cancer.
In conclusion, we showed that miR-320a was downregulated and played a tumor- suppressing role by regulating the oncogene PBX3 in GC. miR-320a overexpression inhibited cell proliferation, invasion and migration, and induced cell apoptosis. miR-320a expression was regulated by DNA methylation and was partially reversed by 5-Aza-CdR and TSA. These data indicate that miR-320a may be a potential therapeutic target in GC.
Gastric cancer (GC) is a common type of malignant tumor with poor prognosis and presents a serious threat to human health. In February 2018 , the latest statistics from the Chinese National Cancer Center showed that although the overall incidence of GC is declining, it remains second in terms of incidence among all malignancies in China, just below lung cancer. At present, there is no efficient early diagnosis and curative treatment strategy for GC. As precision medicine is the future direction in cancer therapy, it is important to identify an early diagnostic or therapeutic biomarker of GC.
To explore the expression pattern, biological function and potential mechanism of miRNA (miR)-320a in GC, and to determine whether miR-320a functions as an early diagnostic or therapeutic biomarker in GCaccording to its expression and biological functions.
miR-320a mimic and inhibitor were transfected into GC cells for bidirectional regulation of the expression level of miR-320a. The effect of miR-320a on cell viability, migration, invasion and apoptosis was determined through a series of functional experiments. These results would provide scientific evidence for clinical treatment of GC.
Quantitative real-time polymerase chain reaction (PCR) and Western blotting were performed to determine the levels of related factors. Methylation-specific PCR was applied for analysis of methylation status of the miR-320a promoter CpG islands in GC cell lines and tissues. CCK8, flow cytometry, Transwell invasion and wound healing assays were performed to determine the effect of miR-320a on cell behavior. Dual luciferase assay was performed to identify whether pre-B-cell leukemia homeobox (PBX) 3 was the target gene of miR-320a. TargetScanHuman7.1, miRDB, microrna.org and MethPrimer were used for bioinformatics analysis. Student’s t test (two-tailed) and analysis of variance (ANOVA) were used for statistical analysis.
miR-320a was downregulated in GC, and its expression deficiency was partly due to hypermethylation of the promoter CpG islands. miR-320a overexpression inhibited cell viability, migration and invasion, and induced apoptosis through targeting PBX3 in GC cells. miR-320a functioned as a tumor suppressor in vitro. However, its biological effect in a preclinical model should be further determined.
miR-320a was downregulated in GC tissues and cells, and its abnormal expression was related to GC cell behavior. We confirmed that miR-320a overexpression inhibited cell viability, migration and invasion, and induced apoptosis in GC cells. miR-320a deficiency showed the opposite results. Consistent with previous research, miR-320a functioned as a tumor suppressor. miR-320a could be a biomarker for GC diagnosis and treatment. We found that miR-320a downregulation in GC was due to the elevated methylation level of the miR-320a promoter CpG islands. The elevation was partly reversed by 5-Aza-2’-deoxycytidine and trichostatin A. In addition, PBX3 was firstly identified as the target gene of miR-320a in GC.
For future research, we will focus on the related signaling pathways through which miR-320a regulates GC cell biological behaviors. Screening for related signaling pathways of GC will be performed using the Cancer Genome Atlas database, followed by related factor detection using Western blotting and quantitative real-time PCR. Activator and inhibitor for target signaling will be applied to GC cells, and cell viability, cell cycle, cell migration, invasion and apoptosis will be determined.
We are grateful to the surgeons and general practitioners for their participation and patient contacts.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lo GH, Mathur A, Ko E S-Editor: Wang JL L-Editor: A E-Editor: Qi LL
| 1. | Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 2. | Tang H, Liu X, Wang Z, She X, Zeng X, Deng M, Liao Q, Guo X, Wang R, Li X, Zeng F, Wu M, Li G. Interaction of hsa-miR-381 and glioma suppressor LRRC4 is involved in glioma growth. Brain Res. 2011;1390:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Ivey KN, Srivastava D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell. 2010;7:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 4. | Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353:1768-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 602] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 5. | Zhou W, Zou B, Liu L, Cui K, Gao J, Yuan S, Cong N. MicroRNA-98 acts as a tumor suppressor in hepatocellular carcinoma via targeting SALL4. Oncotarget. 2016;7:74059-74073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Zhou L, Liang X, Zhang L, Yang L, Nagao N, Wu H, Liu C, Lin S, Cai G, Liu J. MiR-27a-3p functions as an oncogene in gastric cancer by targeting BTG2. Oncotarget. 2016;7:51943-51954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5705] [Cited by in RCA: 6023] [Article Influence: 317.0] [Reference Citation Analysis (0)] |
| 8. | Xu L, Wang F, Xu XF, Mo WH, Xia YJ, Wan R, Wang XP, Guo CY. Down-regulation of miR-212 expression by DNA hypermethylation in human gastric cancer cells. Med Oncol. 2011;28 Suppl 1:S189-S196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Zhang S, Zhang C, Liu W, Zheng W, Zhang Y, Wang S, Huang D, Liu X, Bai Z. MicroRNA-24 upregulation inhibits proliferation, metastasis and induces apoptosis in bladder cancer cells by targeting CARMA3. Int J Oncol. 2015;47:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, Liu C, Song W, Wang F, Zhang J, Shen J, Yu J. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 11. | Xishan Z, Ziying L, Jing D, Gang L. MicroRNA-320a acts as a tumor suppressor by targeting BCR/ABL oncogene in chronic myeloid leukemia. Sci Rep. 2015;5:12460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Lu Y, Wu D, Wang J, Li Y, Chai X, Kang Q. miR-320a regulates cell proliferation and apoptosis in multiple myeloma by targeting pre-B-cell leukemia transcription factor 3. Biochem Biophys Res Commun. 2016;473:1315-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Zhao H, Dong T, Zhou H, Wang L, Huang A, Feng B, Quan Y, Jin R, Zhang W, Sun J, Zhang D, Zheng M. miR-320a suppresses colorectal cancer progression by targeting Rac1. Carcinogenesis. 2014;35:886-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Qi X, Li J, Zhou C, Lv C, Tian M. MicroRNA-320a inhibits cell proliferation, migration and invasion by targeting BMI-1 in nasopharyngeal carcinoma. FEBS Lett. 2014;588:3732-3738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Wang B, Yang Z, Wang H, Cao Z, Zhao Y, Gong C, Ma L, Wang X, Hu X, Chen S. MicroRNA-320a inhibits proliferation and invasion of breast cancer cells by targeting RAB11A. Am J Cancer Res. 2015;5:2719-2729. [PubMed] |
| 16. | Shang C, Zhang H, Guo Y, Hong Y, Liu Y, Xue Y. MiR-320a down-regulation mediates bladder carcinoma invasion by targeting ITGB3. Mol Biol Rep. 2014;41:2521-2527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Ayala-Ortega E, Arzate-Mejía R, Pérez-Molina R, González-Buendía E, Meier K, Guerrero G, Recillas-Targa F. Epigenetic silencing of miR-181c by DNA methylation in glioblastoma cell lines. BMC Cancer. 2016;16:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Li X, Wu Y, Liu A, Tang X. MiR-27b is epigenetically downregulated in tamoxifen resistant breast cancer cells due to promoter methylation and regulates tamoxifen sensitivity by targeting HMGB3. Biochem Biophys Res Commun. 2016;477:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | He DX, Gu XT, Jiang L, Jin J, Ma X. A methylation-based regulatory network for microRNA 320a in chemoresistant breast cancer. Mol Pharmacol. 2014;86:536-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Zeng J, Pan J, Geng X, Li L, Wu J, Song P, Wang Y, Liu J, Wang L. MiR-320a inhibits gastric carcinoma by targeting activity in the FoxM1-P27KIP1 axis. Oncotarget. 2016;7:29275-29286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Xie N, Wang C, Zhuang Z, Hou J, Liu X, Wu Y, Liu H, Huang H. Decreased miR-320a promotes invasion and metastasis of tumor budding cells in tongue squamous cell carcinoma. Oncotarget. 2016;7:65744-65757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Zhang G, Jiang G, Wang C, Zhong K, Zhang J, Xue Q, Li X, Jin H, Li B. Decreased expression of microRNA-320a promotes proliferation and invasion of non-small cell lung cancer cells by increasing VDAC1 expression. Oncotarget. 2016;7:49470-49480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Milech N, Kees UR, Watt PM. Novel alternative PBX3 isoforms in leukemia cells with distinct interaction specificities. Genes Chromosomes Cancer. 2001;32:275-280. [PubMed] |
| 24. | Han HB, Gu J, Ji DB, Li ZW, Zhang Y, Zhao W, Wang LM, Zhang ZQ. PBX3 promotes migration and invasion of colorectal cancer cells via activation of MAPK/ERK signaling pathway. World J Gastroenterol. 2014;20:18260-18270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 25. | Ramberg H, Alshbib A, Berge V, Svindland A, Taskén KA. Regulation of PBX3 expression by androgen and Let-7d in prostate cancer. Mol Cancer. 2011;10:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Han H, Du Y, Zhao W, Li S, Chen D, Zhang J, Liu J, Suo Z, Bian X, Xing B, Zhang Z. PBX3 is targeted by multiple miRNAs and is essential for liver tumour-initiating cells. Nat Commun. 2015;6:8271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Li Y, Sun Z, Zhu Z, Zhang J, Sun X, Xu H. PBX3 is overexpressed in gastric cancer and regulates cell proliferation. Tumour Biol. 2014;35:4363-4368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Li Z, Huang H, Li Y, Jiang X, Chen P, Arnovitz S, Radmacher MD, Maharry K, Elkahloun A, Yang X, He C, He M, Zhang Z, Dohner K, Neilly MB, Price C, Lussier YA, Zhang Y, Larson RA, Le Beau MM, Caligiuri MA, Bullinger L, Valk PJ, Delwel R, Lowenberg B, Liu PP, Marcucci G, Bloomfield CD, Rowley JD, Chen J. Up-regulation of a HOXA-PBX3 homeobox-gene signature following down-regulation of miR-181 is associated with adverse prognosis in patients with cytogenetically abnormal AML. Blood. 2012;119:2314-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Lujambio A, Calin GA, Villanueva A, Ropero S, Sánchez-Céspedes M, Blanco D, Montuenga LM, Rossi S, Nicoloso MS, Faller WJ, Gallagher WM, Eccles SA, Croce CM, Esteller M. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:13556-13561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 822] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 30. | Li Z, Li D, Zhang G, Xiong J, Jie Z, Cheng H, Cao Y, Jiang M, Lin L, Le Z, Tan S, Zou W, Gong B, Lin S, Yang K. Methylation-associated silencing of MicroRNA-335 contributes tumor cell invasion and migration by interacting with RASA1 in gastric cancer. Am J Cancer Res. 2014;4:648-662. [PubMed] |
| 31. | Grady WM, Parkin RK, Mitchell PS, Lee JH, Kim YH, Tsuchiya KD, Washington MK, Paraskeva C, Willson JK, Kaz AM, Kroh EM, Allen A, Fritz BR, Markowitz SD, Tewari M. Epigenetic silencing of the intronic microRNA hsa-miR-342 and its host gene EVL in colorectal cancer. Oncogene. 2008;27:3880-3888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |