Copyright
©The Author(s) 2021.
World J Gastrointest Oncol. Oct 15, 2021; 13(10): 1213-1228
Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1213
Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1213
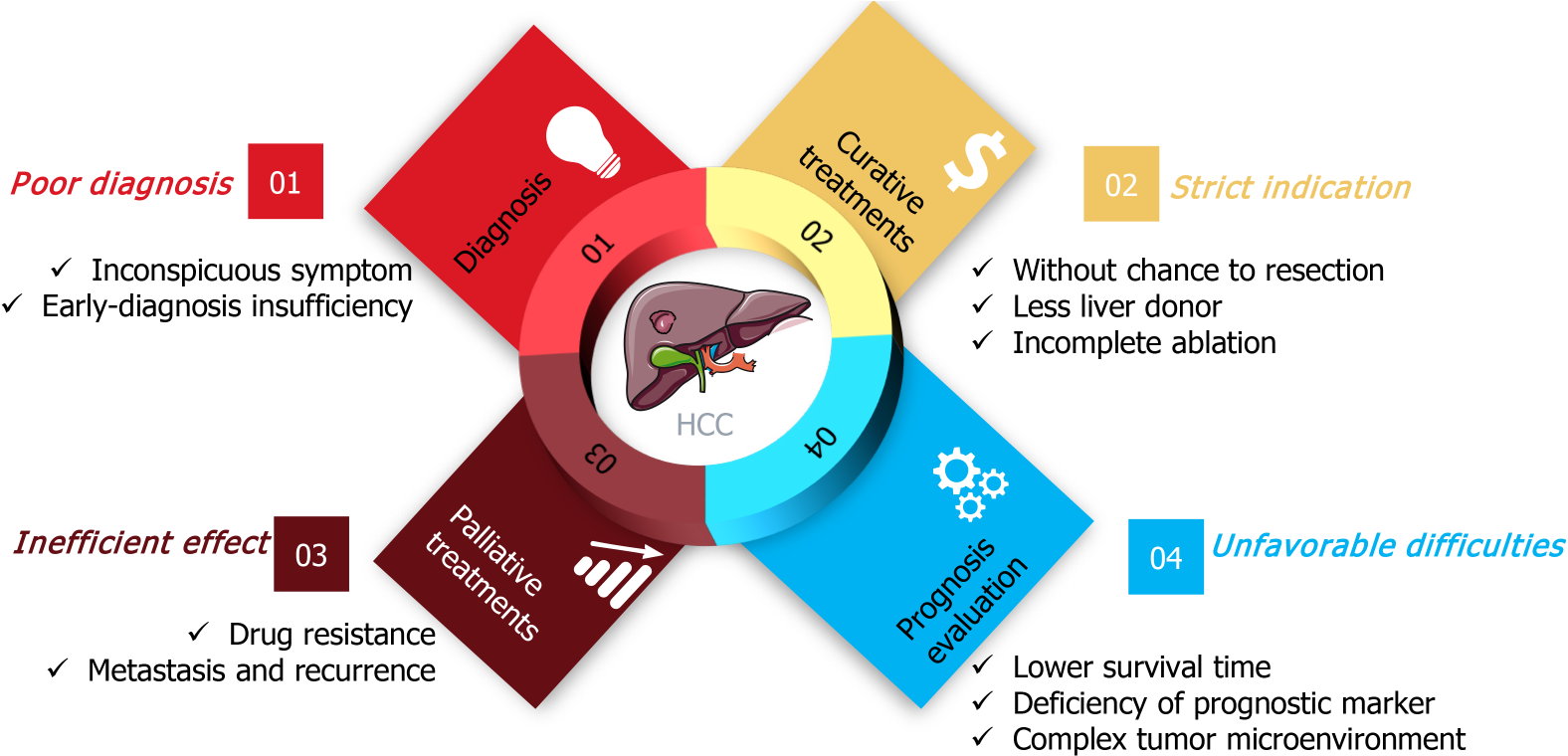
Figure 1 The dilemma of clinical hepatocellular carcinoma treatment.
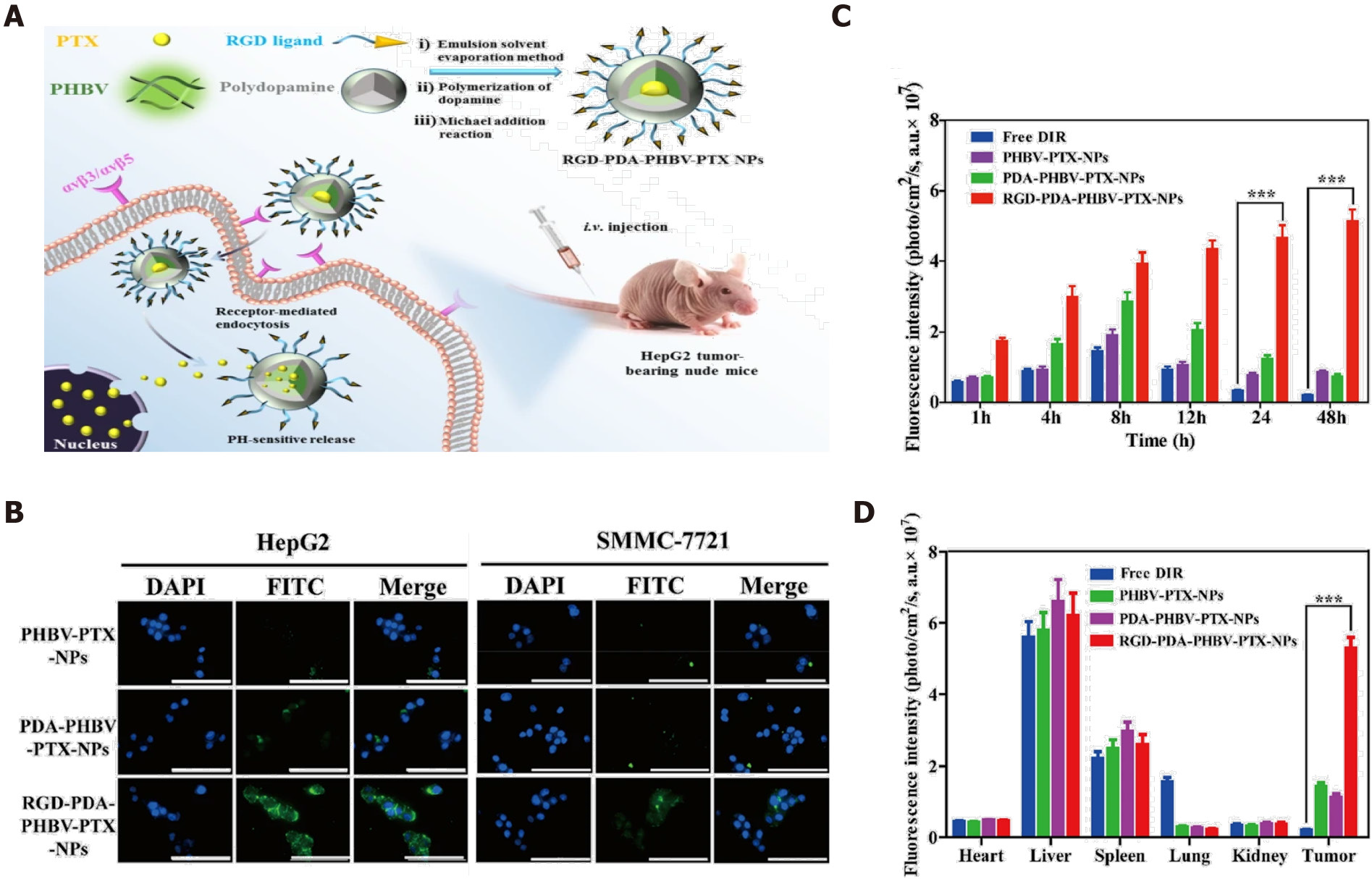
Figure 2 RGD-modified nanoparticles for targeted hepatocellular carcinoma therapy.
A: Illustration of RGD-PDA-PHBV-PTX-NPs and targeted therapy; B: Specific targeting of RGD-modified PDA-PHBV-PTX-NPs for liver cancer cells; C: Distribution of various NPs in tumors in a HepG2 xenograft tumor model; D: Bio-distribution of NPs in major organs. Citation: Wu M, Zhong C, Zhang Q, Wang L, Wang L, Liu Y, Zhang X, Zhao X. pH-responsive delivery vehicle based on RGD-modified polydopamine-paclitaxel-loaded poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanoparticles for targeted therapy in hepatocellular carcinoma. J Nanobiotechnology 2021; 19: 39. Copyright © The Authors 2021. Published by BioMed Central Ltd.
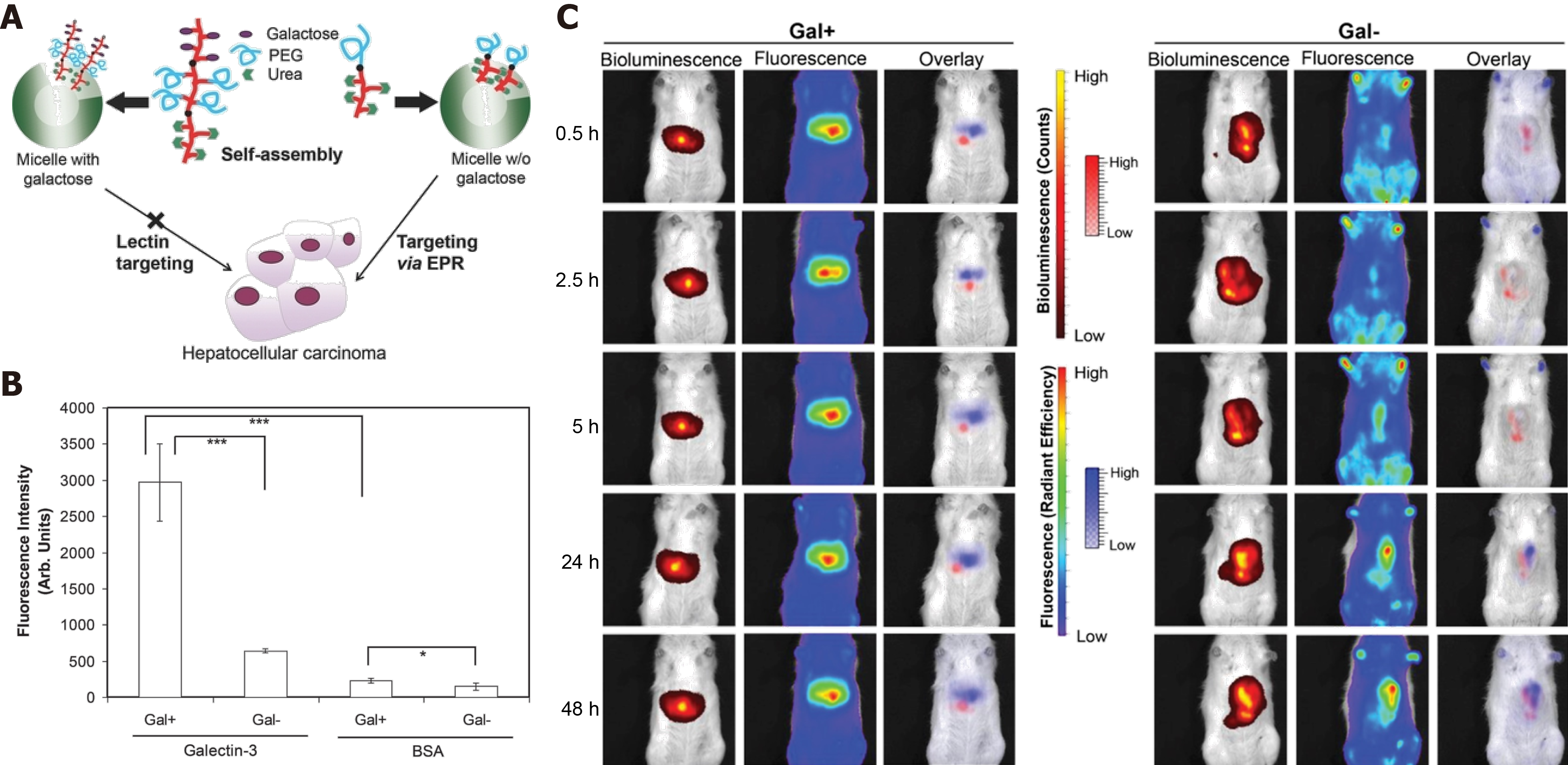
Figure 3 Enhanced permeability and retention effect favors polymeric micelles as an ideal drug delivery platform.
A: Illustration of polymeric micelles with or without galactose for hepatocellular carcinoma (HCC) targeting; B: Galactose-functionalized micelles bind to galectin-3; C: Specific HCC targeting powered by enhanced permeability and retention. Citation: Ebrahim Attia AB, Oh P, Yang C, Tan JP, Rao N, Hedrick JL, Yang YY, Ge R. Insights into EPR effect vs lectin-mediated targeted delivery: biodegradable polycarbonate micellar nanoparticles with and without galactose surface decoration. Small 2014; 10: 4281-4286. Copyright © The Authors 2014. Published by John Wiley & Sons, Inc.
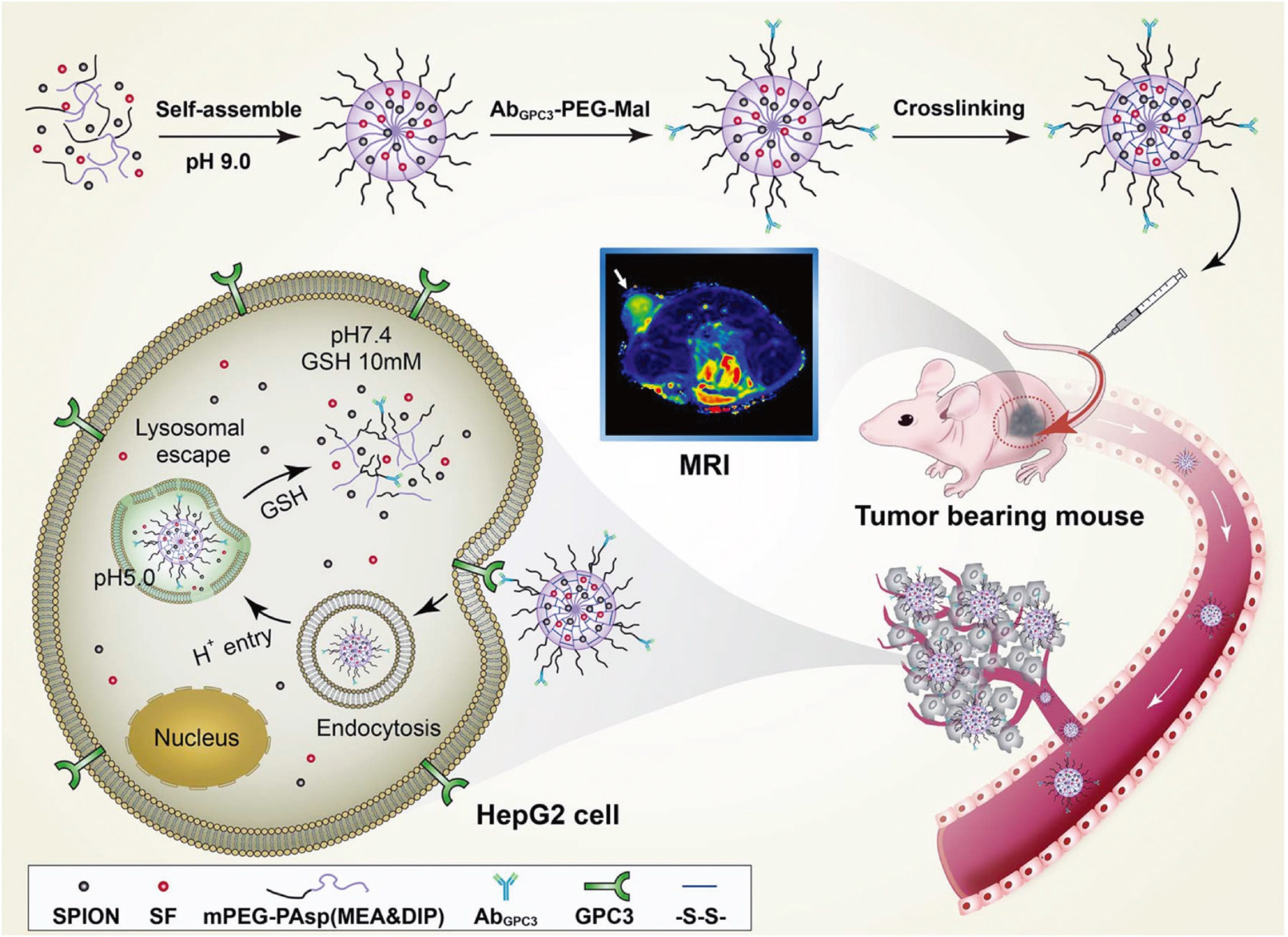
Figure 4 Dual-sensitive nanodrug delivery for treatment of hepatocellular carcinoma.
Based on the tumor microenvironment, a copolymer system was designed to rapidly release sorafenib in response to high cytoplasmic glutathione and low pH. Citation: Cai M, Li B, Lin L, Huang J, An Y, Huang W, Zhou Z, Wang Y, Shuai X, Zhu K. A reduction and pH dual-sensitive nanodrug for targeted theranostics in hepatocellular carcinoma. Biomater Sci 2020; 8: 3485-3499. Copyright © The Authors 2020. Published by Royal Society of Chemistry.
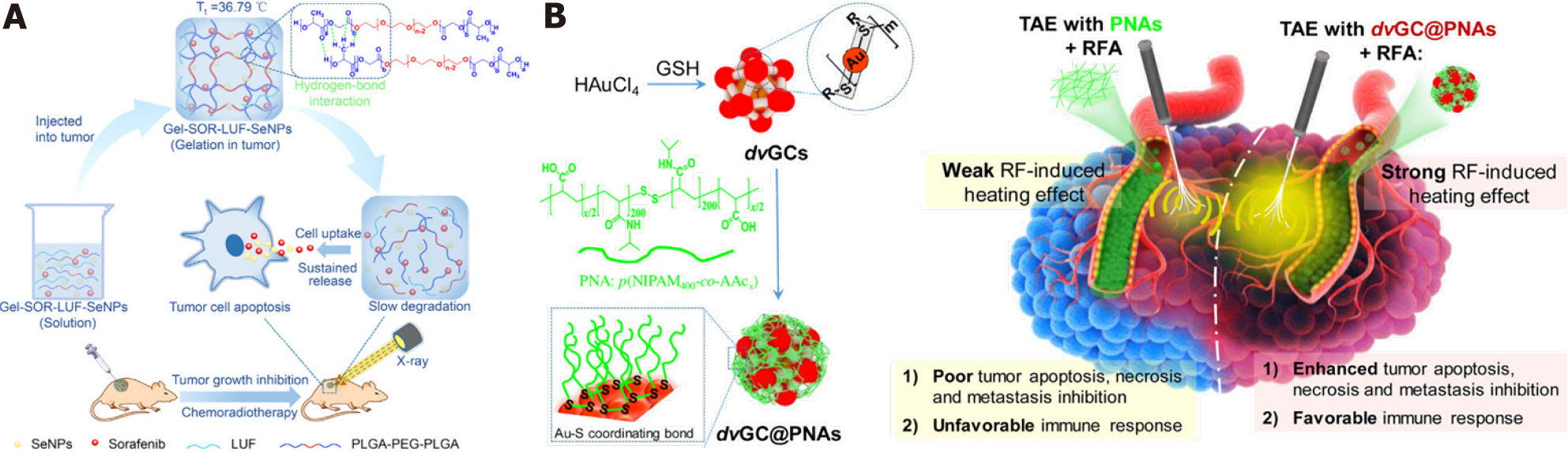
Figure 5 Enhanced therapeutic strategies of in situ treatment.
A: Temperature-sensitive hydrogel with sustained drug release capacity for orthotopic hepatocellular carcinoma therapy. Citation: Zheng L, Li C, Huang X, Lin X, Lin W, Yang F, Chen T. Thermosensitive hydrogels for sustained-release of sorafenib and selenium nanoparticles for localized synergistic chemoradiotherapy. Biomaterials 2019; 216: 119220. Copyright © The Authors 2019. Published by Elsevier; B: Gold nanoclusters in thermosensitive hydrogel for radiofrequency ablation and transcatheter artery embolization. Citation: Li L, Guo X, Peng X, Zhang H, Liu Y, Li H, He X, Shi D, Xiong B, Zhao Y, Zheng C, Yang X. Radiofrequency-responsive dual-valent gold nanoclusters for enhancing synergistic therapy of tumor ablation and artery embolization. Nano Today 2020; 35: 100934. Copyright © The Authors 2020. Published by Elsevier.
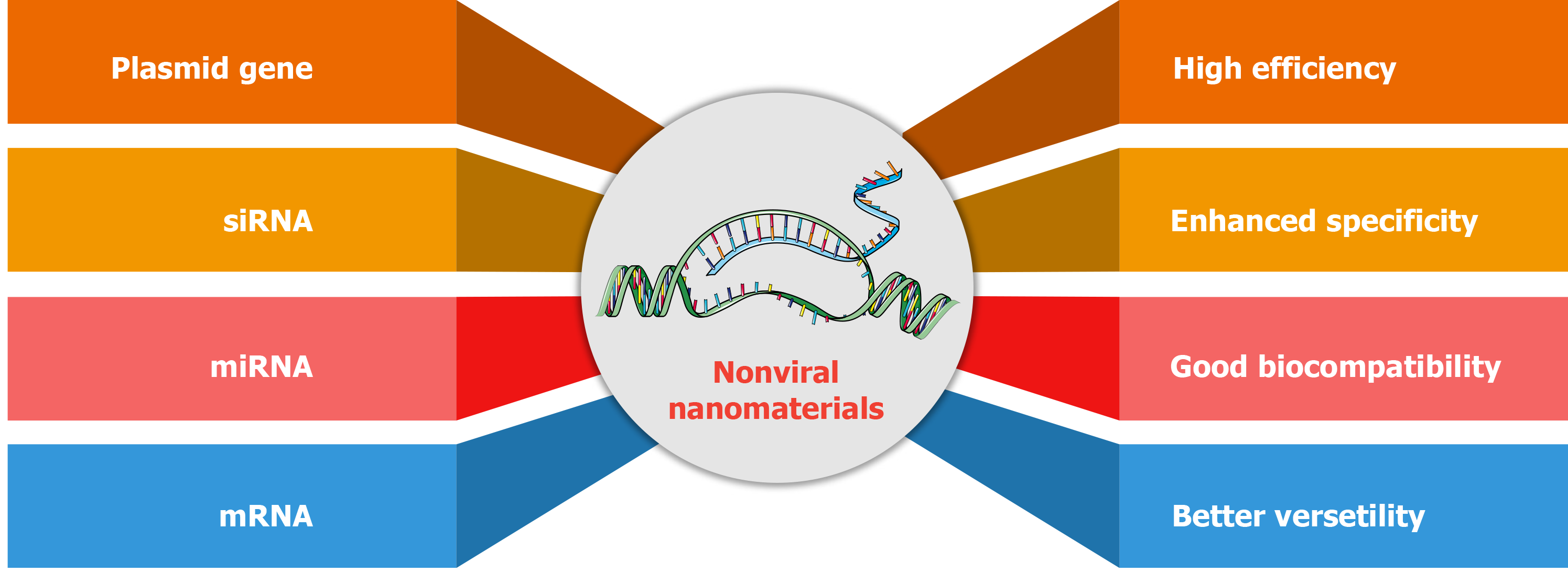
Figure 6 Advantages of nonviral nanomaterials for gene delivery.
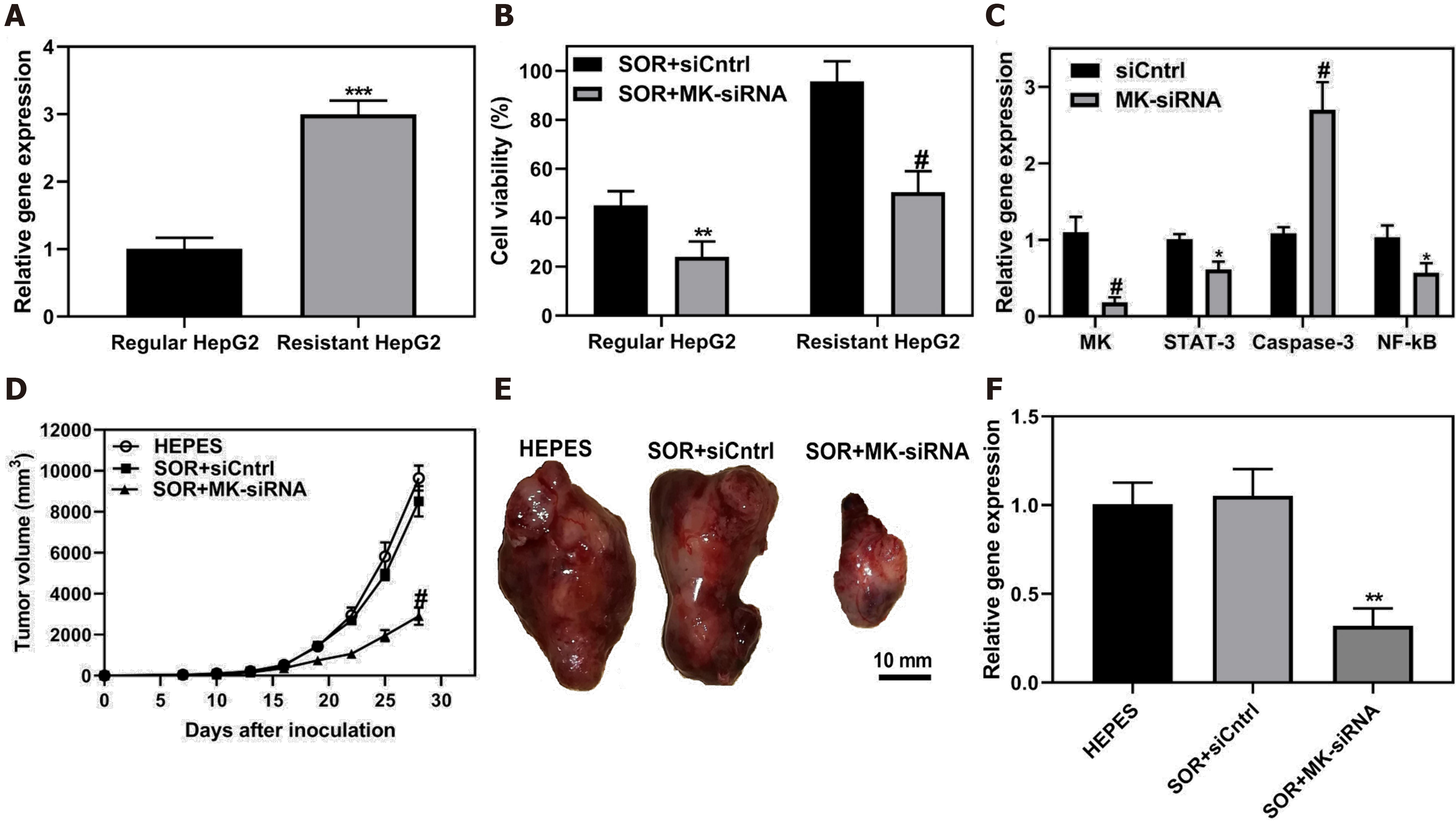
Figure 7 Co-delivery of siRNA and chemotherapeutic sorafenib by ultra-small lipid nanoparticles to overcome drug resistance of hepatocellular carcinoma.
A: MK gene expression was evaluated in HepG2 and sorafenib-resistant HepG2 cells; B: HepG2 and resistant HepG2 cells were treated with sorafenib and MK-siRNA or siCntrl, and subjected test for cell viability; C: Sorafenib-resistant HepG2 cells were treated usLNPs encapsulating MK-siRNA or control. STAT-3, Caspase-3 and NF-κB signaling were assayed; D-E: Sorafenib-resistant HCC tumors in mice were treated with sorafenib and MK-siRNA, and tumor volume and resected tumors were evaluated; F: After treatment, tumor tissue was collected and MK gene expression level was assayed. Citation: Younis MA, Khalil IA, Elewa YHA, Kon Y, Harashima H. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo. J Control Release 2021; 331: 335-349. Copyright © The Authors 2021. Published by Elsevier.
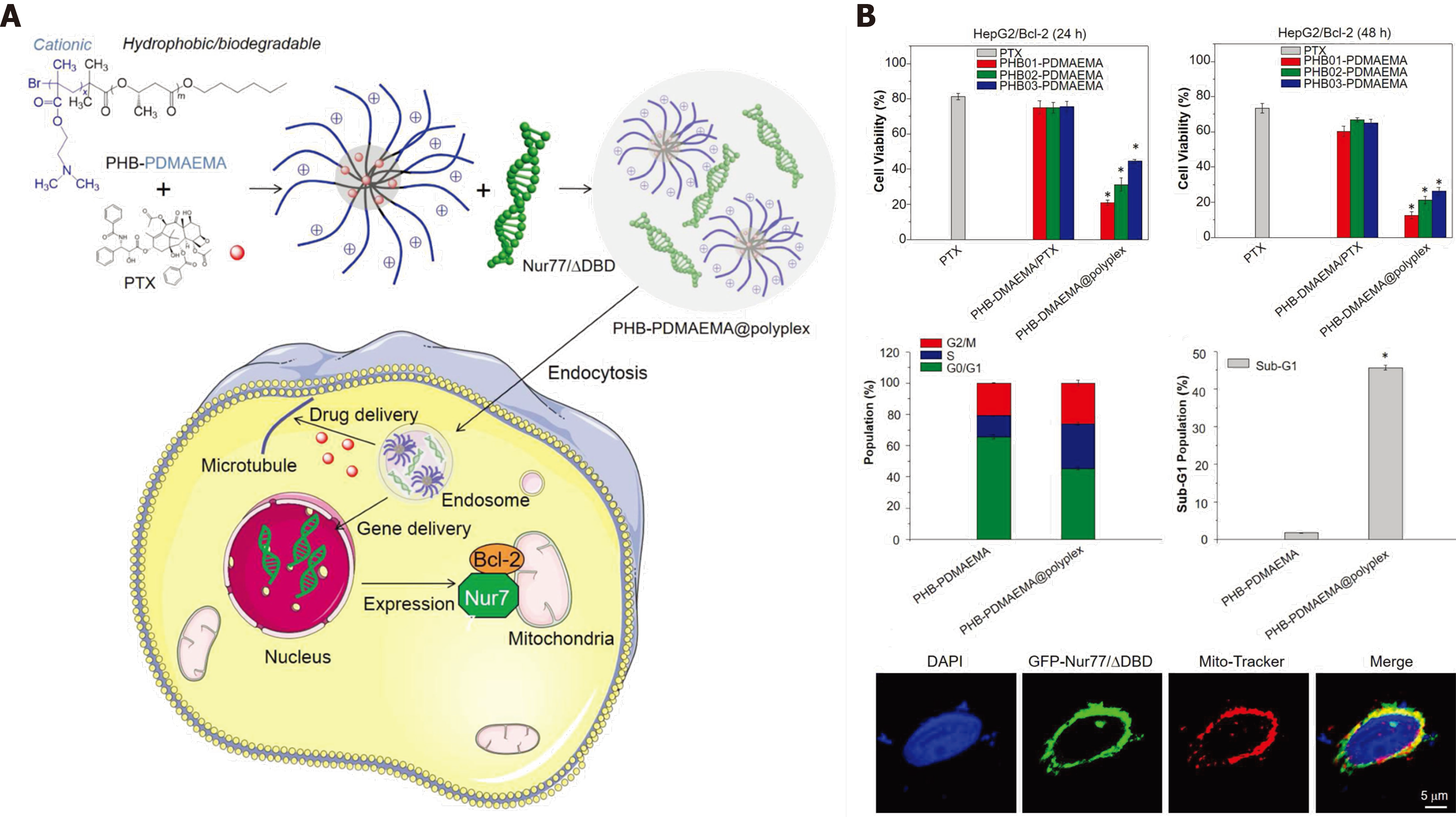
Figure 8 Co-delivery of Bcl-2 conversion gene and chemotherapeutic agents for non-pump drug resistance.
A: The diagram shows the PHB-PDMAEMA@polyplex with chemotherapeutic PTX and Nur77/△DBD to inhibit Bcl-2-mediated drug resistance through the reversal of Bcl-2 from protector to killer in liver tumor cells; B: Significant tumor inhibition of the PHB-PDMAEMA@polyplex of cell viability and cell cycle in Bcl-2-related drug-resistant tumor cells. Citation: Wang X, Liow SS, Wu Q, Li C, Owh C, Li Z, Loh XJ, Wu YL. Co-delivery for Paclitaxel and Bcl-2 Conversion Gene by PHB-PDMAEMA Amphiphilic Cationic Copolymer for Effective Drug Resistant Cancer Therapy. Macromol Biosci 2017; 17. Copyright © The Authors 2017. Published by Wiley.
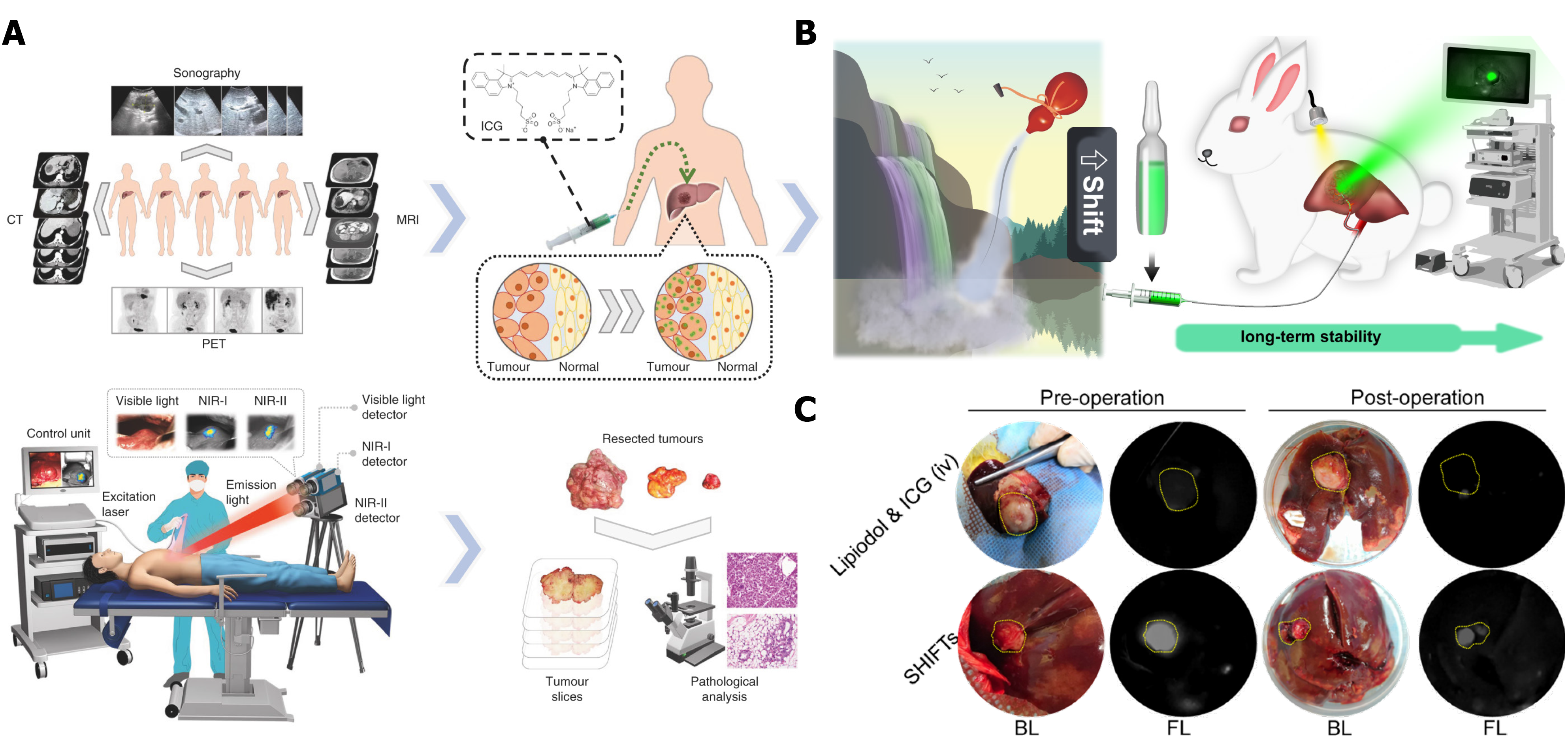
Figure 9 Imaging-guided tumor surgery.
A: ICG fluorescence imaging-guided tumor resection in hepatoma patients; B: Illustration of a superstable homogeneous lipiodol-ICG formulation for HCC therapy; C: Long-term stability of ICG fluorescence in tumors through a combination of transcatheter arterial embolization. Citation: Hu Z, Fang C, Li B, Zhang Z, Cao C, Cai M, Su S, Sun X, Shi X, Li C, Zhou T, Zhang Y, Chi C, He P, Xia X, Chen Y, Gambhir SS, Cheng Z, Tian J. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat Biomed Eng 2020; 4: 259-271. Copyright © The Authors 2020. Published by Springer Nature Limited. Citation: Chen H, Cheng H, Dai Q, Cheng Y, Zhang Y, Li D, Sun Y, Mao J, Ren K, Chu C, Liu G. A superstable homogeneous lipiodol-ICG formulation for locoregional hepatocellular carcinoma treatment. J Control Release 2020; 323: 635-643. Copyright © The Authors 2020. Published by Elsevier.
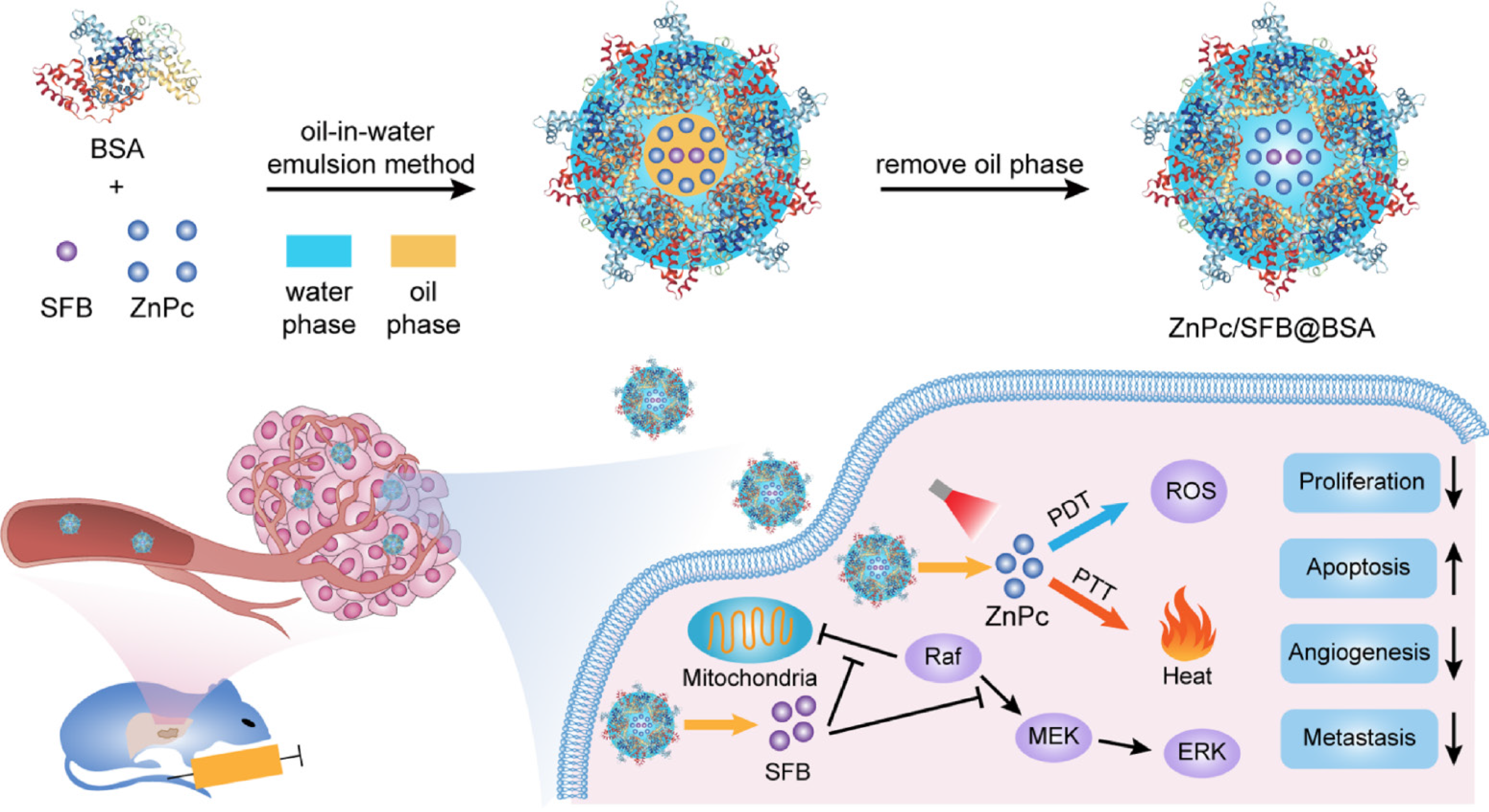
Figure 10 Trimodal therapy of ZnPc/SFB@BSA for orthotopic hepatocellular carcinoma.
Bovine serum albumen (BSA)-coated zinc phthalocyanine and sorafenib (ZnPc/SFB@BSA) nanoparticles for photodynamic therapy (PDT), photothermal therapy (PTT) and chemotherapy with 730 nm light irradiation. Citation: Yu XN, Deng Y, Zhang GC, Liu J, Liu TT, Dong L, Zhu CF, Shen XZ, Li YH, Zhu JM. Sorafenib-Conjugated Zinc Phthalocyanine Based Nanocapsule for Trimodal Therapy in an Orthotopic Hepatocellular Carcinoma Xenograft Mouse Model. ACS Appl Mater Interfaces 2020; 12: 17193-17206. Copyright © The Authors 2020. Published by American Chemical Society.
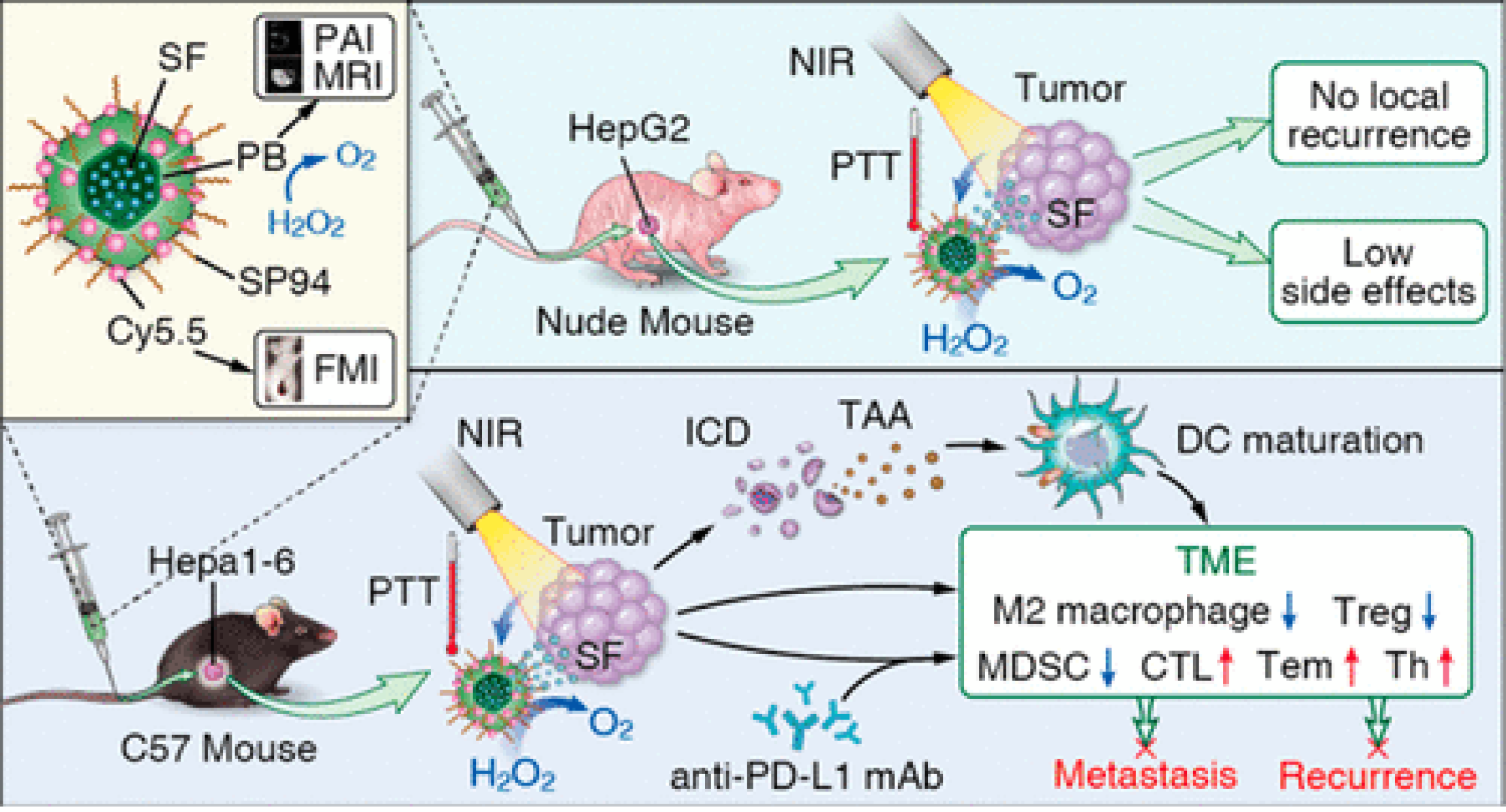
Figure 11 Phototherapy combined with immunotherapy to inhibit hepatocellular carcinoma metastasis and recurrence.
Multifunctional Prussian blue nanoparticles loading sorafenib are conjugated with hepatocellular carcinoma-specific targeting peptide SP94 and Cy5.5. The photothermal treatment induces immunogenic cell death, activating the systemic immune response, and enhance the treatment of anti-PD-L1 therapy. Citation: Zhou T, Liang X, Wang P, Hu Y, Qi Y, Jin Y, Du Y, Fang C, Tian J. A Hepatocellular Carcinoma Targeting Nanostrategy with Hypoxia-Ameliorating and Photothermal Abilities that, Combined with Immunotherapy, Inhibits Metastasis and Recurrence. ACS Nano 2020; 14: 12679-12696. Copyright © The Authors 2020. Published by American Chemical Society.
- Citation: Cao L, Zhu YQ, Wu ZX, Wang GX, Cheng HW. Engineering nanotheranostic strategies for liver cancer. World J Gastrointest Oncol 2021; 13(10): 1213-1228
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1213.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1213