Published online Sep 26, 2023. doi: 10.4252/wjsc.v15.i9.947
Peer-review started: August 14, 2023
First decision: August 22, 2023
Revised: August 31, 2023
Accepted: September 14, 2023
Article in press: September 14, 2023
Published online: September 26, 2023
Processing time: 41 Days and 16.2 Hours
Rapid wound healing remains a pressing clinical challenge, necessitating studies to hasten this process. A promising approach involves the utilization of human umbilical cord mesenchymal stem cells (hUC-MSCs) derived exosomes. The hypothesis of this study was that these exosomes, when loaded onto a gelatin sponge, a common hemostatic material, would enhance hemostasis and accelerate wound healing.
To investigate the hemostatic and wound healing efficacy of gelatin sponges loaded with hUC-MSCs-derived exosomes.
Ultracentrifugation was used to extract exosomes from hUC-MSCs. Nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), and western blot techniques were used to validate the exosomes. In vitro experiments were performed using L929 cells to evaluate the cytotoxicity of the exosomes and their impact on cell growth and survival. New Zealand rabbits were used for skin irritation experiments to assess whether they caused adverse skin reactions. Hemolysis test was conducted using a 2% rabbit red blood cell suspension to detect whether they caused hemolysis. Moreover, in vivo experiments were carried out by implanting a gelatin sponge loaded with exosomes subcutaneously in Sprague-Dawley (SD) rats to perform biocompatibility tests. In addition, coagulation index test was conducted to evaluate their impact on blood coagulation. Meanwhile, SD rat liver defect hemostasis model and full-thickness skin defect model were used to study whether the gelatin sponge loaded with exosomes effectively stopped bleeding and promoted wound healing.
The NTA, TEM, and western blot experimental results confirmed that exosomes were successfully isolated from hUC-MSCs. The gelatin sponge loaded with exosomes did not exhibit significant cell toxicity, skin irritation, or hemolysis, and they demonstrated good compatibility in SD rats. Additionally, the effectiveness of the gelatin sponge loaded with exosomes in hemostasis and wound healing was validated. The results of the coagulation index experiment indicated that the gelatin sponge loaded with exosomes had significantly better coagulation effect compared to the regular gelatin sponge, and they showed excellent hemostatic performance in a liver defect hemostasis model. Finally, the full-thickness skin defect healing experiment results showed significant improvement in the healing process of wounds treated with the gelatin sponge loaded with exosomes compared to other groups.
Collectively, the gelatin sponge loaded with hUC-MSCs-derived exosomes is safe and efficacious for promoting hemostasis and accelerating wound healing, warranting further clinical application.
Core Tip: In this study, we loaded exosomes derived from human umbilical cord mesenchymal stem cells onto a gelatin sponge, a common hemostatic substance in clinics, to stop bleeding and promote wound healing. The fabricated material appears relatively safe, provides better hemostatic activity than gelatin sponge alone, and promotes good wound healing.
- Citation: Hu XM, Wang CC, Xiao Y, Jiang P, Liu Y, Qi ZQ. Enhanced wound healing and hemostasis with exosome-loaded gelatin sponges from human umbilical cord mesenchymal stem cells. World J Stem Cells 2023; 15(9): 947-959
- URL: https://www.wjgnet.com/1948-0210/full/v15/i9/947.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i9.947
In recent years, biomaterials have increasingly been used in clinical practice. Notably, gelatin sponges have been widely used in various surgical procedures owing to their excellent characteristics. Gelatin sponges are highly accessible, cheap, and easy to use, allowing use as a dry material or impregnated with saline or thrombin prior to use. Furthermore, gelatin sponges exhibit a robust absorption capacity and can absorb approximately 40 times their own weight of water or liquid and expand to 200% of their original volume, thus increasing the local platelet concentration[1]. Moreover, the expanded gelatin particles help limit blood flow and provide a packing effect in the limited space, thereby promoting the formation of blood clots during wound healing.
Gelatin sponges were first used in neurosurgery in the 1940s and have since been widely used in various surgical procedures. In recent years, many new applications have been developed for absorbable gelatin sponges. Wang et al[2] have fabricated an Au/Ag gelatin sponge by adding Au/Ag to the gelatin matrix, which improved its antibacterial performance. Yang et al[3] have used a cocktail of ropivacaine, dexamethasone, and vitamin B12 to impregnate a gelatin sponge for promoting leg pain relief and functional recovery after percutaneous endoscopic lumbar discectomy. Notably, Jogo et al[4] have loaded gelatin sponge particles with 5% ethanolamine oleate iopamidol and used the mixture to examine the effect of retrograde occlusion of veins in gastric varices. Incorporating colloidal Ag into gelatin sponges was beneficial in promoting bone healing in infected skull defects[5]. Accordingly, gelatin sponges can be used to promote wound healing and combined with various drugs or substances to improve their therapeutic effects.
Mesenchymal stem cells (MSCs) have been shown to markedly improve wound closure, angiogenesis, and wound healing. However, stem cell therapy is complex, expensive, and time-consuming. Recent studies have demonstrated the effectiveness of stem cell-derived exosomes in treating wounds. Exosomes are emerging as a new mode of intercellular communication. Additionally, exosomes play an important role in wound repair[6]. Exosomes are membrane-derived vesicles interacting with target receptor cells, enabling inter cellular communication[7]. Growing evidence suggests that exosomes are crucial in coagulation, intercellular signaling, and waste metabolism[8]. The biological characteristics of exosomes derived from MSCs are similar to those of umbilical cord (UC)-MSCs, making them valuable for tissue repair.
Recently, an increasing number of studies have explored the combined application of exosomes and biological materials. Wang et al[9] have fabricated an injectable, viscous, heat-sensitive, multifunctional polysaccharide-based wound dressing capable of sustained exosomes release to accelerate wound healing by stimulating the angiogenic process of the wound tissue. Tao et al[10] have combined exosomes released by synovial MSCs overexpressing miR-126-3p with chitosan, demonstrating that this strategy could be used to treat skin wounds. Xu et al[11] have assembled exosomes on chitosan/silk hydrogel sponges based on platelet-rich plasma to examine wound healing in a diabetic rat model, revealing their capacity to promote wound healing during diabetes. Furthermore, Nooshabadi et al[12] have employed an exosome-loaded chitosan hydrogel to study wound healing and dermal reconstruction in mice. The authors found that the exosome-loaded chitosan hydrogel exhibited a wound closure capacity of approximately 83.6% and a high degree of re-epithelization[12]. Based on the above literature, novel dressings made by combining exosomes and a variety of biological materials afford better effects than conventional dressings.
In the present study, we loaded a common clinically used gelatin sponge with exosomes to synthesize a novel gelatin sponge with improved characteristics. The safety and effectiveness of the improved gelatin sponge in hemostasis and promotion of healing were studied both in vivo and in vitro. The findings will provide novel avenues for further research on the potential applications of human UC MSCs (hUC-MSCs)-derived exosomes in tissue repair. Furthermore, our study provided evidence for the feasibility of using a bioscaffold synthesized by combining exosomes and biological materials simply and non-invasively to stop bleeding and promote wound healing, thereby supporting the further use of exosomes for clinical applications.
All animals were obtained from Changsha Tianqin Biotechnology Co., Ltd. (Changsha, China) and were housed under ordinary conditions. The animals were maintained under a 12-h light/12-h dark cycle at stable temperature (22-26 °C) and humidity (50%-70%). They were given free access to food and water throughout the study period.
hUC-MSCs were purchased from Guangxi TaiMeiRenSheng Biotechnology Co. Ltd. Fourth-passage cells at logarithmic phase were inoculated in T175 air-permeable cell culture flasks with a dedicated medium (HUXUC-90011, Cyagen). The cells were cultured at 37 °C in an incubator containing 5% CO2. When the adherent cells reached a confluence of 80%-90%, the used cell medium was discarded and replaced with a serum-free medium. After 48 h, cells were centrifuged and cell supernatants were collected. Exosomes in the cell supernatants were extracted by ultracentrifugation. Impurities in the cell supernatants were removed by centrifugation at 300 × g for 10 min, followed by the removal of dead cells by centrifugation at 2000 × g for 20 min; then, cell debris was removed by centrifugation at 10000 × g for 30 min. Subsequently, cell supernatants were filtered using a 0.22 μM membrane filter and centrifuged in an ultracentrifuge (Beckman, America) for 120 min at 120000 × g, and the supernatants were discarded. The precipitated exosomes were re-suspended in phosphate buffered saline (PBS) and centrifuged again for 120 min at 120000 × g, and the exosomes precipitate were finally re-suspended in filtered PBS. All centrifugations were performed at 4 °C.
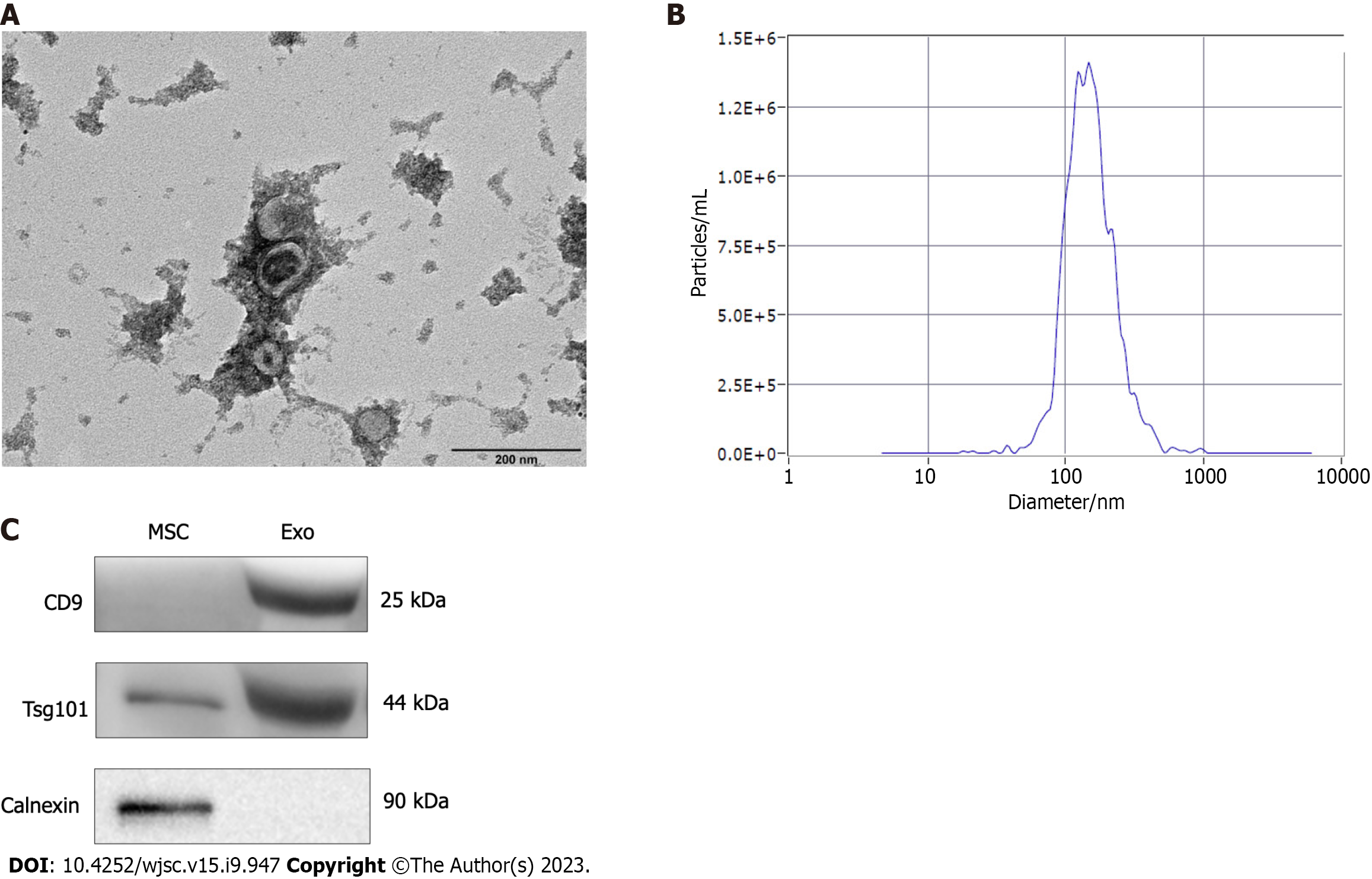
Exosomes were identified using a nanoparticle tracking analyzer (ZetaView, Particle Metrix, Germany), which was used to measure their concentration and particle size. A transmission electron microscope (HT-7700, Hitachi High-tech Company) was used to observe the shape and size of exosomes. Western blot was used to detect the surface marker proteins, CD9, Tsg101, and Calnexin, in exosomes. The protein concentration of hUC-MSCs-derived exosomes was determined using a BCA protein quantitation kit (Zoman, Biotechnology, ZD301). After adding the required proportion of loading buffer, the sample was heated at 95 °C for protein denaturation. Protein from each sample was separated on a sodium dodecyl sulfate-polyacrylamide gel and then transferred to polyvinylidene fluoride membranes. After blocking with 5% skim milk for 2 h, the membranes were incubated overnight with anti-CD9 (20597-1-AP, Proteintech), anti-Tsg101 (28283-1-AP, Proteintech), and anti-Calnexin (10427-2-AP, Proteintech) antibodies. Subsequently, the secondary antibody (SA00001-2, Proteintech) was added, followed by incubation for 1.5 h. Band visualization was achieved using a chemiluminescence kit (P0018FM, Beyotime, China) and observed on a chemiluminescent imaging system (MiniChemi 610, Sagecreation, China).
An absorbable gelatin sponge was purchased from Jiangxi Xiangen Medical Technology Development Co., Ltd. It was sliced into appropriate sizes, and immersed in the exosome solution mixed with trehalose, to ensure that the gelatin sponge fully absorbed the exosomes. Subsequently, the loaded gelatin sponge was freeze-dried and characterized via scanning electron microscopy.
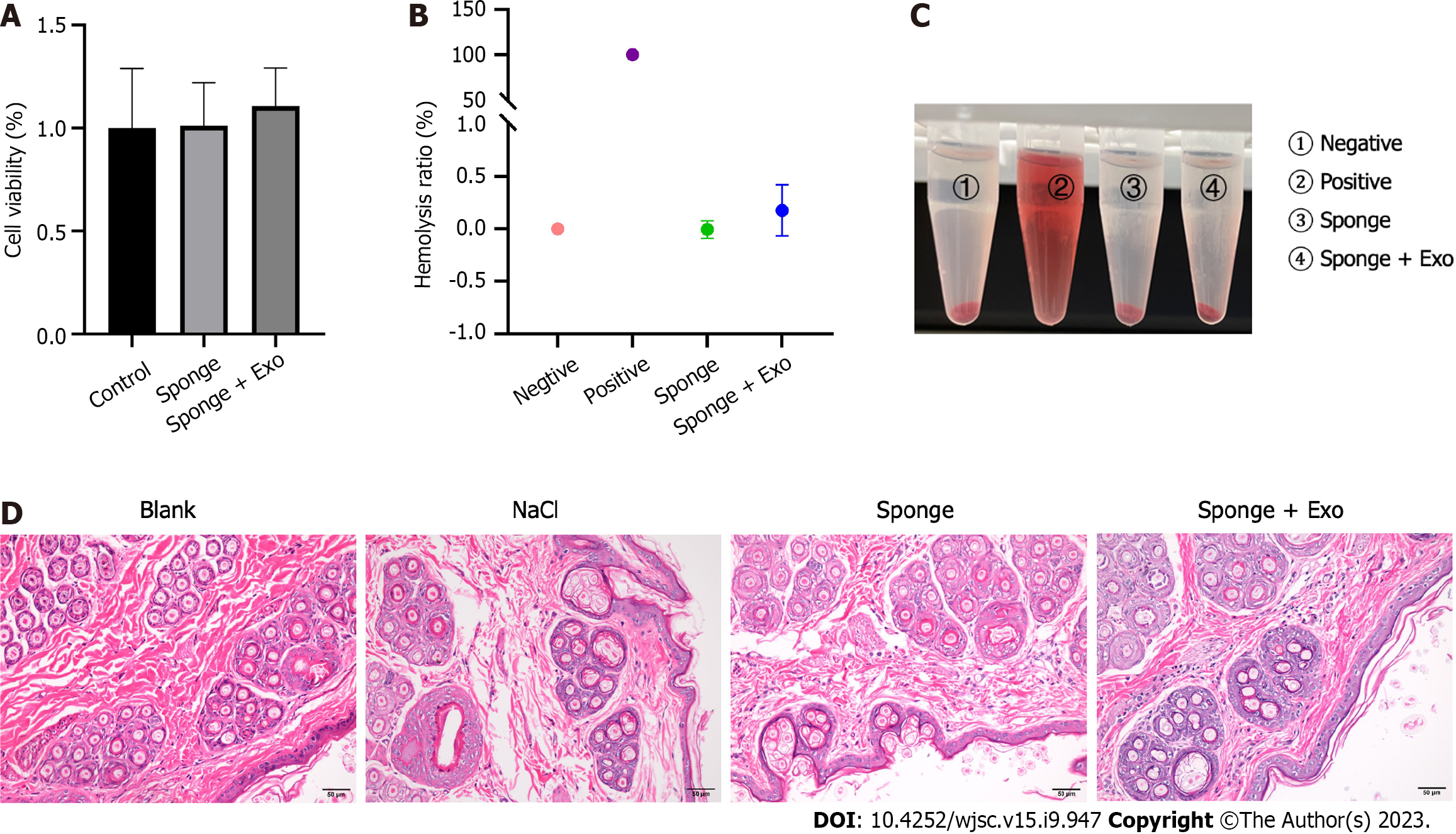
The effect of the hUC-MSCs-derived exosomes on mouse epithelioid fibroblasts (L929) was evaluated using the CCK-8 assay. Briefly, L929 cells were inoculated into 96-well plates and divided into control, sponge extract, and exosome groups. The cell density was adjusted to 10000 cells/well and then incubated at 37 °C in a 5% CO2 incubator for 12 h. Subsequently, the medium in the exosome group was adjusted to ensure that each well contained 6 × 106 particles of exosomes and then incubated at 37 °C under 5% CO2 for a further 6 h. After 6 h, 10 μL of CCK-8 reagent was added to all groups, followed by incubation in the dark for 1 h. Finally, the absorbance of each well was measured at 450 nm using a microplate reader.
Briefly, 5 mL of fresh blood from New Zealand rabbits was stirred in one direction with a glass rod, followed by fibrin removal; then, 20 mL of normal saline was added. The mixture was mixed well and centrifuged at 2000 rpm for 10 min. The supernatant was discarded, normal saline was added, and the process was repeated 3-4 times until the supernatant was clear. Finally, the supernatant was discarded, and 1 mL of fresh blood was added to 49 mL of normal saline to obtain a 2% rabbit red blood cell suspension for subsequent use. The assay was divided into negative control, positive control, sponge, and exosome-loaded sponge groups. Subsequently, 500 μL of the 2% rabbit red blood cell suspension was added to each group, and an equal volume of liquid was added (normal saline was added to the negative control group, and Triton X-100 with a mass fraction of 0.5% was added to the positive control group. Gelatin sponge extract at a concentration of 10 mg/mL was added to the sponge group, and gelatin sponge extract with 1 × 109 exosomes was added to the exosome-loaded sponge group). Then, the solutions were mixed evenly and incubated at 37 °C for 3 h. The samples were then centrifuged at 3000 rpm for 3 min. The optical density (OD) of the supernatant was measured at a wavelength of 545 nm, and the hemolysis rate was calculated by photography. The hemolysis rate was calculated as (ODsam - ODneg)/(ODpos - ODneg) × 100% (1)[13].
The skin irritation test was performed using three New Zealand rabbits. The hair on their backs was carefully removed. Four specific areas on their skin were identified and treated with different fluids. They were blank group without any articles, NaCl group with 0.9%NaCl solution, sponge group with 10 mg/mL sponge extract, and exosome group with 5 × 108 exosomes solution. The application was repeated every 24 h until absorption was complete. The rabbits were closely monitored to assess any changes in their skin condition, such as redness, swelling, inflammation, or other related symptoms. After 72 h, skin samples were harvested from treated sites for histopathological examination to observe any signs of inflammation in the skin tissue.
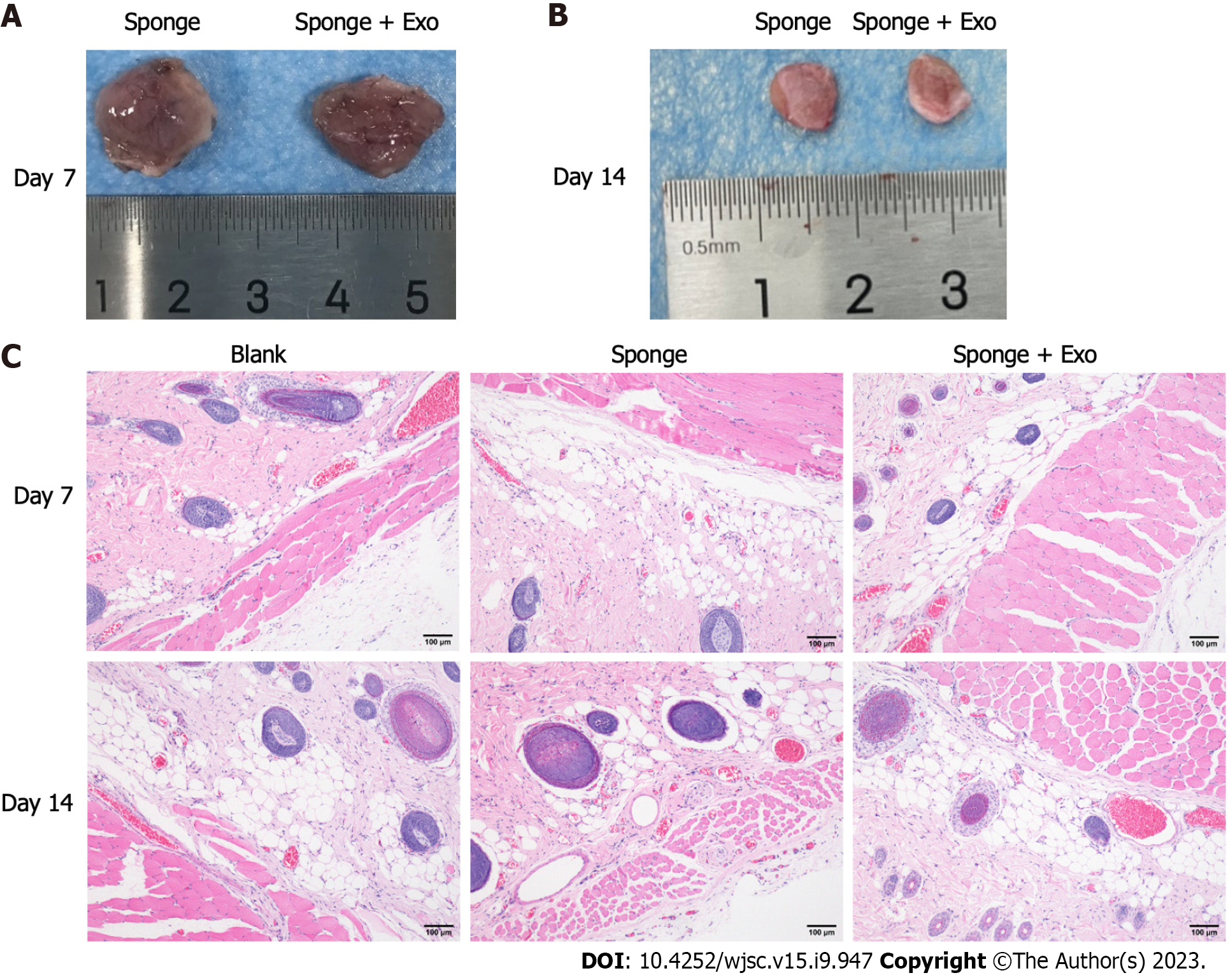
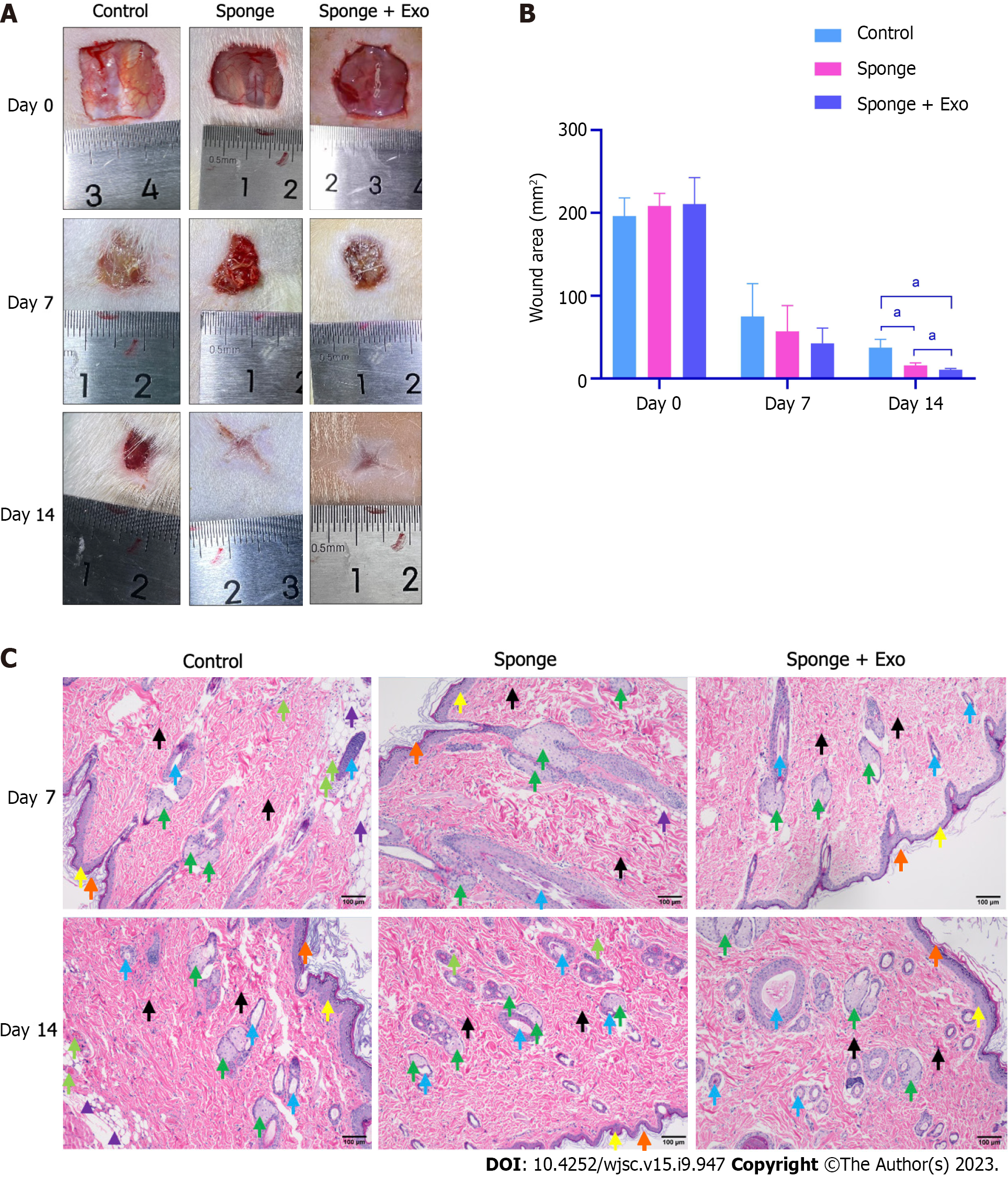
Eighteen male Sprague-Dawley (SD) rats were divided into blank control, sponge, and exosome-loaded sponge groups. After anesthesia with 10% chloral hydrate, the hair on their back was removed, the subcutaneous bags were excised, and 1 cm × 1 cm × 0.5 cm of either gelatin sponges or gelatin sponges loaded with exosomes were implanted into them, while the blank control group was not implanted with any gelatin sponge. The adjacent skin was collected for pathological analysis on day 7 and 14, respectively, to evaluate the in vivo biocompatibility of the gelatin sponges.
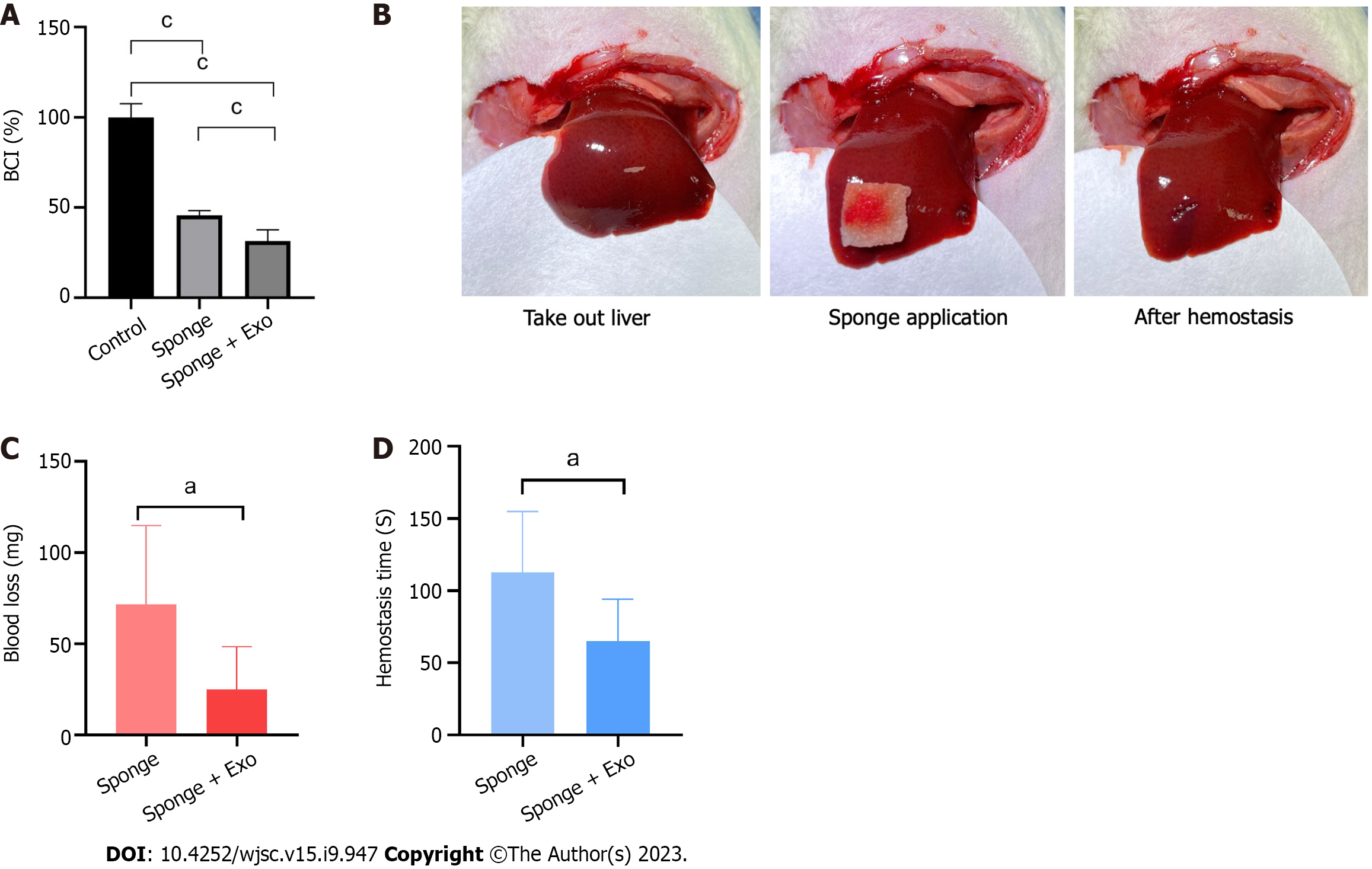
The gelatin sponges were cut into 1 cm × 1 cm × 0.5 cm sections, and preheated in an oven at 37 °C for 5 min. In the sponge and exosome groups, the gelatin sponges were infiltrated with 100 μL of normal saline and 100 μL of exosome solution containing 5 × 108 exosomes, respectively. Next, 100 μL of rat whole blood was added to each gelatin sponge in both groups for adequate absorption, followed by 10 μL of 0.2 M calcium chloride solution, and incubated at 37 °C for 15 min. Subsequently, 25 mL of deionized water was used to rinse blood from the gelatin sponge surface without coagulation. Finally, the absorbance (Abs1) of the washed liquid was measured at 540 nm by using a microplate reader. For the blank control (Abs0), 100 μL of whole blood was added to 25 mL of deionized water, and the blood coagulation index (BCI) was calculated using the formula: BCI (%) = Abs1/Abs0 × 100% (2).
The hemostatic capacity of the exosome-loaded gelatin sponge was evaluated using a rat liver defect hemostasis model[14]. Twelve SD rats were divided into sponge and exosome-loaded sponge groups (n = 6 rats/group), with an equal distribution of male and female rats. After anesthesia induction with 10% chloral hydrate, an abdominal incision was made to expose the liver, and the tissue fluid around the liver was carefully removed using a dry cotton ball to prevent inaccurate blood weight measurements. The liver was placed on a pre-weighed filter paper, and a 0.5 cm long and 0.2 cm deep wound was created on the liver using a scalpel. The gelatin sponge loaded with exosomes was immediately placed at the bleeding site and bleeding was observed. After the bleeding stopped completely, the bleeding time was recorded, and the blood-absorbed filter paper was weighed. The weight difference between the front and rear of the filter paper was considered as the weight of blood loss from the rat liver.
Eighteen male SD rats (220-280 g) were randomly divided into control, sponge, and exosome-loaded sponge groups. After anesthesia induction with 10% chloral hydrate, the hair on the back of rats was removed, and a full-thickness skin defect wound (approximately 1.5 cm × 1.5 cm) was created on their backs. Photographs were taken to record the original area, and the wound was covered with either a gelatin sponge or an exosome-loaded gelatin sponge, and wrapped with a transparent dressing; the control group received only a transparent dressing. On day 7 and 14 post the procedure, the gauze was opened to observe and record wound healing. All wounds were photographed, the area was analyzed using Image J software (National Institutes of Health, Bethesda, MD), and skin samples around the wounds were collected and fixed with 4% paraformaldehyde for pathological analysis.
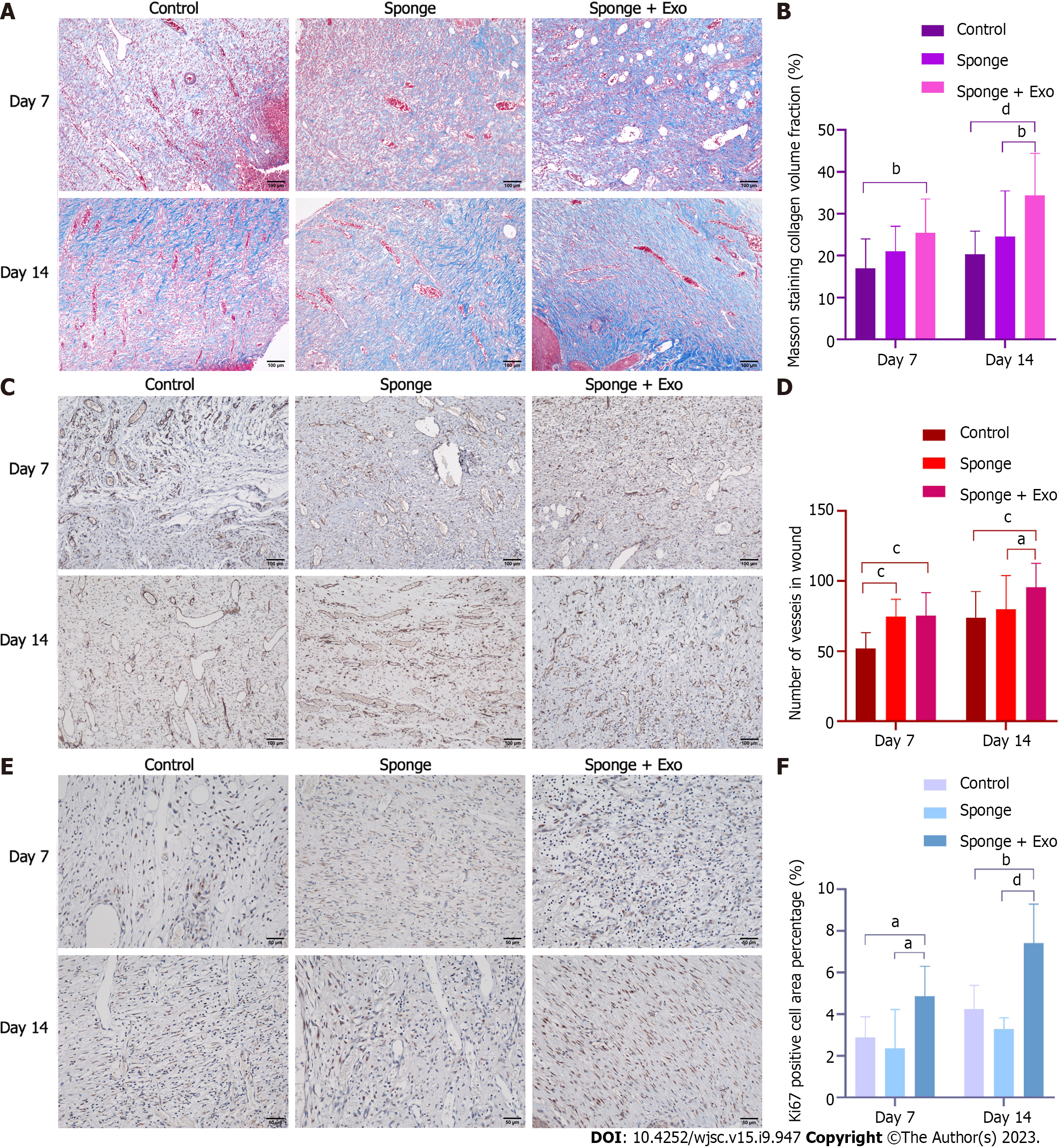
Masson’s trichrome staining was used to determine collagen content and wound maturity. Image J software was used to analyze the areas of interest. The collagen content was quantified. Ki67 was used to evaluate local whole-cell proliferation and CD31 staining was used to evaluate angiogenesis. Sections were incubated with antibodies against Ki67 (1:600, #AF0198, Affinity) or CD31 (1:1000, Cat No.28083-1-AP, Proteintech). Subsequently, the secondary antibody was added (2118D1104, Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.). Six randomly selected areas were imaged using an inverted microscope for each specimen, and the number of Ki67- and CD31- positive cells/positive blood vessels was detected using image J software.
SPSS 26.0 (IBM Corp., Armonk, NY, United States) and GraphPad Prism 8.0 (GraphPad Software Inc.; San Diego, CA, United States) were used for statistical analyses. Paired or unpaired t-tests were used for comparisons between two groups, and one-way analysis of variance (ANOVA) was used for comparisons among three or more groups. P < 0.05 was considered statistically significant.
We successfully isolated exosomes from hUC-MSCs by ultracentrifugation. After successful separation, the morphology of the exosomes was observed by using TEM, and a typical cup-shaped structure was observed (Figure 1A). The exosome concentration and particle size were measured using a nano-particle size analyzer. The average exosome particle size was 144.7 nm, consistent with the particle size of exosomes (30-150 nm) (Figure 1B). Western blot results showed that the extracted exosomes successfully expressed the surface marker proteins CD9 and Tsg101, but not Calnexin (Figure 1C).
The exosomes on the gelatin sponge were detected via scanning electron microscopy. The gelatin sponge with exosomes was coarser than the sponge without exosomes, and exosome particles could be observed (Figures 2A and B).
The gelatin sponge loaded with exosomes did not induce significant toxicity toward L929 cells as the cells remained viable following exposure to them (Figure 3A). Moreover, the hemolytic assay showed that no hemolysis was observed with the naked eye using the gelatin sponge loaded with exosomes (Figure 3C). Measuring the OD value of the solution and calculating the hemolysis rate, we found that the hemolysis rates of the gelatin sponge and exosome-loaded gelatin sponge in whole blood were -0.01% and 0.18%, respectively, which were within the permissible range for biological materials (less than 5%), indicating that the exosome-loaded gelatin sponge was safe for use (Figure 3B). All experimental rabbits had an intact epidermis, with normal sebaceous glands, hair follicles, and hair structures; there were no notable pathological changes, such as inflammatory cell infiltration, tissue congestion, and edema (Figure 3D).
To investigate the in vivo biocompatibility of gelatin sponge loaded with exosomes, we implanted the gelatin sponge into the dorsal subcutaneous space of SD rats, and observed the condition of the implant on day 7 and day 14 (Figures 4A and B). A portion of the gelatin sponge was retained in the body on day 7 and day 14; however, the gelatin sponge on day 14 was obviously smaller than that on day 7, indicating that the gelatin sponge could be degraded in the body. Adjacent skin tissue samples were collected for pathological analysis. After 7 and 14 d of implantation, there was no obvious inflammatory response around the tissue, and no obvious tissue fibrosis or necrosis was observed (Figure 4C), indicating that the gelatin sponge loaded with exosomes showed good biocompatibility.
We evaluated the clotting ability of gelatin sponge and gelatin sponge loaded with exosomes. Our findings showed that the BCI values of the gelatin sponge and exosome-loaded gelatin sponge groups were 45.63% and 31.47%, respectively, indicating that both groups had significant clotting ability, and the clotting ability of the exosome-loaded gelatin sponge was significantly higher than that of the gelatin sponge group (Figure 5A).
After establishing a liver defect model, we covered the wound with a gelatin sponge and recorded blood loss and hemostasis time. The blood loss and hemostasis time of the gelatin sponge group loaded with exosomes were significantly lower than those of the gelatin sponge group, indicating that the gelatin sponge loaded with exosomes had a better hemostatic effect than the conventional gelatin sponge (Figures 5B-D).
After establishing a rat full-thickness skin defect model, the wound was covered with a gelatin sponge, and the gauze was opened on day 7 and day 14 post the procedure to observe and record the wound healing status. The wound size of all groups decreased with time. Moreover, the wound size of the gelatin sponge group loaded with exosomes was smaller than that of the other two groups, indicating that the gelatin sponge loaded with exosomes promoted wound healing better than the conventional gelatin sponge (Figures 6A and B). Histopathological results showed that on day 14, the gelatin sponge loaded with exosomes group demonstrated better regeneration of hair follicles and sebaceous glands than the other two groups (Figure 6C).
Masson’s trichrome staining was performed on the skin adjacent to the wound to evaluate the formation and distribution of collagen, which is important for promoting wound healing. Microscopic observations showed that collagen fibers in the skin of all three groups were relatively few on day 7. However, on day 14, the collagen fibers in the skin of the three groups gradually increased along with gradual healing of the wound (Figure 7A). Statistical analysis showed that the collagen fibers in the exosome-loaded gelatin sponge group were significantly higher than those in the other two groups on day 14; on day 7, the collagen fibers in the gelatin sponge and exosome-loaded gelatin sponge groups were significantly higher than those in the control group (Figure 7B). These findings suggested that the exosome-loaded gelatin sponge promoted wound healing by promoting collagen fiber synthesis.
To explore the possible mechanism of action of gelatin sponges loaded with exosomes, we employed immunohistochemical techniques to evaluate angiogenesis and cell proliferation in the granulation tissue. To evaluate angiogenesis, we stained the skin near the wound for CD31. On day 7 and day 14, the number of CD31-positive blood vessels in the exosome-loaded gelatin sponge group was significantly higher than that in the control group; on day 14, this number was significantly higher than that in the gelatin sponge group (Figures 7C and D). Subsequently, we detected the proliferation of whole cells of granulation tissue in the skin near the wound and found that the proportion of Ki67-positive cells in the exosome-loaded gelatin sponge group was significantly higher than that in the control and gelatin sponge groups on day 7 and day 14. However, there was no statistically significant difference in the proportion of Ki67-positive cells between the gelatin sponge and control groups (Figures 7E and F). These results indicated that the gelatin sponge loaded with exosomes could promote cell proliferation and angiogenesis, thereby accelerating wound healing.
Wound healing presents an important challenge for both clinicians and patients. Poor wound healing can negatively impact the patient’s quality of life and exacerbate pain, stress, and depression[15,16]. Therefore, methods that effectively promote wound healing are particularly important. Several studies have demonstrated that MSCs accelerate wound closure by promoting skin cell migration, angiogenesis, epithelization, and granulation tissue formation. However, the clinical application of MSCs faces ethical issues, and there is a lack of long-term follow-up evidence regarding their safety. Therefore, alternative cell-free therapies are required. With the emergence of the paracrine hypothesis, the therapeutic applications of exosomes have become more extensive. Exosomes exhibit functions similar to MSCs[17]. Given their immunogenicity, compared with that of MSCs, exosomes can be considered a good alternative[18]. Recently, an increasing number of studies have shown that stem cell-derived exosomes contain mRNA, microRNAs, growth factors, and various other proteins, and potentially participate in processes such as hemostasis, angiogenesis, and wound healing[19,20]. For example, exosomes derived from hUC-MSCs containing miR-21, miR-23a, miR-125b, and miR-145 inhibit fibroblast proliferation and reduce scar formation during wound healing[21].
In the current study, we proposed a new method for loading exosomes into gelatin sponges to achieve wound healing. Our analyses showed that exosome-loaded gelatin sponge was safe and reliable for use. In vivo and in vitro experiments showed that exosome-loaded gelatin sponge exhibited a good hemostatic effect and promoted the formation of wound granulation tissue, collagen deposition, and angiogenesis, thereby promoting wound healing. Although several studies have shown that exosomes are well-tolerated in animal models, their clinical safety needs to be verified. Considering the goal of exosome-loaded gelatin sponges for clinical application, the safety of exosome-loaded gelatin sponges was verified in this study. We confirmed that the exosome-loaded gelatin sponge was safe for use, as determined by hemolysis, skin irritation, and histocompatibility experiments, consistent with the results of Sun et al[22]. These findings provide further evidence for the clinical application of exosomes.
Healing of skin injuries is a complex process that mainly includes four stages: Hemostasis, inflammation, proliferation, and remodeling[23]. The first stage involves hemostasis and clot formation to prevent blood loss. The second stage is the inflammatory stage, which includes coagulation, phagocytosis, removal of foreign bodies, and recruitment of growth factors and anti-inflammatory cells to injury site. The third stage involves proliferation, including fibrous proliferation, angiogenesis, and cell migration. Finally, fibroblasts continue to secrete collagen during the remodeling phase[24,25]. Type I collagen replaces type III collagen at the wound site. Subsequently, scar formation occurs via apoptosis.
In this study, we conducted in vitro and in vivo experiments to verify the potential role of a gelatin sponge loaded with exosomes in coagulation and hemostasis. We found that the coagulation index of the gelatin sponge loaded with exosomes was significantly lower than that of the control group and the gelatin sponge group, thereby suggesting that the gelatin sponge loaded with exosomes had significant coagulation ability; this finding was also confirmed in the liver injury hemostasis experiment, where the gelatin sponge group loaded with exosomes reduced bleeding in rats and shortened the bleeding time. These data proved that the gelatin sponge loaded with exosomes afforded a better hemostatic effect than the conventional gelatin sponge, and had a significant effect in promoting wound healing during this stage of hemostasis.
Angiogenesis is essential for wound repair. Blood vessels provide progenitor cells, oxygen, and nutrients to maintain proliferation and remodeling of the wound site[26]. CD31 is an endothelial cell marker demonstrating the degree of tissue vascularization. In the current study, we evaluated angiogenesis by immunohistochemical staining for CD31 in the skin adjacent to the wound. The results showed that the number of CD31-positive blood vessels in the exosome-loaded gelatin sponge group was significantly higher than that in the control and gelatin sponge groups, indicating that the exosome-loaded gelatin sponge had a better angiogenic effect than the conventional gelatin sponge. This may be related to the provascular effects of exosomes. Many studies have shown that exosomes participate in angiogenesis signaling pathways and affect the occurrence, development, and maturation of blood vessels[27]. Zhang et al[28] have extracted exosomes from induced pluripotent stem cells and studied their effects on the proliferation, migration, and angiogenesis of hUC blood venous endothelial cells. They found that in a rat model of full-thickness skin defects, exosomes could promote not only angiogenesis at the wound site but also the maturation of blood vessels[28]. Fat-derived exosomes can also be ingested by the vascular endothelium to promote blood vessel formation in vivo and in vitro[29].
Masson’s trichrome staining is a classical technique used to distinguish collagen and muscle fibers. In the current study, we performed Masson’s trichrome staining on the skin near the wounds of rats. We found that the collagen fibers produced in the exosome-loaded gelatin sponge group were significantly higher than those in the gelatin sponge and control groups at the end of the observation period. This indicates that the exosome-loaded gelatin sponge could promote the formation of collagen fibers. This finding is consistent with that of Kim et al[30], who employed exosomes to directly treat human skin tissue and found that exosomes can facilitate the migration of fibroblasts and nearby collagen synthesis, as well as promote the synthesis of collagen and elastin in human skin tissue. Ki67 is a marker of cell proliferation, and, in general, angiogenesis begins with endothelial cell proliferation, followed by their isolation, migration, adhesion, and differentiation from adjacent tissues[31]. Cell proliferation and migration are the two key steps in angiogenesis. In this study, we detected the proliferation of integral cells of granulation tissue in the skin near the wound and found that the proportion of Ki67-positive cells in the exosome-loaded gelatin sponge group was significantly higher than that in the control and gelatin sponge groups. This finding was consistent with the results reported by Wang et al[9], who found that in diabetic rats, the expression of the Ki67 in the FEP@exo group was significantly higher than that in the FEP and control groups. These observations may be related to the promotion of cell proliferation. Moreover, the application of exosomes derived from hUC-MSCs has been explored in a rat skin burn model, revealing that exosomes could dose-dependently promote fibroblast proliferation[6,32].
In summary, using an exosome-loaded gelatin sponge for wound healing could be safe and reliable and promote wound healing by promoting cell proliferation, collagen fiber formation, and blood vessel formation.
In conclusion, our study has proposed a novel method that uses hUC-MSC-derived exosomes loaded onto gelatin sponges for wound healing, which afforded good hemostatic and wound healing-promoting effects. The exosome-loaded gelatin sponge is safe and could provide new avenues for the clinical application of exosomes. Gelatin sponges are a common hemostatic material used in clinical practice. Moreover, they are inexpensive and readily available. Therefore, combining exosomes and gelatin sponges has strong clinical adaptability and is conducive to the clinical transformation and application of exosomes.
Hemostasis and wound healing are one of the common problems in clinics which need to be paid attention to. Gelatin sponge is often used as a hemostatic material in clinics. Exosomes have been proved to play an important role in wound repair. Therefore, it is worth studying whether the combination of exosomes derived from human umbilical cord mesenchymal stem cells (hUC-MSCs) and a gelatin sponge could promote hemostasis and wound healing more efficiently.
Poor wound healing would contribute a negative impact on patients’ quality of life and aggravate pain, stress and depression, so it is of great significance to find ways to effectively promote wound healing.
The present study aimed to investigate the hemostatic and wound healing efficacy of a gelatin sponge loaded with hUC-MSCs-derived exosomes.
After the exosomes were extracted and characterized by ultracentrifugation, we loaded the exosomes on a gelatin sponge. Then, in vitro and in vivo experiments, including cell viability assay, hemolysis assay, skin irritation test, and histocompatibility assay were performed to verify the safety of the exosome-loaded gelatin sponge. Subsequently, whole blood coagulation index test, hemostatic assay using a rat liver defect hemostasis model, and full-thickness skin defect healing promoting test were performed to verify the effects of the exosome-loaded gelatin sponge in hemostasis and wound healing.
We successfully extracted exosomes from hUC-MSCs. The safety experiments showed that the gelatin sponge loaded with exosomes would not cause abnormal proliferation of L929 cells, hemolysis, or irritation to skin and tissues. In addition, the exosome-loaded gelatin sponge had a better hemostatic effect than the traditional gelatin sponge, which can promote the formation of collagen fibers and blood vessels around the wound and increase the proportion of Ki67-positive cells, thus promoting the wound healing.
In a word, gelatin sponge loaded with hUC-MSCs-derived exosomes is safe. It is better than traditional gelatin sponge in stopping bleeding and promoting wound healing.
The gelatin sponge loaded with exosomes derived from hUC-MSCs may be a potential material to stop bleeding and promote wound healing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Silva-Junior AJD, Brazil; Wang S, China; Li SC, United States S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Zhang XD
| 1. | Sae-Jung S, Apiwatanakul P. Chitosan Pad, Cellulose Membrane, or Gelatin Sponge for Peridural Bleeding: An Efficacy Study on a Lumbar Laminectomized Rat Model. Asian Spine J. 2018;12:195-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Wang X, Guo J, Zhang Q, Zhu S, Liu L, Jiang X, Wei DH, Liu RS, Li L. Gelatin sponge functionalized with gold/silver clusters for antibacterial application. Nanotechnology. 2020;31:134004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Yang JS, Liu KX, Chu L, Chan YK, Fan H, Li XM, Liu P, Liu TJ, Hao DJ. Cocktail Treatment with a Gelatin Sponge Impregnated with Ropivacaine, Dexamethasone, and Vitamin B12 Promotes Early Postoperative Recovery after Percutaneous Endoscopic Lumbar Discectomy: A Retrospective, Case-Controlled Study. Pain Physician. 2020;23:E211-E218. [PubMed] |
| 4. | Jogo A, Yamamoto A, Kaminoh T, Nakano M, Kageyama K, Sohgawa E, Hamamoto S, Sakai Y, Hamuro M, Nishida N, Miki Y. Utility of low-dose gelatin sponge particles and 5% ethanolamine oleate iopamidol mixture in retrograde transvenous obliteration (GERTO) for gastric varices. Br J Radiol. 2020;93:20190751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Dong Y, Liu W, Lei Y, Wu T, Zhang S, Guo Y, Liu Y, Chen D, Yuan Q, Wang Y. Effect of gelatin sponge with colloid silver on bone healing in infected cranial defects. Mater Sci Eng C Mater Biol Appl. 2017;70:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells. 2015;33:2158-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 574] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 7. | Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1475] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 8. | van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1346] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 9. | Wang M, Wang C, Chen M, Xi Y, Cheng W, Mao C, Xu T, Zhang X, Lin C, Gao W, Guo Y, Lei B. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano. 2019;13:10279-10293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 364] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 10. | Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem Cells Transl Med. 2017;6:736-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 294] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 11. | Xu N, Wang L, Guan J, Tang C, He N, Zhang W, Fu S. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol. 2018;117:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 12. | Nooshabadi VT, Khanmohamadi M, Valipour E, Mahdipour S, Salati A, Malekshahi ZV, Shafei S, Amini E, Farzamfar S, Ai J. Impact of exosome-loaded chitosan hydrogel in wound repair and layered dermal reconstitution in mice animal model. J Biomed Mater Res A. 2020;108:2138-2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 13. | Yu Y, Fan P, Li J, Wang S. Preparation of Biocompatible Manganese Selenium-Based Nanoparticles with Antioxidant and Catalytic Functions. Molecules. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Tang J, Yi W, Yan J, Chen Z, Fan H, Zaldivar-Silva D, Agüero L, Wang S. Highly absorbent bio-sponge based on carboxymethyl chitosan/poly-γ-glutamic acid/platelet-rich plasma for hemostasis and wound healing. Int J Biol Macromol. 2023;247:125754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 15. | Eaglstein WH, Falanga V. Chronic wounds. Surg Clin North Am. 1997;77:689-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle). 2015;4:560-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1534] [Cited by in RCA: 1434] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 17. | Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142-4157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 589] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 18. | Moghadasi S, Elveny M, Rahman HS, Suksatan W, Jalil AT, Abdelbasset WK, Yumashev AV, Shariatzadeh S, Motavalli R, Behzad F, Marofi F, Hassanzadeh A, Pathak Y, Jarahian M. A paradigm shift in cell-free approach: the emerging role of MSCs-derived exosomes in regenerative medicine. J Transl Med. 2021;19:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 19. | Rackov G, Garcia-Romero N, Esteban-Rubio S, Carrión-Navarro J, Belda-Iniesta C, Ayuso-Sacido A. Vesicle-Mediated Control of Cell Function: The Role of Extracellular Matrix and Microenvironment. Front Physiol. 2018;9:651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 20. | Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 21. | Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, Qian X, Wu M, Ji K, Zhao Y, Wang Y, Liu H, Xing X. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl Med. 2016;5:1425-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 439] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 22. | Sun L, Xu R, Sun X, Duan Y, Han Y, Zhao Y, Qian H, Zhu W, Xu W. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy. 2016;18:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 23. | Monaco JL, Lawrence WT. Acute wound healing an overview. Clin Plast Surg. 2003;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 24. | Diegelmann RF. Analysis of collagen synthesis. Methods Mol Med. 2003;78:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Madden JW, Smith HC. The rate of collagen synthesis and deposition in dehisced and resutured wounds. Surg Gynecol Obstet. 1970;130:487-493. [PubMed] |
| 26. | Okonkwo UA, DiPietro LA. Diabetes and Wound Angiogenesis. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 622] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 27. | Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular Vesicles in Angiogenesis. Circ Res. 2017;120:1658-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 463] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 28. | Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 556] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 29. | Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129:2182-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 433] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 30. | Kim YJ, Yoo SM, Park HH, Lim HJ, Kim YL, Lee S, Seo KW, Kang KS. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem Biophys Res Commun. 2017;493:1102-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 31. | Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 1125] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 32. | Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, Zhu Y, Wu L, Pan Z, Zhu W, Qian H, Xu W. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 364] [Article Influence: 36.4] [Reference Citation Analysis (0)] |