Published online Jul 26, 2023. doi: 10.4252/wjsc.v15.i7.768
Peer-review started: April 28, 2023
First decision: June 7, 2023
Revised: June 9, 2023
Accepted: July 11, 2023
Article in press: July 11, 2023
Published online: July 26, 2023
Processing time: 87 Days and 22.1 Hours
Scar formation and loss of cutaneous appendages are the greatest challenges in cutaneous wound healing. Previous studies have indicated that antler reserve mesenchyme (RM) cells and their conditioned medium improved regenerative wound healing with partial recovery of cutaneous appendages.
To develop hydrogels from the antler RM matrix (HARM) and evaluate the effect on wound healing.
We prepared the hydrogels from the HARM via enzymatic solubilization with pepsin. Then we investigated the therapeutic effects of HARM on a full-thickness cutaneous wound healing rat model using both local injections surrounding the wound and topical wound application.
The results showed that HARM accelerated wound healing rate and reduced scar formation. Also, HARM stimulated the regeneration of cutaneous appendages and blood vessels, and reduced collagen fiber aggregation. Further study showed that these functions might be achieved via creating a fetal-like niche at the wound site. The levels of fetal wound healing-related genes, including Collagen III and TGFβ3 treated with HARM were all increased, while the expression levels of Collagen I, TGFβ1, and Engrailed 1 were decreased in the healing. Moreover, the number of stem cells was increased in the fetal-like niche created by HARM, which may contribute to the regeneration of cutaneous appendages.
Overall, we successfully developed an injectable hydrogel made from antler RM matrix for the regenerative repair of full-thickness cutaneous wounds. We uncovered the molecular mechanism of the hydrogels in promoting regenerative wound healing, and thus pave the way for HARM to be developed for the clinic use.
Core Tip: Our study developed an injectable hydrogel made from antler reserve mesenchyme (a tissue suitable for stem cells) matrix for the regenerative repair of full-thickness cutaneous wounds. Moreover, we uncovered the molecular mechanism of the hydrogels in promoting regenerative wound healing, and thus pave the way for HARM to be developed for the clinic use.
- Citation: Zhang GK, Ren J, Li JP, Wang DX, Wang SN, Shi LY, Li CY. Injectable hydrogel made from antler mesenchyme matrix for regenerative wound healing via creating a fetal-like niche. World J Stem Cells 2023; 15(7): 768-780
- URL: https://www.wjgnet.com/1948-0210/full/v15/i7/768.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i7.768
As the biggest organ, skin serves multiple critical protective functions, including preventing harmful microorganism invasion, minimizing body fluid evaporation, and blockage of UV damage[1,2]. Healing in adult skin wounds usually results in scar formation, and scar consists of thick bundles of collagen fibers in the dermis and lacks functional cutaneous appendages (such as hair follicles and sebaceous glands). Scarring can result in patients experiencing bodily discomfort, including heat intolerance, pruritus, thermoregulatory and sensory disabilities, and psychological distress[3,4]. Therefore, realization of regenerative wound healing is the dream in clinics.
Biomaterials, especially the extracellular matrix (ECM) hydrogels, have become a promising strategy for treating severe skin injury[5-7]. ECM provides specific physical and chemical cues that regulate cell behaviors such as cell survival, proliferation, differentiation, and migration[8,9]. Thus far, quite a few types of ECM hydrogels from different animal tissues have been developed, including pig skin, bladder, and human placenta[10,11], and applied for inducing appendage regeneration in adult rat/mouse skin injury models. These hydrogels, however, have limited potential to maintain proliferation, differentiation, and migration of the cells resident in them, resulting in insufficient regeneration of nerves, blood vessels and cutaneous appendages in the healing wounds[10,11]. Therefore, the development of more effective alternative ECM hydrogels is highly desirable.
Deer antlers are the only mammalian organ that can fully regenerate after being lost[12-14]. Antler regeneration begins with regenerative healing of the wounds left after the previous antler casting. Studies show that this regenerative wound healing depends entirely on the adjacent pedicle periosteum (PP) or the PP-derived reserve mesenchyme (RM)[15,16]. The RM cells are a type of adult mesenchymal stem cell but with some embryonic stem cell properties[12,13]. The RM-derived paracrine factors are potential candidates that stimulate cell proliferation, migration and regenerative wound healing[17]. Recently, we applied RM cells through injection to treat rat cutaneous wounds and found that the healed skin consisted of basket-wave-like collagen fibers and numerous cutaneous appendages[18], thus at least partially achieving regenerative wound healing. The ability of the RM cells to stimulate regenerative wound healing is believed to rely on paracrine factors, because the RM cells did not survive in rats for more than 14 d[18]. Subsequently, we topically applied RM-cell-conditioned medium to treat the full-thickness wounds in rats and found that outcome of the healed wounds was comparable to that of using RM cells in a regenerative manner[19]. Therefore, we speculated that the ECM hydrogels prepared from the RM tissue should have similar if not better effects, as the ECM is the secretion of RM cells. Importantly, as a novel injectable hydrogel, antler RM matrix (HARM) is simpler to apply compared to RM cells or RM cell conditioned medium, as it does not need other carrier materials. At the same time, it can also be used as a carrier material for other factors for skin injury treatment.
This study reported the effects of HARM on the healing of full-thickness cutaneous wounds in rats. HARM had a stronger potential to promote skin cell proliferation and migration compared with the hydrogels from porcine urinary bladder matrix (HPUB, as a control), resulting in better wound healing quality. We believe that the regenerative wound healing achieved using the HARM is highly likely through creating a fetal-like niche in the wound area. Thus, HARM has the potential to be developed as a treatment for regenerative wound healing in clinics.
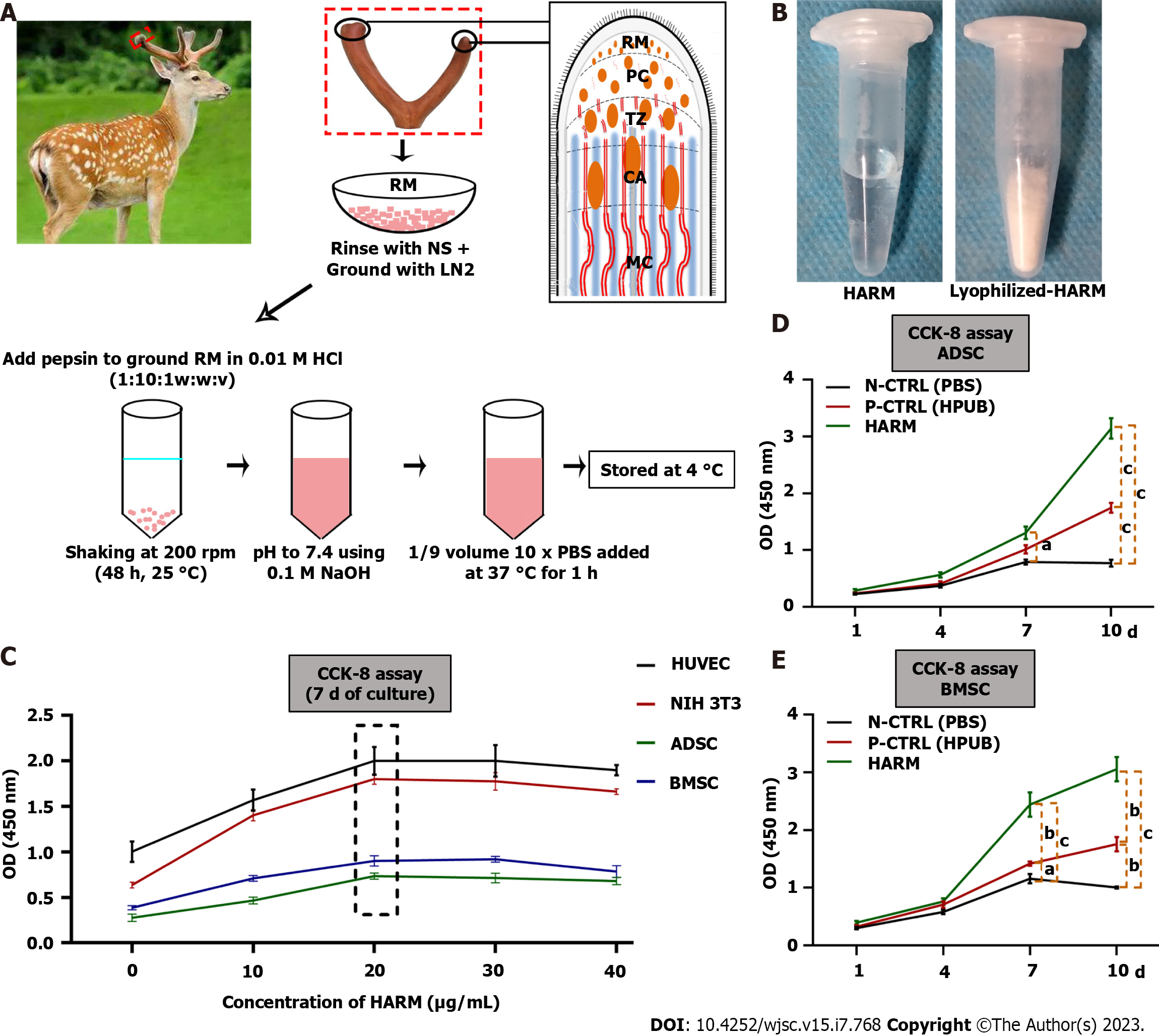
The preparation of HARM is described in the previous study[5]. Briefly, three 2-branch-antlers were collected from three healthy sika deer (Cervus nippon) about 30 d after casting of the previous hard antler buttons in a commercial deer farm (Purchased from Dongao Deer Farm). RM tissue located in the antler growth center of each antler tip was sampled and cut into small pieces (1 mm3) using two handheld scalpel blades and rinsed with normal saline (NS), ground in liquid nitrogen to obtain RM powder. The RM powder was further mixed with pepsin in 0.01 M HCl with the ratio of 10:1:1 (w:w:v) and the solution was kept shaking at a constant rate of 200 rpm for 48 h at 25 °C. The final pH of the resultant solution (3.0 to 4.0) was adjusted to 7.4 by adding 0.1 M NaOH to inactivate pepsin. HARM was formed when 10 × PBS was added into the solution and stored at 37 °C for 1 h (Supplementary Figure 1A). The predetermined concentration of the hydrogels was made by adding cool 1 × PBS (4 °C). HPUB, a previously reported ECM hydrogel[20], was prepared by the same procedure as HARM.
Surface morphology of the hydrogels was visualized using a scanning electron microscope (SEM, JEO JSM-5600LV, Japan) at a voltage of 10 kV. Lyophilized-HARM (Supplementary Figure 1B) were sputter-coated with a thin layer of gold before observation.
The cell lines of human umbilical vein endothelial cells (HUVEC) and NIH 3T3 fibroblasts were cryopreserved in our laboratory. The mouse adipose mesenchymal stem cells (ADSC) and bone marrow mesenchymal stem cells (BMSC) were obtained from Prof. Yimin Wang of China-Japan Union Hospital of Jilin University. The cells were expanded in DMEM media supplemented with 10% FBS and 1% penicillin-streptomycin in an incubator at 37°C, 5% CO2.
Hydrogels were sterilized with UV for 30 min; then cells (1 × 105/mL) were mixed into the hydrogels and seeded in 96-well plates incubated at 37 °C with 5% CO2 for 30 min. The growth medium was then added to each hydrogel and changed every 2 d. The related cells (HUVEC, NIH 3T3, ADSC, BMSC, ADSC, and BMSC) and assays were listed in Table 1.
| Test purposes | Cell type | Culture system | Culture time | Test type | Required reagents | |||||
| Screen the optimum concentration of HARM on cell culture | HUVEC | HARM (0 μg/mL) | HARM (10 μg/mL) | HARM (20 μg/mL) | HARM (30 μg/mL) | HARM (40 μg/mL) | 7 d | CCK-8 assay | CCK-8 (Beyotime, Shanghai, China) | |
| NIH 3T3 | ||||||||||
| ADSC | ||||||||||
| BMSC | ||||||||||
| Compare the performance of HARM to HUPB on cell proli | ADSC | HARM (20 μg/mL) | P-CTRL (HUPB, 20 μg/mL) | N-CTRL (PBS) | 1, 4, 7, 10 d | CCK-8 assay | Nuclear staining | CCK-8 (Beyotime, Shanghai, China) | ||
| BMSC | ||||||||||
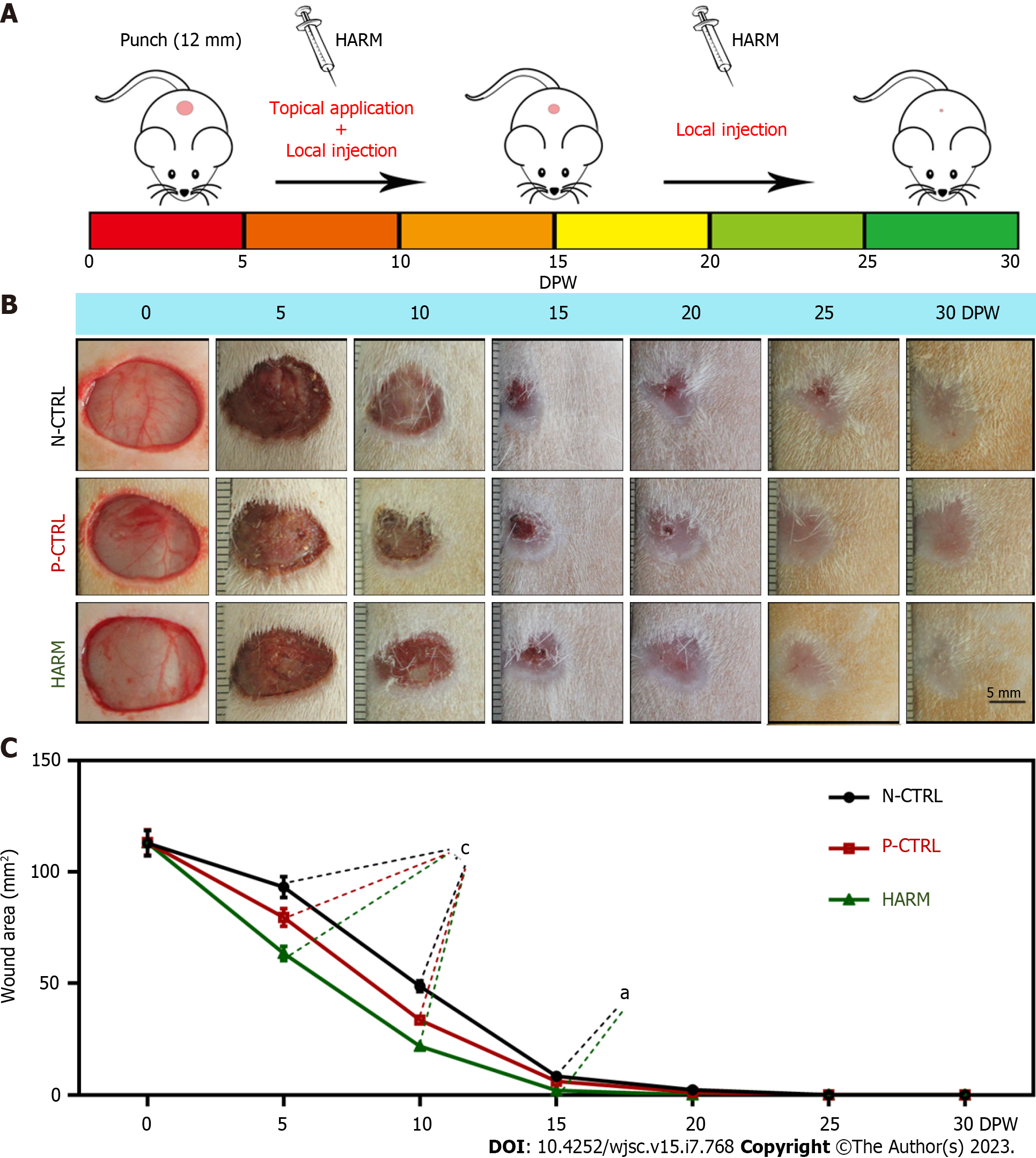
Thirty 8-wk-old female SD rats (200-220 g/rat) were purchased from Liaoning Changsheng Biotechnology Co., Ltd. (Liaoning, China) and were raised under standard laboratory animal feeding conditions. All the rats received humane care, and all study protocols and procedures were approved by the Animal Ethics Committee of Changchun Sci-Tech University (Approval No. CKARI2020012). For skin wounding, rats were anesthetized with 3% pentobarbital sodium (30 mg/kg), and then the hair was removed from the area to be operated (dorsal surface). A 12-mm diameter skin biopsy punch was used to cut the full-thickness skin and make wounds.
The model rats were randomly divided into three groups (HARM, HPUB, and PBS), with each group having 10 rats. HARM was diluted with cold 1 × PBS to the final concentration of 20 μg/mL before use. HARM was administered via injection around the wound margins (25 μL/injection × 4 injections) together with topical application over each wound (100 μL/wound) once every 5 d in the first 15 d, thereafter via local injection (100 μL) only in the remaining 15 d. The wound healing status was photographed every 5 d and the healing area was calculated using Image-Pro Plus software. PBS solution and HPUB were used as negative control (N-CTRL) and positive control (P-CTRL), respectively. The healed tissues were collected on 15 d post wounding (DPW) and 30 DPW respectively, and each tissue sample was divided into two halves: (1) For protein and RNA extraction; and (2) fixed in 4% paraformaldehyde for histological examination.
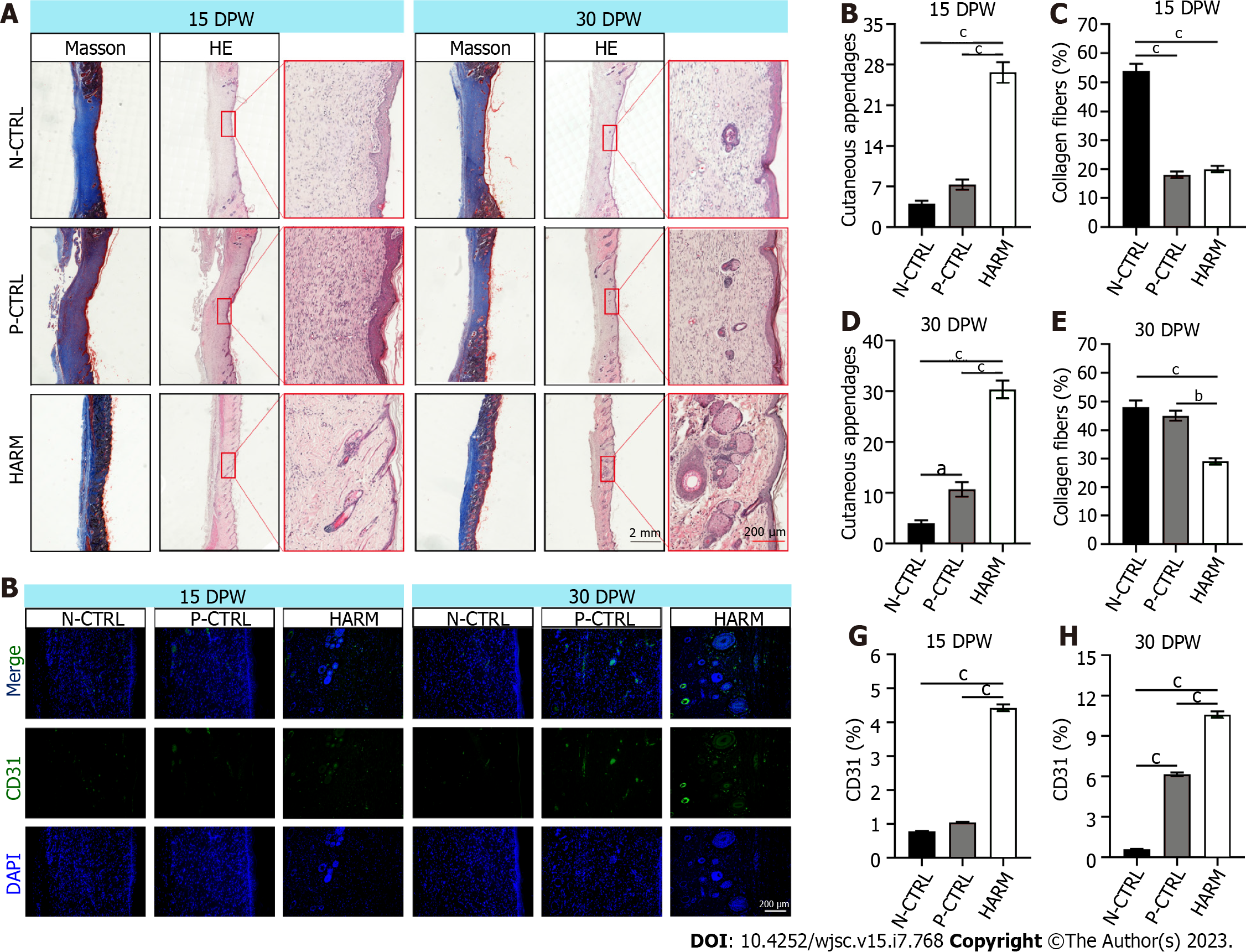
Healed skin tissue was embedded in paraffin and sliced into 5 μm sections. The sections were stained with hematoxylin and eosin (HE), Masson, or immunofluorescence (IF). All antibodies used in IF were listed in Supplementary Table 1. Sections stained with HE and Masson were photographed using the Digital Imaging Scanning System (Precipoint M8; Freising, Germany); and stained with IF and photographed using Fluorescence Microscopes (BX63, Olympus, Japan). The collagen fibers (blue area on micrograph) in Masson staining and the positive expression (fluorescence area on microphage) of the target genes in IF staining were analyzed statistically using Image-Pro Plus software; then the ratio of the target area to the whole area was calculated.
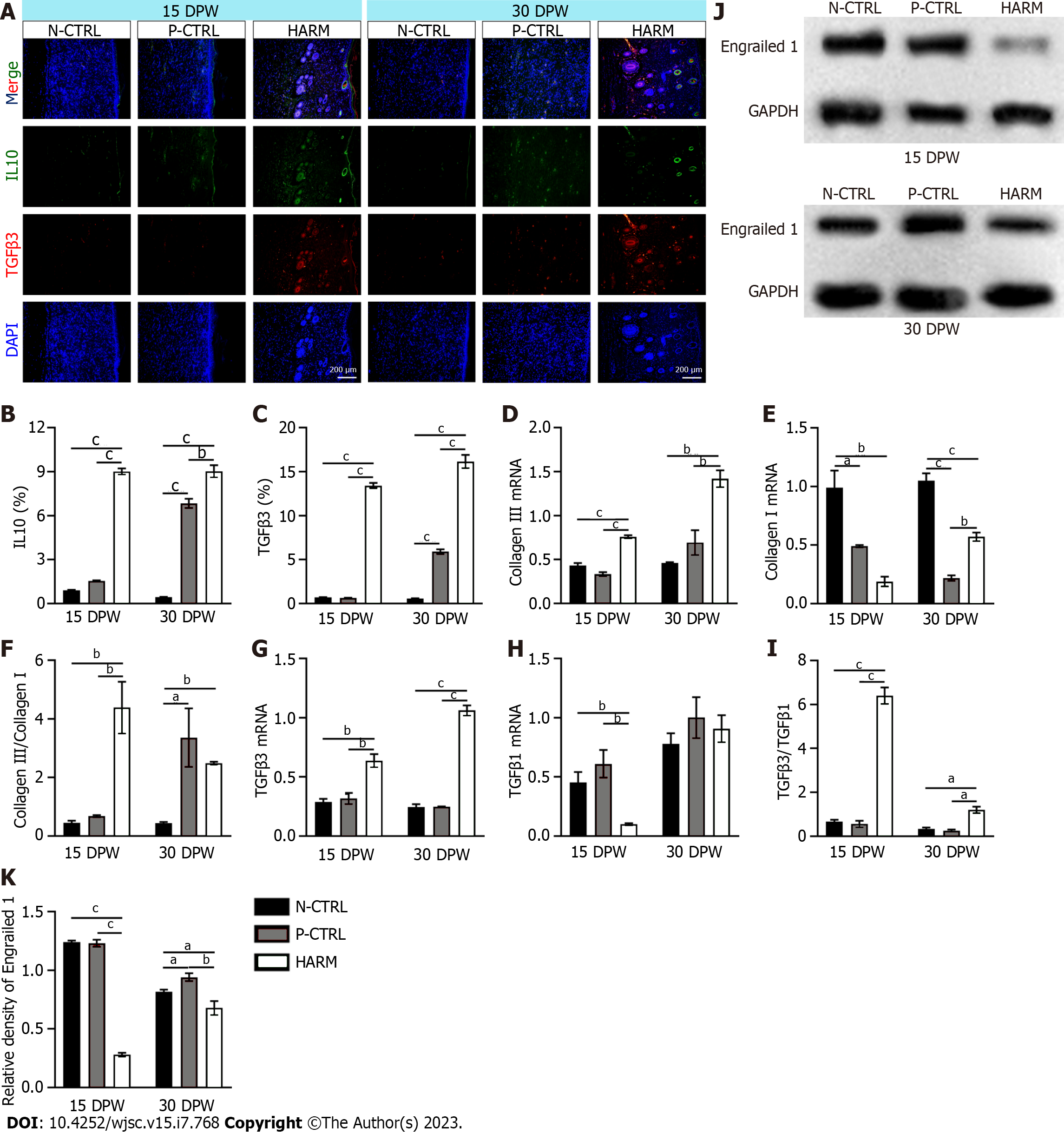
Trizol reagent (Sigma-Aldrich, United States) was used for total RNA isolation from the healed skin tissue samples. cDNA was prepared via reverse transcription of RNA. cDNA, primers (Supplementary Table 2) and SYBR premix (Roche, Basel, Switzerland) were mixed on quantitative real time PCR (qRT-PCR) using the qTOWER 3G (Analykit Jena AG, Jena, Germany). Results of mRNA quantification were normalized against GAPDH and calculated using the ΔΔCt method. Every experiment was repeated three times.
Total proteins were isolated from the healed tissues: 20 μg of total protein was separated via polyacrylamide SDS gel and then transferred to a polyvinylidene fluoride membrane (Millipore, MA). After blocking in 5 % (w/v) non-fat milk, the membrane was first incubated with primary antibodies (Engrailed 1, Bioss; GAPDH, ProteinTech) at 4 °C overnight and then with HRP-conjugated secondary antibody at 25 °C for 2 h. The bands were visualized using an ECL system and quantified using Image J software.
Results were expressed as mean ± SEM. Multi-comparisons were made using ANOVA in the package of GraphPad Prism 9.0. Significant differences were evaluated using t-test and one-way ANOVA. The difference was considered statistically significant (aP < 0.05), or highly significant (bP < 0.01, or cP < 0.001).
We prepared HARMs (Figure 1A), which had typical gel properties (Figure 1B; Supplementary Figure 1). The effects of HARM on cell proliferation were evaluated in vitro. Firstly, the optimum concentration of HARM was determined based on cell proliferation rates. The results showed that proliferation rates of HUVEC, NIH 3T3, ADSC, and BMSC cultured in the medium containing 20 μg/mL HARM all reached their peaks across the concentration range from 0 to 40 μg/mL 7 d after culture (Figure 1C). Further, the proliferation rate of ADSC cultured in 20 μg/mL HARM was significantly higher than in N-CTRL (P < 0.05; Figure 1D), and that of BMSC cultured in 20 μg/mL HARM was significantly higher than N-CTRL and P-CTRL (P < 0.001; Figure 1E). The differences in cell proliferation rate between cells cultured in HARM and those cultured in N-CTRL or P-CTRL were increased at 10 d after further culture. These results suggest that HARM has great potential to stimulate cell proliferation in vitro.
To determine the effects of HARM on the rate and quality of cutaneous wound healing, HARM (20 μg/mL) was used to treat full-thickness skin wounds in rats (Figure 2A). The results showed that the areas of the wound in the HARM group were significantly smaller than those in the N-CTRL and P-CTRL groups (P < 0.001; Figure 2B and C) on 5 or 10 DPW; wound healing in the HARM group was completed on 20 DPW, but those in N-CTRL and P-CTRL groups were not completed until 30 DPW. These results suggest that HARM can effectively accelerate wound healing rate in the full-thickness skin wounds in rats.
Quality of wound healing was examined at histological level. The results showed significantly more appendages in the HARM group than in the N-CTRL or P-CTRL groups (P < 0.001; Figure 3A and B). The collagen fibers of healed skin in the HARM group were fewer and thinner than those in the N-CTRL group (P < 0.001; Figure 3A and C) on 15 DPW; moreover, the collagen fibers in the HARM group consisted of basket-wave-like collagens, which were drastically different from the thick-bundle-like collagens in the N-CTRL and P-CTRL groups. Likewise, healed skin in the HARM group on 30 DPW was similar to that in the N-CTRL and P-CTRL groups (P < 0.01; Figure 3A, D, and E). The expression levels of CD31 (the surface marker of neovascular endothelial cells[21]) and Ki67 (the marker of cell proliferation and division[22]) in the HARM group were significantly more than those in the N-CTRL or P-CTRL groups both on 15 DPW and 30 DPW (CD31: P < 0.001; Figure 3F-H); Ki67: (P < 0.001; Supplementary Figure 2). Overall, the results suggest that the HARM treatment can effectively improve the quality of wound healing in rats, and the effects are even better than the HPUB treatment.
The expression levels of IL10 and TGFβ3, the fetal wound healing-related genes[23,24] were detected in the healed tissues. The results showed that expression levels of IL-10 and TGFβ3 in the healed skin of the HARM group were significantly higher than in those of the N-CTRL or P-CTRL groups on 15 DPW (P < 0.001; Figure 4A-C). Likewise, on 30 DPW, these gene expressions had a similar trend to those on 15 DPW (P < 0.01; Figure 4A-C). We further evaluated the status of expression of some other fetal wound healing-related genes using qRT-PCR. The results showed that the healed skin of the HARM group favored expression of fetal wound healing genes including collagen Ⅲ (15 DPW: P < 0.001, P < 0.001; 30 DPW: P < 0.01, P < 0.01) and TGFβ3 (15 DPW: P < 0.01, P < 0.01; 30 DPW: P < 0.001, P < 0.001); but did not favor expression of scar healing genes including collagen I (15 DPW: P < 0.01, P < 0.05; 30 DPW: P < 0.001, P < 0.01) and TGFβ1 (15 DPW: P < 0.01, P < 0.01) when compared with those in the N-CTRL and P-CTRL groups to varying degrees (Figure 4D, E, G and H). The ratios of collagen III to collagen I and TGFβ3 to TGFβ1 in the healed tissue of the HARM group were all significantly higher than in those of N-CTRL or P-CTRL groups (P < 0.05; Figure 4F and I). Furthermore, the expression of Engrailed 1, activated in adult wound healing and prevented in fetal wound healing[25], was also down-regulated with the HARM treatment in the healed tissues (Figure 4J and K). These results suggest that HARM promotes regenerative wound healing which may be achieved via creating a fetal-like wound healing niche.
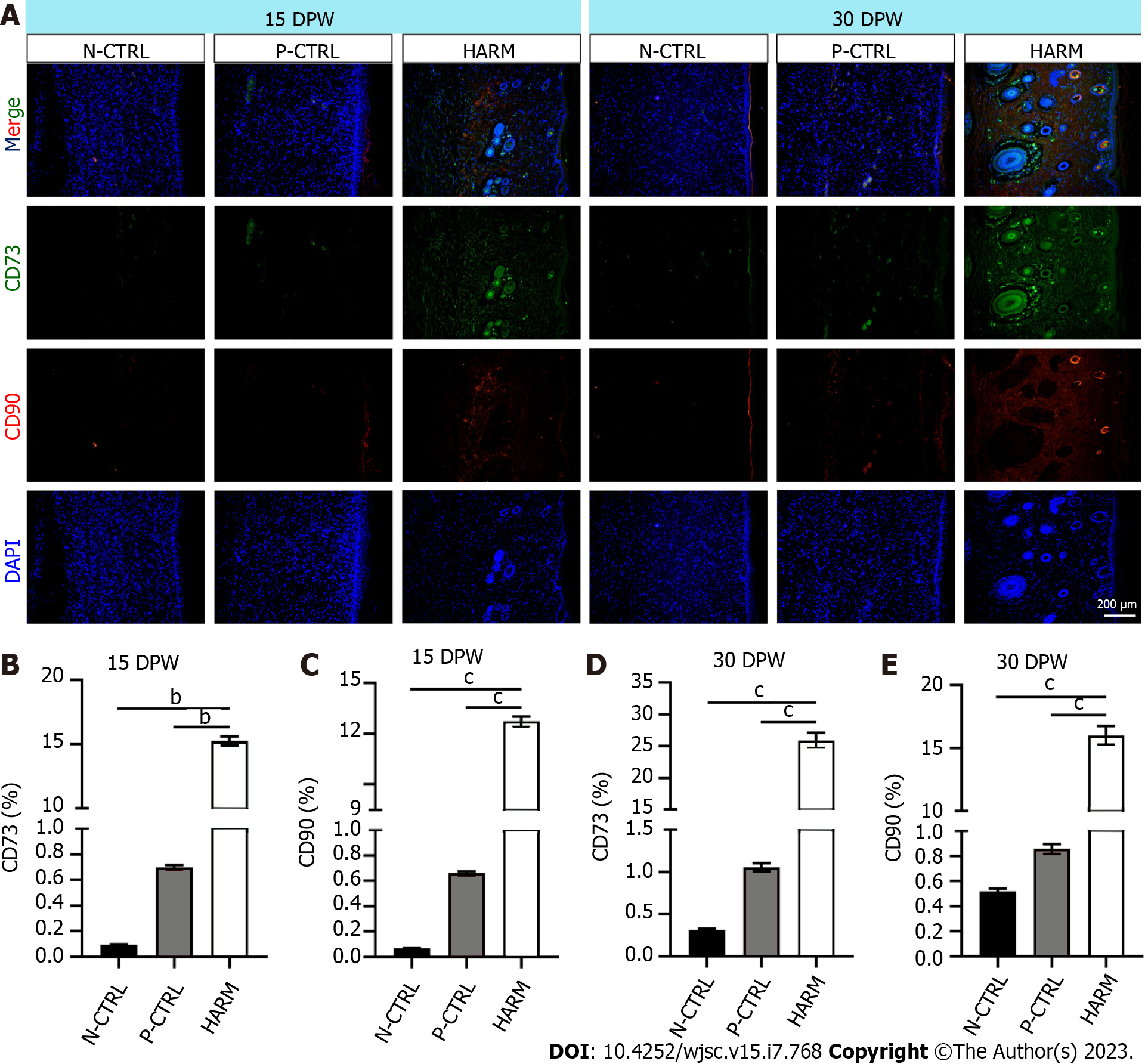
Next, the effects of the fetal-like wound healing niche created by HARM on recruitment of stem cells and maintenance of plasticity were detected. IF staining results showed that healed skin of the HARM group expressed significantly higher CD73 and CD90 than that of the N-CTRL or P-CTRL groups (P < 0.01, P < 0.001; Figure 5A-C) on 15 DPW. Likewise, on 30 DPW, a similar expression trend was observed as on 15 DPW (P < 0.001; Figure 5A, D and E). These results suggest that HARM may promote recruitment of stem cells or maintenance of plasticity which grows in the fetal-like wounding healing niche.
Scarring in wound healing is still a challenge in the clinic, where the goal is regeneration. The regeneration of functional cutaneous appendages and organized collagen is a complex process involving multiple factors and cells related to the microenvironment. Keeping the cells that regenerate the injured tissues in the optimum environment may be a practical approach to induce cutaneous regeneration and prevent abnormal scar formation. Although many approaches, such as synthetic hydrogels or ECM hydrogels, have been evaluated to improve the environment during wound healing, the outcomes have been unsatisfactory. This study is the first to report the role of an acellular ECM hydrogel prepared from stem cell tissue (antler RM) in cutaneous wound healing. Through a full-thickness rat model, we found that HARM improved the regeneration of cutaneous appendages and blood vessels and reduced the aggregation of disorganized collagen fiber, which may be achieved via creating a fetal-like niche at the wound site.
Injectable hydrogels, as a promising strategy, are being used with increasing frequency for the treatment of wound healing. They have several desirable features, including targeted delivery by minimally invasive techniques, ability to fill an irregularly shaped space quickly, and polymerization to form a support structure suitable for host cell infiltration and remodeling[26]. The injectable hydrogels derived from naturally occurring biologic materials are claimed to possess superior biocompatibility and bioactivity compared to their synthetic counterparts, which are usually composed of the ECM of decellularized tissues, and can be partially digested with pepsin, solubilized, and polymerized in situ to form a hydrogel[20,27,28]. Intact ECM hydrogels retain numerous molecular constituents found in the native tissue, such as cell adhesion proteins and growth factors, and these hydrogels support a constructive, site appropriate, remodeling response when implanted in a variety of anatomical sites[29]. It is possible that a hydrogel formed from enzymatically degraded and solubilized ECM may maintain some of the biological activity found in the intact ECM. ECM hydrogels have been prepared from various tissues, including urinary bladder[20], dermis[27], cardiac tissues[28], adipose tissue[30], small intestine[31], and skeletal muscle[32]. The hydrogels derived from these tissue types differ in their composition and activity. However, a common problem is the lack of practical factors to stimulate tissue regeneration/repair, although they do induce a certain level of repair on tissue injury. Therefore, selecting suitable tissue for ECM hydrogel preparation is essential, especially if the tissue is rich in regeneration-stimulated factors; a tissue suitable for stem cell survival may be suitable and advantageous. In the annual regeneration of deer antlers, the RM tissue layer in the growth center is responsible for the growth of deer antlers with a growth rate of up to 2.75 cm/d[33]. RM-derived cells have the characteristics of both mesenchymal and embryonic stem cells with strong abilities of proliferation/division and paracrine function, and low immunogenicity[12,13,17].
In the present study, we developed a hydrogel from the antler RM matrix, which may retain similar components and characteristics of the RM tissue. It can be kept as a solution at 4 °C and quickly transitions to a hydrogel at 37 °C (average human body temperature; Supplementary Figure 1A). This is clinically important since the biomaterial as a solution can be used in the injection method and quickly reach a gel state that protects the integrity of the tissue and allows for native cells to adhere and grow. Importantly, HARM exhibits pores on the order of 100 μm (Supplementary Figure 1), providing an ideal space for cell division and proliferation. Further, we found that the concentration of 20 μg/mL HARM showed the strongest stimulation over the other concentrations on the proliferation rate of HUVEC, NIH 3T3, ADSC, and BMSC; moreover, proliferation rates of ADSC and BMSC cultured in HARM in vitro and cutaneous cells treated with HARM in vivo were both stronger than the previously reported hydrogel, HPUB. In general, our results demonstrated the advantage of HARM for cell proliferation, which is essential for reconstructing the lamellar stromal structure using acellular biomaterial.
Based on the above characteristics of HARM, we used the full-thickness rat wound to evaluate the effects of HARM on wound healing. As expected, HARM effectively promoted the healing rate and improved the healing quality; these effects were better overall than the HPUB. To the best of our knowledge, this is the first report that a hydrogel derived from a tissue matrix can promote not only the healing rate but also healing quality. However, hydrogels from other tissue matrices can only promote wound healing[34,35]. The microenvironment of wound healing has been reported to be critical in influencing the type of healing. An ideal microenvironment, such as fetal wound healing, results in scarless regenerative healing; a normal microenvironment, such as adult wound healing, results in scarring[23,24]. There are many differences between fetal and adult wound healing, such as inflammatory reactions, growth factors and collagen synthesis[23,24]. It has been reported that IL10 expression is up-regulated in fetal wounds without scars, and wound healing occurs with scar formation in fetal mice with IL-10 deficiency. However, in clinical trials by intradermal injection of recombinant human IL-10 into the wound edge for 1 mo, IL-10 treatment reduced scar formation[36]. In the present study, we found that the expression of IL10 was increased in the healed tissues that had been treated with HARM. TGFβ, including TGFβ1, TGFβ2, and TGFβ3, signals are required for wound repair and regeneration. TGFβ1 causes scar formation after skin injury; on the contrary, TGFβ3 has an anti-fibrotic effect because the ratio of TGFβ3/TGFβ1 in fetal wounds was found to be higher than that in adults[36-38]. We also found that TGFβ3 was increased by HARM in the wound, while TGFβ1 was the opposite. Fetal wound healing differs from adult wound healing in collagen synthesis in terms of the speed of deposition, variation in collagen type ratios, and quantity of collagen. Most striking is the persistence of excess collagen III over collagen I, with healed wounds in the fetus; higher levels of collagen III yield smaller, reticular structures with more cross-linking than collagen I and contribute toward scarless wound healing. In the present study, we found that collagen III was increased by HARM in the wound, while TGFβ1 was the opposite. Engrailed 1 is another typical gene that is activated in adult wound healing, and preventing the activation would inhibit the scarring and achieve fetal-like wound healing[25]. This gene was also down-regulated with HARM treatment in the healed tissues. Above all, the HARM treatment favored expressions of fetal wound healing genes, including IL10, collagen III, and TGFβ3; but did not favor expression of scar healing genes, including collagen IS, TGFβ1, and Engrailed 1, in the healed tissues. Thus, we speculate that the effects of HARM on regenerative wound healing may be achieved via creating a fetal-like niche.
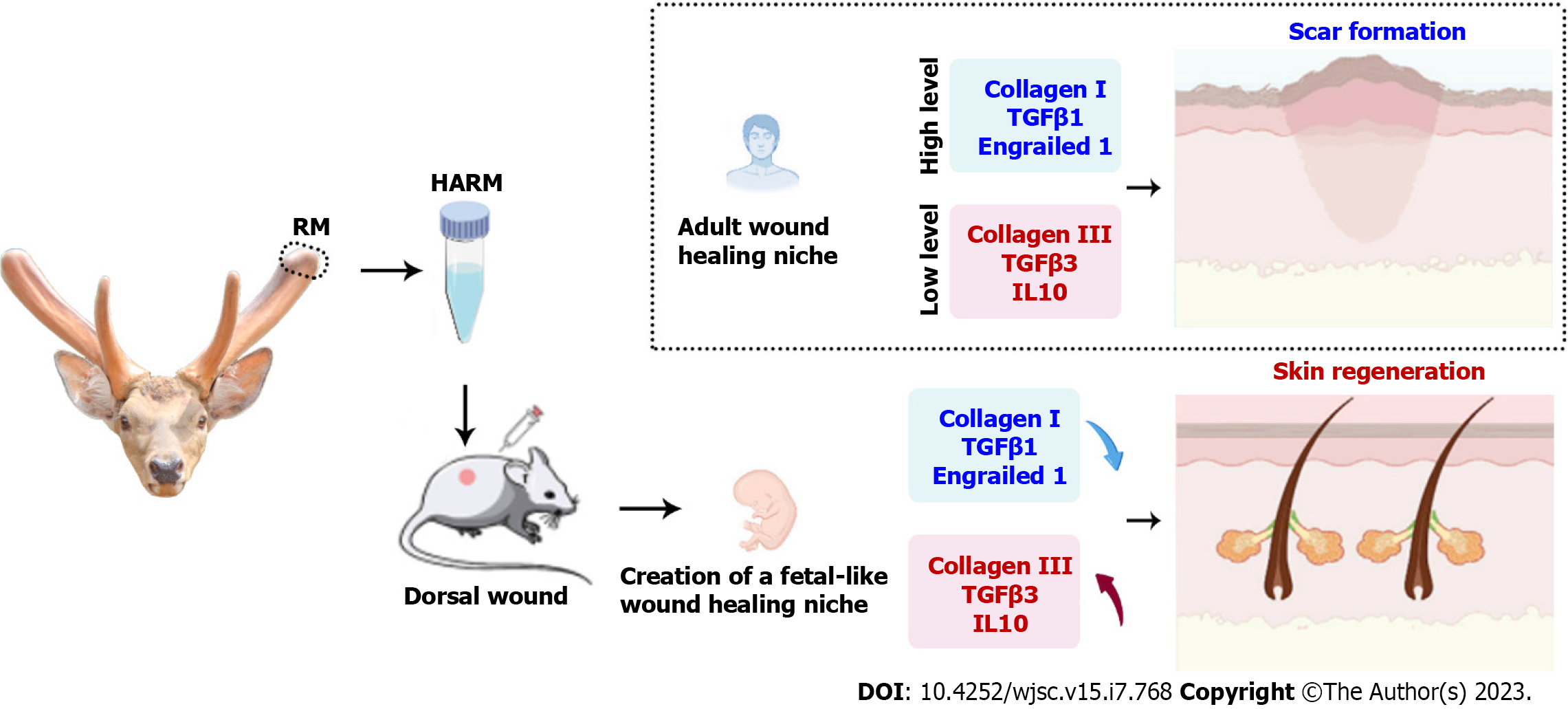
This study prepared a new injectable hydrogel using a stem cell tissue matrix, the antler reserve mesenchymal matrix. The therapeutic effects of the hydrogels were evaluated using full-thickness rats. The results revealed that the hydrogels effectively increased the healing rate and improved the healing quality of the wound, including regeneration of the cutaneous appendages and formation of the basketweave-like pattern of collagens. Further study showed that these effects of the hydrogels were likely achieved through creating a niche similar to the regeneration of fetal skin, thereby promoting skin regeneration without scars (Figure 6). Overall, we believe that our prepared hydrogels may have clinical benefits for stimulating regenerative wound healing, especially those large cutaneous wounds caused by burns, scalds, or machinery.
It believes that hydrogels from the antler reserve mesenchyme matrix (HARM) may have clinical benefits for stimulating regenerative wound healing, especially those large cutaneous wounds caused by burns, scalds, or machinery.
This study prepared a new injectable hydrogel from antler reserve mesenchyme (RM) for regenerative wound healing via creating a fetal-like niche.
HARM was successfully prepared from antler RM. Through a full-thickness rat model, it was found that HARM improved the regeneration of cutaneous appendages and blood vessels and reduced the aggregation of disorganized collagen fiber, which may be achieved via creating a fetal-like niche at the wound site.
The HARM was prepared via enzymatic solubilization with pepsin. Then the therapeutic effects of HARM on a full-thickness cutaneous wound healing rat mode were investigated.
To develop an injectable hydrogel made from antler RM matrix for the regenerative repair of full-thickness cutaneous wounds, which may have clinical benefits for stimulating regenerative wound healing, especially those large cutaneous wounds caused by burns, scalds, or machinery.
Deer antlers are the only mammalian organ that can fully regenerate after being lost. Antler regeneration begins with regenerative healing of the wounds left after the previous antler casting. Studies show that this regenerative wound healing depends entirely on the adjacent pedicle periosteum (PP) or the PP-derived RM.
Scarring in wound healing is still a challenge in the clinic, where the goal is regeneration. Keeping the cells that regenerate the injured tissues in the optimum environment may be a practical approach to induce cutaneous regeneration and prevent abnormal scar formation. Although many approaches, such as synthetic hydrogels or extracellular matrix hydrogels, have been evaluated to improve the environment during wound healing, the outcomes have been unsatisfactory.
We thank Dr. Peter Fennessy for his critical reading through the manuscript and valuable suggestions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haque N, Bangladesh; Maurya DK, India; Nagaya M, Japan; Zhang H, China; Li SC, United States S-Editor: Chen YL L-Editor: A P-Editor: Zhao S
| 1. | Gravitz L. Skin. Nature. 2018;563:S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Deniz AAH, Abdik EA, Abdik H, Aydın S, Şahin F, Taşlı PN. Zooming in across the Skin: A Macro-to-Molecular Panorama. Adv Exp Med Biol. 2020;1247:157-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Yannas IV, Tzeranis DS, So PTC. Regeneration of injured skin and peripheral nerves requires control of wound contraction, not scar formation. Wound Repair Regen. 2017;25:177-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Monavarian M, Kader S, Moeinzadeh S, Jabbari E. Regenerative Scar-Free Skin Wound Healing. Tissue Eng Part B Rev. 2019;25:294-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 5. | Jiang D, Huang J, Shao H, Hu X, Song L, Zhang Y. Characterization of bladder acellular matrix hydrogel with inherent bioactive factors. Mater Sci Eng C Mater Biol Appl. 2017;77:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Francis MP, Breathwaite E, Bulysheva AA, Varghese F, Rodriguez RU, Dutta S, Semenov I, Ogle R, Huber A, Tichy AM, Chen S, Zemlin C. Human placenta hydrogel reduces scarring in a rat model of cardiac ischemia and enhances cardiomyocyte and stem cell cultures. Acta Biomater. 2017;52:92-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Wolf MT, Daly KA, Brennan-Pierce EP, Johnson SA, Carruthers CA, D'Amore A, Nagarkar SP, Velankar SS, Badylak SF. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33:7028-7038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 8. | Muncie JM, Weaver VM. The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. Curr Top Dev Biol. 2018;130:1-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 9. | Birch HL. Extracellular Matrix and Ageing. Subcell Biochem. 2018;90:169-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Badylak SF. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann Biomed Eng. 2014;42:1517-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 11. | Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 444] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Zhang C, Wang N, Li Z, Heller R, Liu R, Zhao Y, Han J, Pan X, Zheng Z, Dai X, Chen C, Dou M, Peng S, Chen X, Liu J, Li M, Wang K, Liu C, Lin Z, Chen L, Hao F, Zhu W, Song C, Zhao C, Zheng C, Wang J, Hu S, Li C, Yang H, Jiang L, Li G, Liu M, Sonstegard TS, Zhang G, Jiang Y, Wang W, Qiu Q. Genetic basis of ruminant headgear and rapid antler regeneration. Science. 2019;364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 13. | Wang D, Berg D, Ba H, Sun H, Wang Z, Li C. Deer antler stem cells are a novel type of cells that sustain full regeneration of a mammalian organ-deer antler. Cell Death Dis. 2019;10:443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Qin T, Zhang G, Zheng Y, Li S, Yuan Y, Li Q, Hu M, Si H, Wei G, Gao X, Cui X, Xia B, Ren J, Wang K, Ba H, Liu Z, Heller R, Li Z, Wang W, Huang J, Li C, Qiu Q. A population of stem cells with strong regenerative potential discovered in deer antlers. Science. 2023;379:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 15. | Li CY, Medical LJIJo, Frontiers B. Exploration of the mechanism underlying neogenesis and regeneration of postnatal mammalian skin: Deer antler velvet. 2010. [cited 3 June 2023]. Available from: https://www.semanticscholar.org/paper/EXPLORATION-OF-THE-MECHANISM-UNDERLYING-NEOGENESIS-Li/5562111ff1fd8ade010c5b17d64c2a6f29adb3c7. |
| 16. | Li C, Suttie JM. Histological studies of pedicle skin formation and its transformation to antler velvet in red deer (Cervus elaphus). Anat Rec. 2000;260:62-71. [PubMed] [DOI] [Full Text] |
| 17. | Lei J, Jiang X, Li W, Ren J, Wang D, Ji Z, Wu Z, Cheng F, Cai Y, Yu ZR, Belmonte JCI, Li C, Liu GH, Zhang W, Qu J, Wang S. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell. 2022;13:220-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Rong X, Zhang G, Yang Y, Gao C, Chu W, Sun H, Wang Y, Li C. Transplanted Antler Stem Cells Stimulated Regenerative Healing of Radiation-induced Cutaneous Wounds in Rats. Cell Transplant. 2020;29:963689720951549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Rong X, Chu W, Zhang H, Wang Y, Qi X, Zhang G, Li C. Antler stem cell-conditioned medium stimulates regenerative wound healing in rats. Stem Cell Res Ther. 2019;10:326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 367] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 21. | Figueiredo CC, Pereira NB, Pereira LX, Oliveira LAM, Campos PP, Andrade SP, Moro L. Double immunofluorescence labeling for CD31 and CD105 as a marker for polyether polyurethane-induced angiogenesis in mice. Histol Histopathol. 2019;34:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Miller I, Min M, Yang C, Tian C, Gookin S, Carter D, Spencer SL. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018;24:1105-1112.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 416] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 23. | Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Adv Clin Chem. 2009;48:137-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Helmo FR, Machado JR, Guimarães CS, Teixeira Vde P, dos Reis MA, Corrêa RR. Fetal wound healing biomarkers. Dis Markers. 2013;35:939-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Mascharak S, desJardins-Park HE, Davitt MF, Griffin M, Borrelli MR, Moore AL, Chen K, Duoto B, Chinta M, Foster DS, Shen AH, Januszyk M, Kwon SH, Wernig G, Wan DC, Lorenz HP, Gurtner GC, Longaker MT. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science. 2021;372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 343] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 26. | Asadi N, Pazoki-Toroudi H, Del Bakhshayesh AR, Akbarzadeh A, Davaran S, Annabi N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular based hydrogels. Int J Biol Macromol. 2021;170:728-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 27. | Cheng MH, Uriel S, Moya ML, Francis-Sedlak M, Wang R, Huang JJ, Chang SY, Brey EM. Dermis-derived hydrogels support adipogenesis in vivo. J Biomed Mater Res A. 2010;92:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409-5416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 369] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 29. | Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478-491. [PubMed] |
| 30. | Young DA, Ibrahim DO, Hu D, Christman KL. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 2011;7:1040-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Okada M, Payne TR, Oshima H, Momoi N, Tobita K, Huard J. Differential efficacy of gels derived from small intestinal submucosa as an injectable biomaterial for myocardial infarct repair. Biomaterials. 2010;31:7678-7683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, Christman KL. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 2010;5:e13039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 33. | Ba H, Wang D, Yau TO, Shang Y, Li C. Transcriptomic analysis of different tissue layers in antler growth Center in Sika Deer (Cervus nippon). BMC Genomics. 2019;20:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Zhang Q, Chang C, Qian C, Xiao W, Zhu H, Guo J, Meng Z, Cui W, Ge Z. Photo-crosslinkable amniotic membrane hydrogel for skin defect healing. Acta Biomater. 2021;125:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 35. | Hsieh CM, Wang W, Chen YH, Wei PS, Liu YH, Sheu MT, Ho HO. A Novel Composite Hydrogel Composed of Formic Acid-Decellularized Pepsin-Soluble Extracellular Matrix Hydrogel and Sacchachitin Hydrogel as Wound Dressing to Synergistically Accelerate Diabetic Wound Healing. Pharmaceutics. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Rose LF, Chan RK. The Burn Wound Microenvironment. Adv Wound Care (New Rochelle). 2016;5:106-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 785] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 38. | Lichtman MK, Otero-Vinas M, Falanga V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016;24:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 420] [Article Influence: 46.7] [Reference Citation Analysis (0)] |