Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.490
Peer-review started: November 9, 2022
First decision: December 25, 2022
Revised: January 21, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: May 26, 2023
Processing time: 197 Days and 23.8 Hours
Mesenchymal stem cells (MSCs) have been applied to treat degenerative articular diseases, and stromal cell-derived factor-1α (SDF-1α) may enhance their therapeutic efficacy. However, the regulatory effects of SDF-1α on cartilage differentiation remain largely unknown. Identifying the specific regulatory effects of SDF-1α on MSCs will provide a useful target for the treatment of degenerative articular diseases.
To explore the role and mechanism of SDF-1α in cartilage differentiation of MSCs and primary chondrocytes.
The expression level of C-X-C chemokine receptor 4 (CXCR4) in MSCs was assessed by immunofluorescence. MSCs treated with SDF-1α were stained for alkaline phosphatase (ALP) and with Alcian blue to observe differentiation. Western blot analysis was used to examine the expression of SRY-box trans
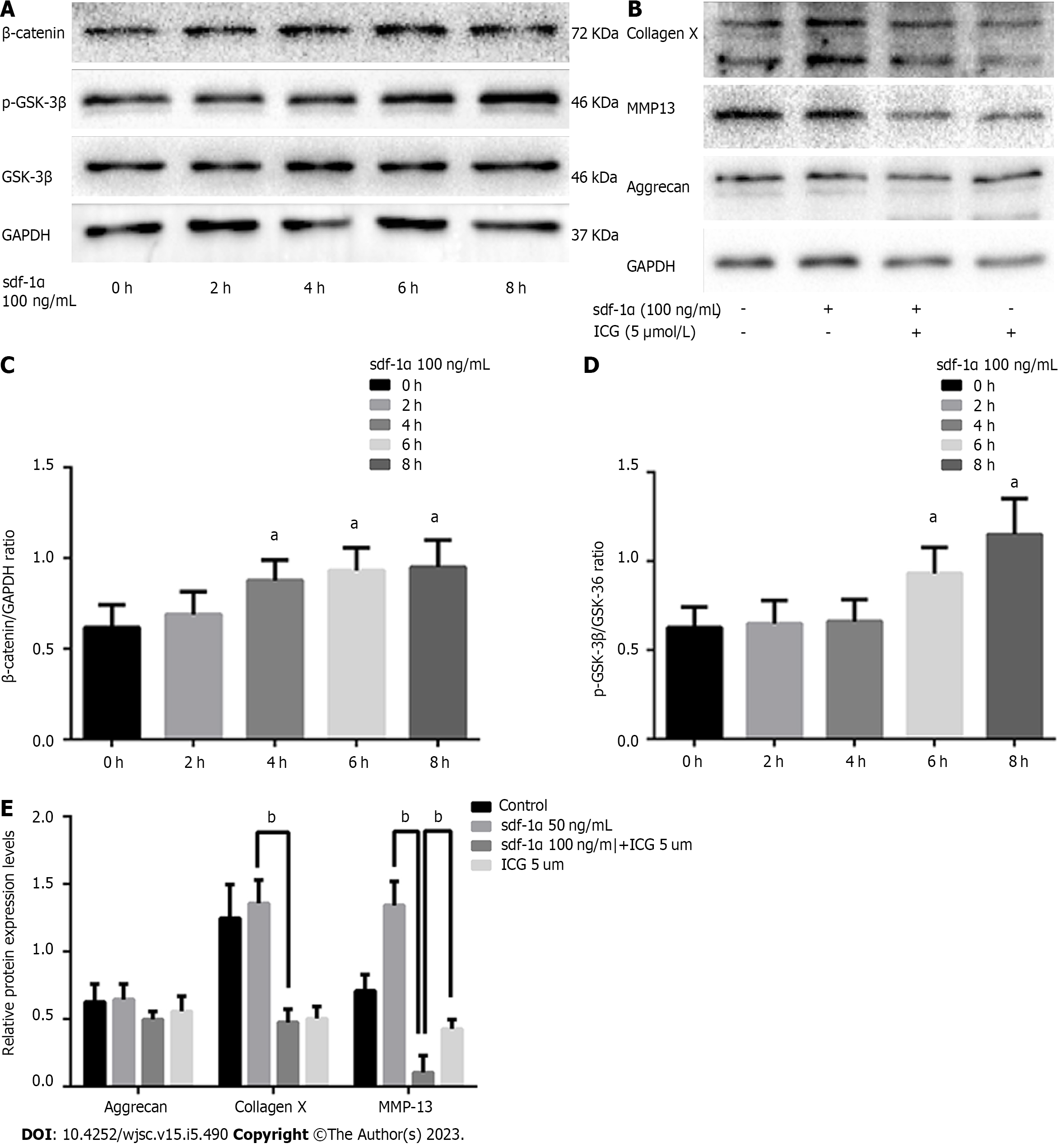
Immunofluorescence showed CXCR4 expression in the membranes of MSCs. ALP stain was intensified in MSCs treated with SDF-1α for 14 d. The SDF-1α treatment promoted expression of collagen X and MMP13 during cartilage differentiation, whereas it had no effect on the expression of collagen II or aggrecan nor on the formation of cartilage matrix in MSCs. Further, those SDF-1α-mediated effects on MSCs were validated in primary chondrocytes. SDF-1α promoted the expression of p-GSK3β and β-catenin in MSCs. And, finally, inhibition of this pathway by ICG-001 (5 µmol/L) neutralized the SDF-1α-mediated up-regulation of collagen X and MMP13 expression in MSCs.
SDF-1α may promote hypertrophic cartilage differentiation in MSCs by activating the Wnt/β-catenin pathway. These findings provide further evidence for the use of MSCs and SDF-1α in the treatment of cartilage degeneration and osteoarthritis.
Core Tip: In this study, we investigated the effect of stromal cell-derived factor-1α (SDF-1α) on the differentiation of bone marrow mesenchymal stem cells (MSCs) and primary chondrocytes in vitro. We demonstrated that SDF-1α promotes the chondrogenic differentiation of MSCs, and similar results were observed in primary chondrocytes. In addition, SDF-1α also activates the Wnt/β-catenin pathway to regulate chondrocyte hypertrophy and maturation in MSCs.
- Citation: Chen X, Liang XM, Zheng J, Dong YH. Stromal cell-derived factor-1α regulates chondrogenic differentiation via activation of the Wnt/β-catenin pathway in mesenchymal stem cells. World J Stem Cells 2023; 15(5): 490-501
- URL: https://www.wjgnet.com/1948-0210/full/v15/i5/490.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i5.490
Osteoarthritis (OA) is a chronic, multifactorial disease characterized by progressive degradation of articular cartilage[1]. The underlying molecular mechanism responsible for the pathogenesis of OA is not yet fully elucidated; as such, a disease-modifying therapy remains elusive[2], although a potential therapeutic strategy of cell-based cartilage regeneration using mesenchymal stem cells (MSCs) has been proposed[3,4]. It is known that following cartilage injury, MSCs undergo proliferation to form new cartilage and repair damage. During this process, chemokines play a role in targeted cell recruitment[5]. The chemokine stromal cell-derived factor-1α [SDF-1α, also known as C-X-C chemokine ligand (CXCL) 12 α][6] binds to the CXC receptor 4 (CXCR4) present in synovial fluid and cartilage tissues[7]. SDF-1α plays an important role in the targeted recruitment and chemotaxis of MSCs[8], and increased SDF-1α levels promote the entry of CXCR4-positive MSCs into damaged cartilage[9]. In addition, MSC recruitment mediated by the SDF-1α/CXCR4 axis has been shown to play an important role in other tissue repair processes[10]. Indeed, a previous study showed that intra-articular injection of meniscus progenitor cells promoted cartilage regeneration and improved OA via the SDF-1α/CXCR4 axis and by inducing progenitor cell homing[11]. Earlier, Hitchon et al[12] had reported the finding of upregulated expression levels of CXCR4 mRNA and protein in chondrocytes of rats with post-traumatic OA, while Kanbe et al[13] reported high SDF-1α expression in human chondrocytes of rheumatoid arthritis and OA joint fluid. This latter study also indicated that synovectomy significantly reduced SDF-1α and matrix metalloproteinase (MMP) concentrations in serum. Finally, Xiang et al[14] reported their study of human OA cartilage and in vitro SDF-1-induced OA chondrocytes, which demonstrated that inhibition of SDF-1α signaling was able to attenuate OA.
MSCs can differentiate into chondrocytes, which are characterized by SRY-box transcription factor 9 (Sox9), aggrecan, and collagen II expression[15]. In vivo, human MSCs used for cartilage repair undergo hypertrophic differentiation, which is characterized by an increase in cell volume and in the expression levels of several markers of hypertrophy, including runt-related transcription factor 2 (RUNX2), collagen X, MMP13, Indian hedgehog homolog, and alkaline phosphatase (ALP)[16]. Under physiological conditions in vivo, hypertrophic chondrocytes exhibit endochondral ossification. Furthermore, SDF-1α mediates several changes in the bone and cartilage[17], with roles in both physiologic and pathogenic processes. For example, SDF-1α/CXCR4 signaling regulates the bone morphogenetic protein-2-induced chondrogenic differentiation of MSCs and enhances chondrocyte proliferation and maturation[18]. However, it also increases the expression of MMP3 in chondrocytes, leading to mechanical destruction of the bound matrix[19]. Therefore, despite its role in MSC recruitment, the direct effect of SDF-1α on cartilage differentiation by MSCs requires further clari
The present study focused on the direct role of SDF-1α in chondrocyte differentiation and demonstrated that SDF-1α participated in chondrocyte differentiation in MSCs. In addition, the Wnt/β-catenin pathway mediated the effects of SDF-1α on cartilage differentiation.
MSCs were obtained from Sprague-Dawley (SD) rats. Ten male 4-8-wk-old SD rats weighing 150-200 g were housed in standard housing conditions with a 12-h light/dark cycle. The rats were euthanized using 20 mg/kg of ketamine intraperitoneally. Bone marrow was flushed from femurs of the SD rats using a 10-mL injector filled with Dulbecco's modified eagle medium (DMEM) and Ham’s F12 medium containing 10% fetal bovine serum (all from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, United States), 100 IU/mL penicillin, and 100 IU/mL streptomycin (Boster Biological Technology, Pleasanton, CA, United States). The cultures were maintained at 37 °C in an atmosphere of 5% CO2. The cells were grown for 48 h, and the medium was replaced. The cells were allowed to reach 70%-80% confluence and passaged by trypsinization using 0.05% trypsin/ ethylene diamine tetraacetic acid (Boster Biological Technology). The culture medium was replaced every 2 d. Rat MSCs cultured to passage 3 were used for the experiments.
Ten male 3-d-old SD rats were euthanized by intraperitoneal ketamine, and their cartilage samples were soaked in a beaker containing 75% alcohol for 15 min. The cartilage surface of the proximal tibia was removed to a depth of 1.0-1.5 mm3 using the micro-shear method and digested with 0.25% trypsin at 37 °C for 30 min. Following 10 min of centrifugation at 500 × g, the tissue pieces were collected and incubated with 0.25% collagenase II (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) at 37 °C for 24 h. After a second centrifugation, the chondrocytes were cultured under the same conditions as described for the MSCs.
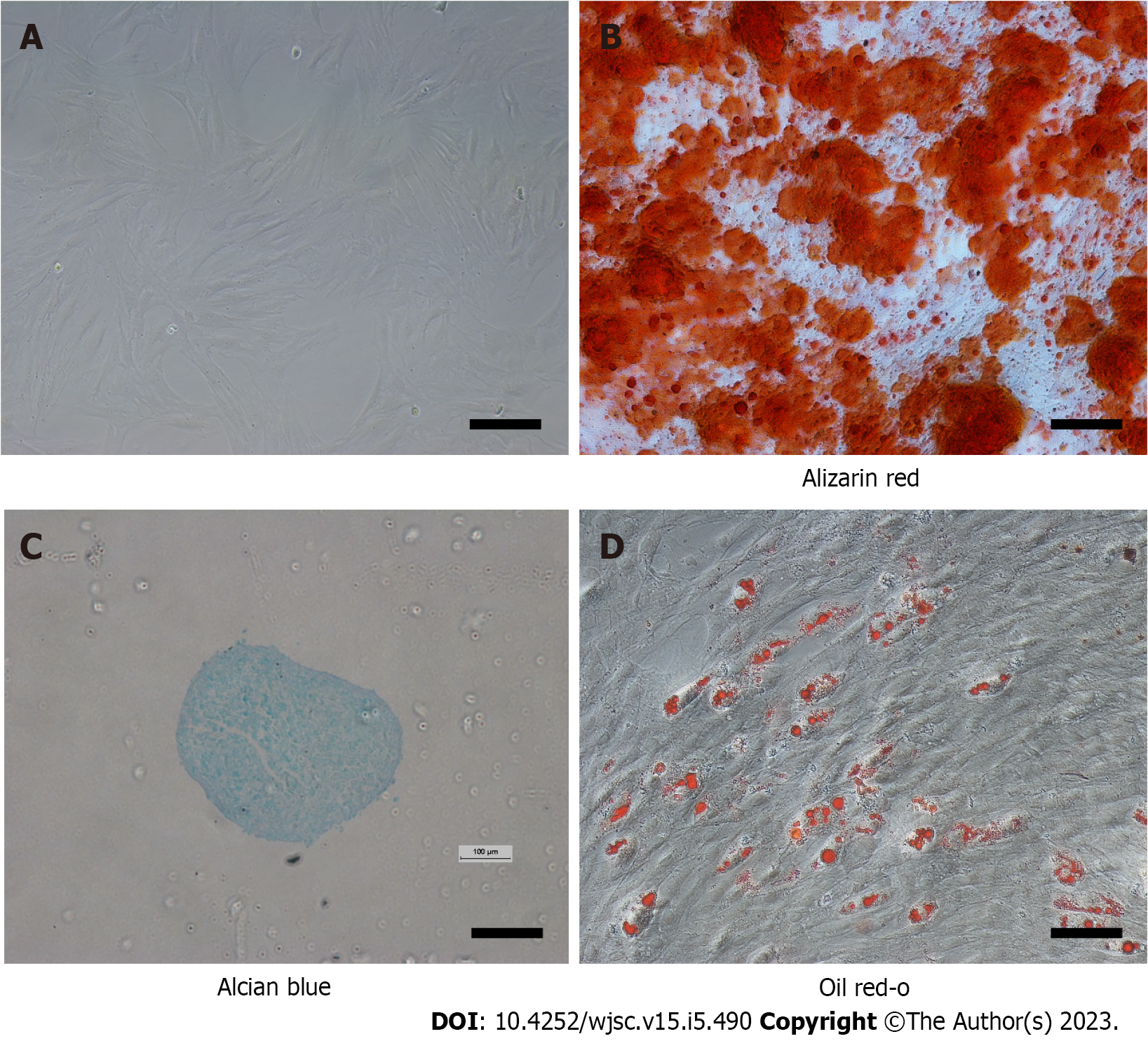
To confirm that the isolated cells were MSCs, their differentiation into bone, cartilage, and adipose cell lineages was induced. For bone differentiation, passage 3 cells were cultured with osteogenic medium (RASMX-90021; Cyagen Biosciences, Inc., Santa Clara, CA, United States). After 21 d, the cells were stained with 0.5% alizarin red S at room temperature. In brief, the cells were washed twice with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 15 min at room temperature, and then stained with alizarin red S solution for 30 min at room temperature. Morphology was evaluated using an inverted microscope (Leica DM IRM; Leica Microsystems, Wetzlar, Germany). Chondrogenic differentiation was achieved by pelleting 2.5 × 105 passage 3 cells in a 15-mL centrifuge tube at 500 × g for 5 min then resuspending the cells in 0.5 mL of chondrogenic induction medium [DMEM high-glucose, 100 nmol/L dexamethasone, 10 ng/mL transforming growth factor (TGF)-β 3, 50 mg/mL ascorbic acid 2-phosphate, 100 mg/mL sodium pyruvate, 40 mg/mL proline and insulin transferrin selenous acid-supplement][20]. The medium was replaced every 3 d. After 21 d, the pellets were fixed with 4% paraformaldehyde for 1 h at room temperature, then embedded in paraffin, cut into 5-µm sections, and stained with Alcian blue. Adipogenesis of MSCs was induced by culturing the cells in 6-well culture plates containing adipogenic medium (Cyagen Biosciences, Inc.). After 21 d, the cultures were fixed with 4% paraformaldehyde, stained with oil red O working solution (60% of 0.5% oil red O/isopropanol in distilled water) for 1 h at room temperature, and observed using light microscopy (Leica DM IRM; Leica Microsystems).
MSCs cultured in 12-well plates were prepared for immunofluorescence analysis (performed at room temperature). First, MSCs were fixed with 4% paraformaldehyde for 15 min at room temperature. The fixed cells were then permeabilized by incubating in 0.1% Triton (Boster Biological Technology, Inc.) in PBS for 10 min. After the cells were blocked with 3% bovine serum albumin (BSA; Boster Biological Technology, Inc.) in 0.1% Triton/PBS for 1 h at room temperature. The cells were initially incubated with anti-CXCR4 antibody (1:200; Abcam, Cambridge, United Kingdom) overnight at 4 °C and subsequently with an fluorescein isothiocyanate-labeled goat anti-rabbit IgG antibody (H + L) (1:200; Beyotime Institute of Biotechnology, Jiangsu, China) for 30 min at room temperature. The labeled cells were mounted with 4',6-diamidino-2-phenylindole (DAPI) at room temperature and observed by fluorescence microscopy.
MSCs were first resuspended in F12-DMEM medium containing 10% fetal bovine serum, 0.25% penicillin-streptomycin, and 0.25% L-glutamine, and plated at a density of 2.5 × 105 cells/10 µL. After incubation for 4 h, a micromass culture medium supplemented with 1 mmol/L β-glycerophosphate and 0.25 mmol/L ascorbic acid with or without SDF-1α (PeproTech, Inc., Rocky Hill, NJ, United States) was added. The cells were cultured in chondrogenic induction medium that was replaced every other day. On day 7, the cells were stained with Alcian blue, and the absorbance of the supernatant was measured at 600 nm.
MSCs and primary chondrocytes were seeded in 6-well plates containing the chondrogenic induction medium. The following three conditions were assessed: Control (cytokine-free); 50 ng SDF-1α; and 100 ng SDF-1α[21]. The expression levels of collagen II, collagen X, aggrecan, MMP13, Sox9, and RUNX2 were determined. The expression levels of Wnt/β-catenin were measured in cells incubated for 24 h with 100 ng SDF-1α and ICG-001, an inhibitor of the Wnt/β-catenin pathway in MSCs.
Collagen II (1:2000), collagen X (1:2000), aggrecan (1:2000), MMP13 (1:1000), Sox9 (1:5000), and RUNX2 (1:2000) antibodies were purchased from Abcam, whereas the p- glycogen synthase kinase 3β (GSK3β) (1:2000), GSK3β (1:2000) and β-catenin (1:2000) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, United States). Secondary mouse IgG (1:10000) or rabbit IgG (1:10000) antibodies were purchased from Abcam, and the anti- glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:1000) antibody was from Boster Biological Technology. Protein was extracted from the cells using 100 mL radio immunoprecipitation assay buffer (Boster Biological Technology, Inc.) supplemented with protease and phosphatase inhibitors. After microcentrifugation for 20 min at 10000 × g, the lysates were prepared as described above. The cell protein concentration was detected with a bicinchoninic acid kit (Boster Biological Technology, Inc.). Briefly, a total of 20 µg of cellular protein per sample was loaded onto a 10% Bis-Tris gel according to the protocol provided by the manufacturer. The separated proteins were then transferred to polyvinylidene fluoride membranes (Thermo Fisher Scientific), which were blocked for 1 h at room temperature with 5% BSA (Boster Biological Technology, Inc.) in Tris-buffered saline containing 0.1% Tween-20 (TBST). The blots were probed overnight at 4 °C with rabbit antibodies against GAPDH, collagen II, collagen X, aggrecan, MMP13, Sox9, RUNX2, p-GSK3β, GSK3β and β-catenin. Following three washes with TBST, the blots were incubated for 1 h at room temperature with anti-mouse or anti-rabbit IgG-horseradish-peroxidase-labeled secondary antibodies and washed three times with TBST. Finally, immunoreactivity was detected with enhanced chemiluminescence, and densitometry was performed using Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA, United States).
Statistical analysis was performed using GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, United States). The results were summarized as mean ± standard deviation. Every experiment contained ≥ 3 replicate and was performed three independent times, unless otherwise stated. One-way analysis of variance and Fisher’s least significant difference post hoc test were performed to compare differences between multiple groups. P < 0.05 indicated a statistically significant difference. we use 1 to express P < 0.05 and 2 to express P < 0.01.
The cells were initially quiescent but began to proliferate rapidly after day 3. Growth yielded a monolayer structure, composed of fibroblasts (Figure 1A). At passage 3, the isolated cells were successfully differentiated into the three skeletal cell lineages: Bone, cartilage, and adipose tissue. After culture in the osteogenic medium, nodules formed that were positive for alizarin red S staining, indicating calcium-bearing mineral deposits (Figure 1B). After culture with cartilage induction medium, cartilage microspheres were positive for Alcian blue staining. Blue granules were also noted in MSCs (Figure 1C). After culture in the adipogenic induction medium, lipid accumulation in the form of lipid droplets was noted in some of the cells, which were stained red by oil red O (Figure 1D).
CXCR4 expression was detected in the membrane of the rat MSCs, while DAPI staining was confined to the nuclei of the MSCs (Figure 2).
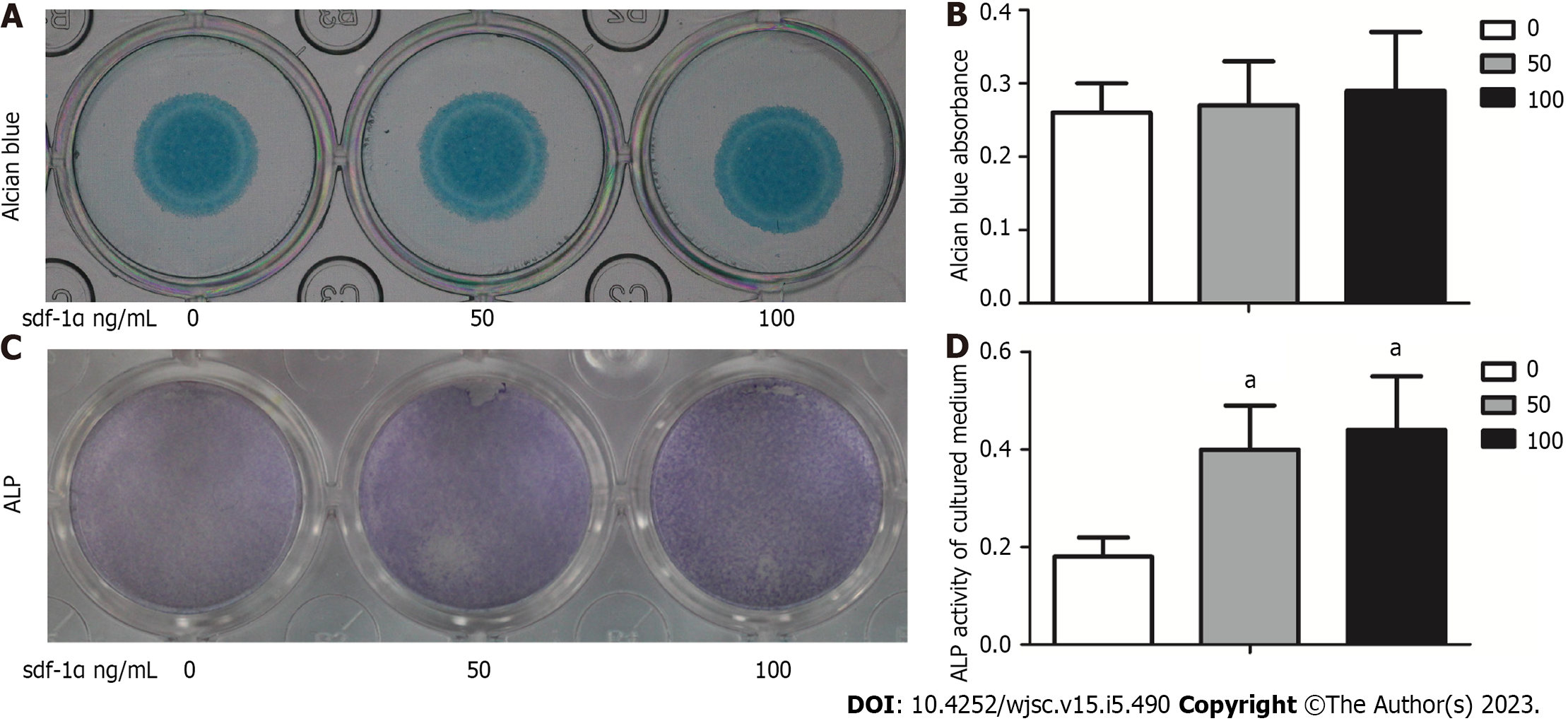
No significant differences were noted between control (untreated) cells and cells treated with 50 ng SDF-1α or 100 ng SDF-1α in regards to the size of the cartilage micelles or the absorbance of Alcian blue (Figure 3A and B). ALP expression and activity levels were increased after 14-d SDF-1α treatment compared to control cells (Figure 3C and D).
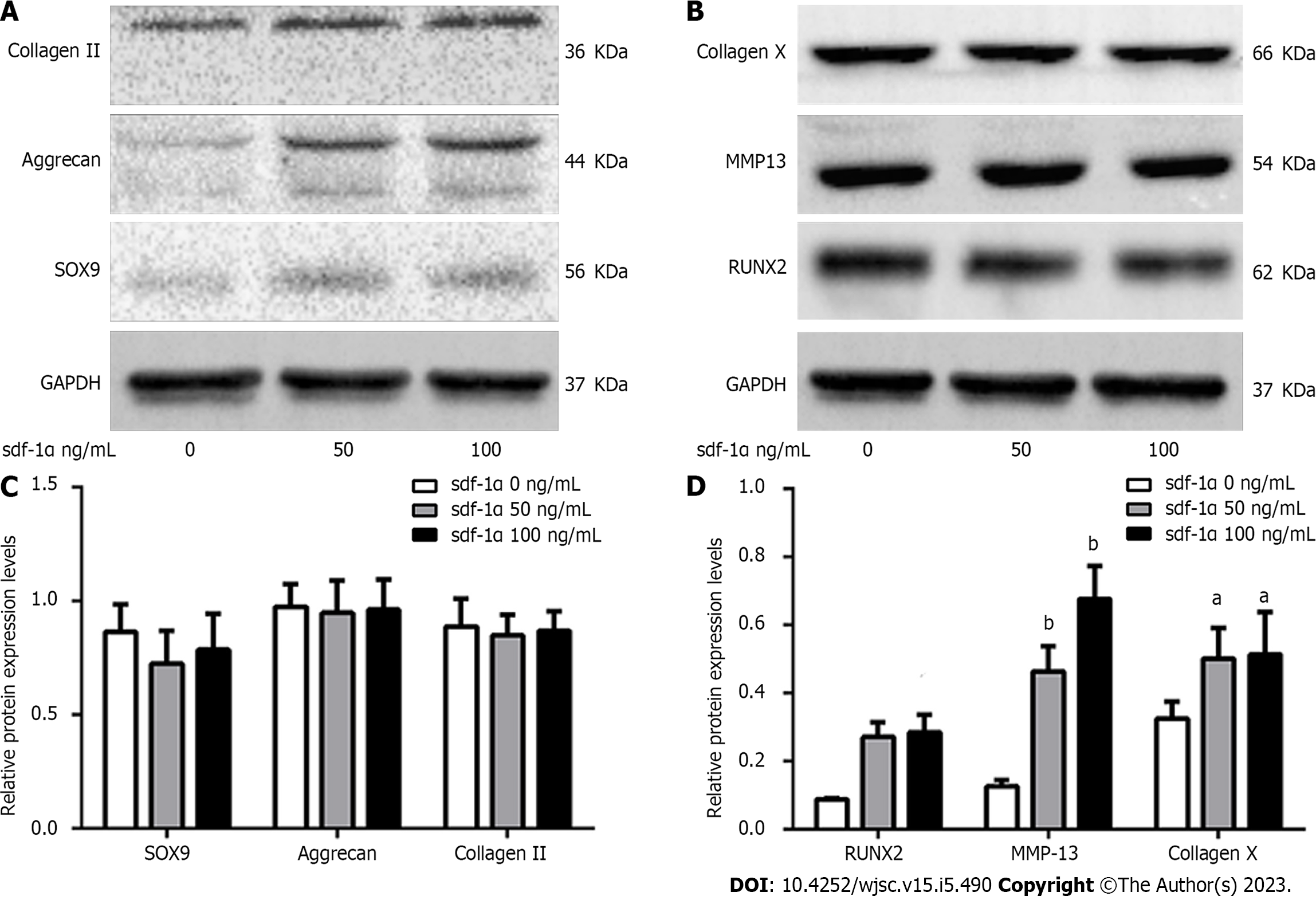
Western blotting indicated no significant differences in the expression levels of early chondrocyte differentiation markers (Sox9, aggrecan, and collagen II) between MSCs treated with SDF-1α and untreated MSCs on day 7 (Figure 4A). On day 14, however, the expression levels of chondrocyte hypertrophy markers (RUNX2, collagen X, and MMP13) were increased in a dose-dependent manner in the SDF-1α-treated group (Figure 4B).
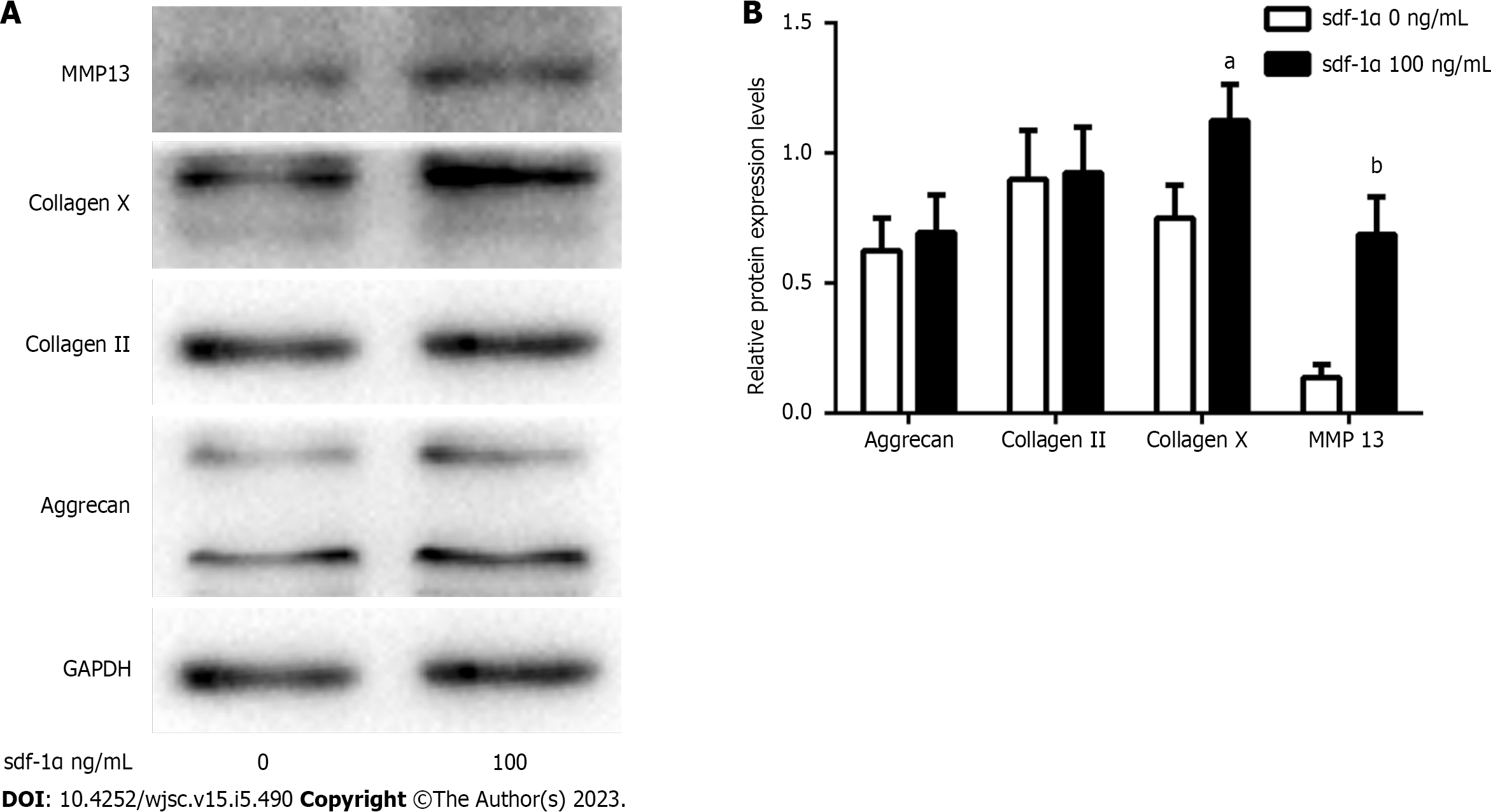
Western blotting showed that SDF-1α treatment did not affect the expression levels of collagen II and aggrecan in primary chondrocytes, whereas it significantly increased the expression levels of collagen X and MMP13 in the MSCs (Figure 5).
SDF-1α promoted the expression of p-GSK3β, decreased degradation of β-catenin, and a gradual increase in β-catenin expression were demonstrated (Figure 6A). Upon blockade of the Wnt/β-catenin pathway via ICG-001, the SDF-1α-mediated increase in the expression levels of collagen X and MMP13 was neutralized (Figure 6B).
In the present study, rat MSCs, which were successfully differentiated into the three skeletal cell lineages and were positive for the expression of the CXCR4 receptors on the cell membrane, were used to assess the effects of SDF-1α on cartilage formation. The results indicated that the size of the cartilage micromass, the absorbance of Alcian blue, and the expression levels of Sox9, aggrecan, and collagen II did not significantly change in response to SDF-1α. However, the expression and activity levels of ALP and the expression levels of RUNX2, collagen X, and MMP13 were significantly increased. These results demonstrated that SDF-1α promoted hypertrophic cartilage differentiation in MSCs, while not affecting the early differentiation of cartilage. Similar results were obtained in primary chondrocytes. The data further indicated that SDF-1α caused a gradual increase in the expression levels of p-GSK-3β in vitro and activated the Wnt/β-catenin pathway, leading to increased collagen X and MMP13 expression levels. These findings demonstrated that the SDF-1α/CXCR4 axis was required in the cartilage differentiation process. Previous studies have implicated other chemokine types, including CXCL8 and CXCL1, as capable of promoting chondrocyte hypertrophy and calcification[22,23].
The Wnt/β-catenin pathway is a classical Wnt signaling pathway involved in tissue development and cell proliferation, differentiation, and apoptosis[24,25]. The signal transduction of the Wnt/β-catenin pathway is well defined and proceeds as follows. Initially, the extracellular Wnt proteins (Wnt-3a, Wnt-4, Wnt-8c, and Wnt-9a) combine with the frizzled and LRP proteins on the cell membrane to form an activation complex. Subsequently, the phosphorylation of GSK-3β blocks the phosphorylation and degradation of β-catenin. Finally, β-catenin enters the cell nucleus and modulates T cell factor/Lymphoid enhancer factor binding, which initiates the transcription of downstream genes, thus causing biological changes[26-30]. Several Wnt signaling components regulate the hypertrophic maturation of chondrocytes. Specifically, Wnt can induce the accumulation of β-catenin, which then enters the nucleus and binds to cell factor/lymphoid enhancer-binding factor to promote the transcription of the collagen X and MMP13 genes. Ultimately, P-GSK3β can add phosphate groups to the serine/threonine residues at the β-catenin N terminus to promote its degradation[4.31].
Overexpression of the Wnt receptor frzb-1 was shown to hinder chondrocyte maturation and mineralization[32]. In a subsequent study, knock-out of the secreted frizzled-related protein 1, a Wnt signaling antagonist, led to a reduced height of the growth plate and increased calcification of hypertrophic areas, indicating that activation of the Wnt signaling pathway accelerated endochondral ossification[33]. The findings of the present study are consistent with the collective previous results indicating that the SDF-1α/CXCR4 axis activates the Wnt/β-catenin signaling pathway in MSCs, which in turn increases the production of collagen X and MMP13. Conversely, when we treated the MSCs with the Wnt/β-catenin inhibitor ICG-001, the effects of SDF-1α were no longer observable, which confirmed the regulatory role of Wnt/β-catenin. Thus, the present study indicates that SDF-1α does not promote the early stages of cartilage differentiation nor increase the expression of Sox9, which is similar to the results of Kim et al[34].
Hypertrophic differentiation of chondrocytes is the primary barrier preventing the use of MSCs in therapeutic cartilage repair[35,36]. Hypertrophy is sometimes noted in OA[37,38]. However, SDF-1α also mediates MSC recruitment and can exert a positive role in OA[31]. The identification of cytokines that block cartilage hypertrophy caused by SDF-1α, promote physiological endochondral ossification, prevent mineralization of the extracellular matrix, and mediate chondrocyte apoptosis will contribute to an improved understanding of the pathogenesis of OA and provide targets for development of future treatment strategies for this disease[39].
There were some limitations in this study, which must be considered when seeking to generalize our findings. First, measuring the stimulation with SDF-1α in MSCs is challenging because the only verification technique is overexpression or knockdown of the CXCR4 receptor. Second, this study primarily used cell experiments and lacked an in vivo perspective to the experimental research. Regardless, through this study, we were able to adequately demonstrate effects of SDF-1α on cartilage differentiation in MSCs and primary chondrocytes.
The present study demonstrated a role of SDF-1α in promoting hypertrophic cartilage differentiation in MSCs and primary chondrocytes in vitro. SDF-1α activated the Wnt/β-catenin pathway in MSCs. Identification of the novel molecular mechanism by which SDF-1α promotes cartilage differentiation in MSCs suggests a therapeutic approach to OA and cartilage repair.
Stromal cell-derived factor-1α (SDF-1α) has a chemotactic effect on mesenchymal stem cells (MSCs), and SDF-1α and MSCs are used together to treat cartilage degeneration and cartilage defects. The specific effects of SDF-1α on cartilage differentiation in MSCs need to be clarified.
Understanding the effects of SDF-1α on MSCs will provide a new theoretical basis for the use of MSCs in the repair of cartilage degeneration.
To explore the role and mechanism of SDF-1α on cartilage differentiation in MSCs and primary chondrocytes.
MSCs were treated with SDF-1α and subsequently stained for alkaline phosphatase and with Alcian blue to demonstrate chondrogenic differentiation. Western blot analysis was used to examine the expression of cartilage differentiation-related and Wnt/β-catenin pathway proteins in MSCs and primary chondrocytes.
After extraction and incubation with the appropriate differentiation media, MSCs differentiated into the three skeletal lineages. SDF-1α exerted no effect on early cartilage formation but enhanced hypertrophic differentiation in MSCs. SDF-1α had no effect on the expression of SRY-box transcription factor 9, aggrecan, and collagen II but increased the expression of runx family transcription factor 2, collagen X, and matrix metalloproteinase 13 in MSCs and primary chondrocytes. SDF-1α increased the expression of p- glycogen synthase kinase 3β and β-catenin.
SDF-1α enhanced hypertrophic differentiation in MSCs and primary chondrocytes. This effect was achieved by activating the Wnt/β-catenin pathway.
These findings provide a new theoretical basis for the treatment of cartilage degeneration with MSCs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salim A, Pakistan; Shamseldeen AA, Egypt S-Editor: Liu GL L-Editor: A P-Editor: Zhang XD
| 1. | Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1087] [Cited by in RCA: 983] [Article Influence: 65.5] [Reference Citation Analysis (5)] |
| 2. | Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage. 2020;28:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 315] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 3. | Richardson SM, Kalamegam G, Pushparaj PN, Matta C, Memic A, Khademhosseini A, Mobasheri R, Poletti FL, Hoyland JA, Mobasheri A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods. 2016;99:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 346] [Article Influence: 34.6] [Reference Citation Analysis (1)] |
| 4. | Samadi P, Saki S, Manoochehri H, Sheykhhasan M. Therapeutic Applications of Mesenchymal Stem Cells: A Comprehensive Review. Curr Stem Cell Res Ther. 2021;16:323-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (3)] |
| 5. | Zhang S, Hu B, Liu W, Wang P, Lv X, Chen S, Liu H, Shao Z. Articular cartilage regeneration: The role of endogenous mesenchymal stem/progenitor cell recruitment and migration. Semin Arthritis Rheum. 2020;50:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 519] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 7. | Chiu YC, Yang RS, Hsieh KH, Fong YC, Way TD, Lee TS, Wu HC, Fu WM, Tang CH. Stromal cell-derived factor-1 induces matrix metalloprotease-13 expression in human chondrocytes. Mol Pharmacol. 2007;72:695-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Marquez-Curtis LA, Janowska-Wieczorek A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed Res Int. 2013;2013:561098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 9. | Kumar S, Ponnazhagan S. Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone. 2012;50:1012-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 10. | Yellowley C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. Bonekey Rep. 2013;2:300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Shen W, Chen J, Zhu T, Chen L, Zhang W, Fang Z, Heng BC, Yin Z, Chen X, Ji J, Chen W, Ouyang HW. Intra-articular injection of human meniscus stem/progenitor cells promotes meniscus regeneration and ameliorates osteoarthritis through stromal cell-derived factor-1/CXCR4-mediated homing. Stem Cells Transl Med. 2014;3:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Hitchon C, Wong K, Ma G, Reed J, Lyttle D, El-Gabalawy H. Hypoxia-induced production of stromal cell-derived factor 1 (CXCL12) and vascular endothelial growth factor by synovial fibroblasts. Arthritis Rheum. 2002;46:2587-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 191] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Kanbe K, Takagishi K, Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis Rheum. 2002;46:130-137. [PubMed] [DOI] [Full Text] |
| 14. | Xiang Y, Li Y, Yang L, He Y, Jia D, Hu X. miR-142-5p as a CXCR4-Targeted MicroRNA Attenuates SDF-1-Induced Chondrocyte Apoptosis and Cartilage Degradation via Inactivating MAPK Signaling Pathway. Biochem Res Int. 2020;2020:4508108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Mrugala D, Dossat N, Ringe J, Delorme B, Coffy A, Bony C, Charbord P, Häupl T, Daures JP, Noël D, Jorgensen C. Gene expression profile of multipotent mesenchymal stromal cells: Identification of pathways common to TGFbeta3/BMP2-induced chondrogenesis. Cloning Stem Cells. 2009;11:61-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Studer D, Millan C, Öztürk E, Maniura-Weber K, Zenobi-Wong M. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur Cell Mater. 2012;24:118-35; discussion 135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Galliera E, Corsi MM, Banfi G. Platelet rich plasma therapy: inflammatory molecules involved in tissue healing. J Biol Regul Homeost Agents. 2012;26:35S-42S. [PubMed] |
| 18. | Guang LG, Boskey AL, Zhu W. Regulatory role of stromal cell-derived factor-1 in bone morphogenetic protein-2-induced chondrogenic differentiation in vitro. Int J Biochem Cell Biol. 2012;44:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Masuko-Hongo K, Sato T, Nishioka K. Chemokines differentially induce matrix metalloproteinase-3 and prostaglandin E2 in human articular chondrocytes. Clin Exp Rheumatol. 2005;23:57-62. [PubMed] |
| 20. | Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 946] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 21. | Murata K, Kitaori T, Oishi S, Watanabe N, Yoshitomi H, Tanida S, Ishikawa M, Kasahara T, Shibuya H, Fujii N, Nagasawa T, Nakamura T, Ito H. Stromal cell-derived factor 1 regulates the actin organization of chondrocytes and chondrocyte hypertrophy. PLoS One. 2012;7:e37163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Merz D, Liu R, Johnson K, Terkeltaub R. IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocyte hypertrophic differentiation. J Immunol. 2003;171:4406-4415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Cecil DL, Rose DM, Terkeltaub R, Liu-Bryan R. Role of interleukin-8 in PiT-1 expression and CXCR1-mediated inorganic phosphate uptake in chondrocytes. Arthritis Rheum. 2005;52:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 842] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 25. | Maeda Y, Nakamura E, Nguyen MT, Suva LJ, Swain FL, Razzaque MS, Mackem S, Lanske B. Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc Natl Acad Sci U S A. 2007;104:6382-6387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 26. | Davis JR, Tapon N. Hippo signalling during development. Development. 2019;146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Pai SG, Carneiro BA, Mota JM, Costa R, Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK, Giles FJ. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 503] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 28. | Baarsma HA, Königshoff M, Gosens R. The WNT signaling pathway from ligand secretion to gene transcription: molecular mechanisms and pharmacological targets. Pharmacol Ther. 2013;138:66-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 29. | MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4613] [Cited by in RCA: 4503] [Article Influence: 281.4] [Reference Citation Analysis (0)] |
| 30. | Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2031] [Cited by in RCA: 3088] [Article Influence: 386.0] [Reference Citation Analysis (0)] |
| 31. | Meng Z, Feng G, Hu X, Yang L, Yang X, Jin Q. SDF Factor-1α Promotes the Migration, Proliferation, and Osteogenic Differentiation of Mouse Bone Marrow Mesenchymal Stem Cells Through the Wnt/β-Catenin Pathway. Stem Cells Dev. 2021;30:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, Koike T, Okada H, Komori T, Yoneda T, Church V, Francis-West PH, Kurisu K, Nohno T, Pacifici M, Iwamoto M. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002;251:142-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Gaur T, Rich L, Lengner CJ, Hussain S, Trevant B, Ayers D, Stein JL, Bodine PV, Komm BS, Stein GS, Lian JB. Secreted frizzled related protein 1 regulates Wnt signaling for BMP2 induced chondrocyte differentiation. J Cell Physiol. 2006;208:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Kim GW, Han MS, Park HR, Lee EJ, Jung YK, Usmani SE, Ulici V, Han SW, Beier F. CXC chemokine ligand 12a enhances chondrocyte proliferation and maturation during endochondral bone formation. Osteoarthritis Cartilage. 2015;23:966-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Zhong L, Huang X, Karperien M, Post JN. The Regulatory Role of Signaling Crosstalk in Hypertrophy of MSCs and Human Articular Chondrocytes. Int J Mol Sci. 2015;16:19225-19247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 36. | Steck E, Fischer J, Lorenz H, Gotterbarm T, Jung M, Richter W. Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009;18:969-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1729] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 38. | Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, Nishida N, Akune T, Yoshimura N, Nakagawa T, Nakamura K, Tokunaga K, Chung UI, Kawaguchi H. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med. 2010;16:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 424] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 39. | Qian G, Zhang L, Wang G, Zhao Z, Peng S, Shuai C. 3D Printed Zn-doped Mesoporous Silica-incorporated Poly-L-lactic Acid Scaffolds for Bone Repair. Int J Bioprint. 2021;7:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |