Published online Aug 26, 2022. doi: 10.4252/wjsc.v14.i8.658
Peer-review started: March 15, 2022
First decision: April 19, 2022
Revised: April 27, 2022
Accepted: July 26, 2022
Article in press: July 26, 2022
Published online: August 26, 2022
Processing time: 163 Days and 16.9 Hours
Bone marrow transplantation (BMT) can be applied to both hematopoietic and nonhematopoietic diseases; nonetheless, it still comes with a number of challenges and limitations that contribute to treatment failure. Bearing this in mind, a possible way to increase the success rate of BMT would be cotransplantation of mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs) to improve the bone marrow niche and secrete molecules that enhance the hematopoietic engraftment.
To analyze HSC and MSC characteristics and their interactions through cotransplantation in murine models.
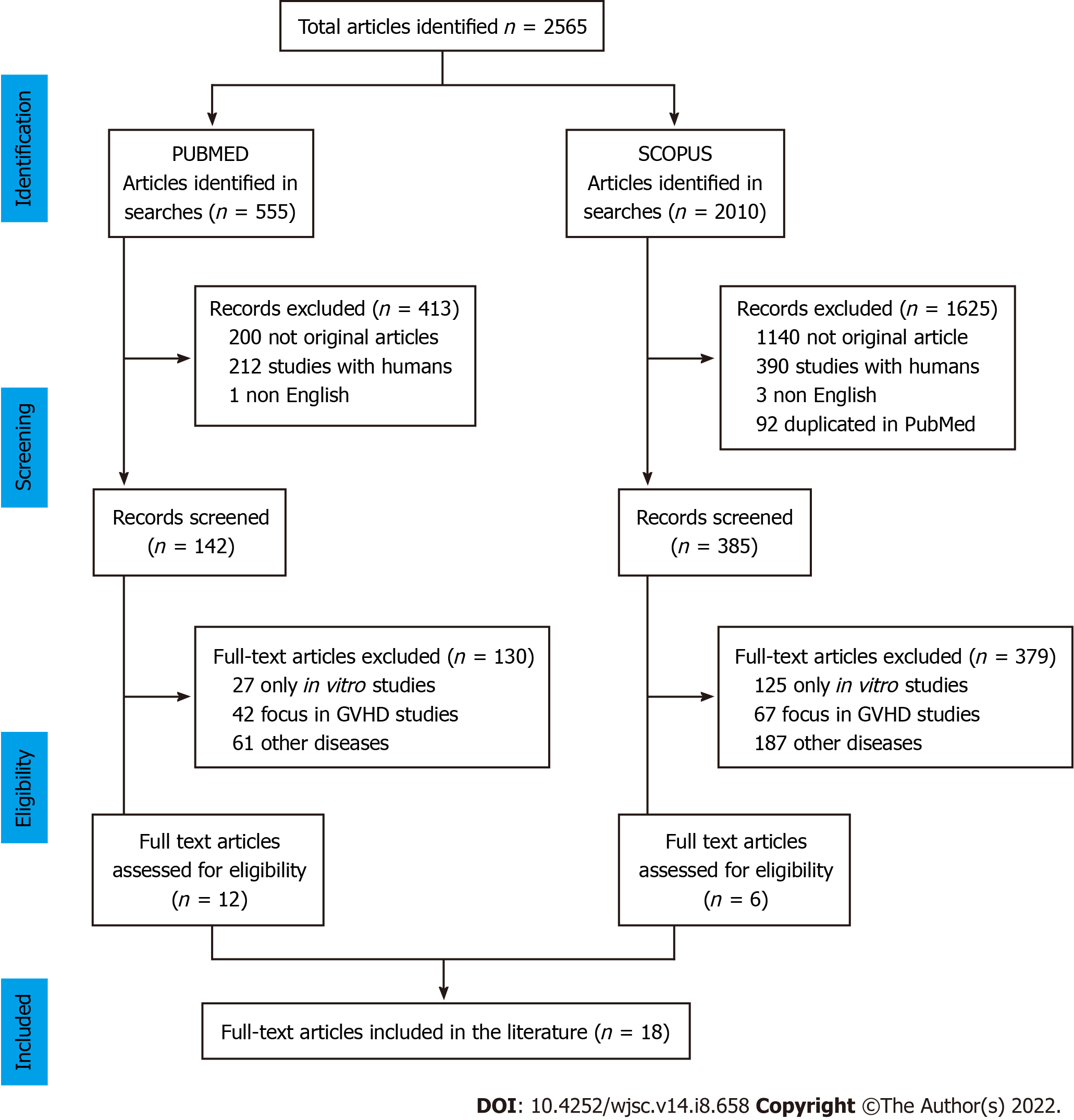
We searched for original articles indexed in PubMed and Scopus during the last decade that used HSC and MSC cotransplantation and in vivo BMT in animal models while evaluating cell engraftment. We excluded in vitro studies or studies that involved graft versus host disease or other hematological diseases and publications in languages other than English. In PubMed, we initially identified 555 articles and after selection, only 12 were chosen. In Scopus, 2010 were identified, and six were left after the screening and eligibility process.
Of the 2565 articles found in the databases, only 18 original studies met the eligibility criteria. HSC distribution by source showed similar ratios, with human umbilical cord blood or animal bone marrow being administered mainly with a dose of 1 × 107 cells by intravenous or intrabone routes. However, MSCs had a high prevalence of human donors with a variety of sources (umbilical cord blood, bone marrow, tonsil, adipose tissue or fetal lung), using a lower dose, mainly 106 cells and ranging 104 to 1.5 × 107 cells, utilizing the same routes. MSCs were characterized prior to administration in almost every experiment. The recipient used was mostly immunodeficient mice submitted to low-dose irradiation or chemotherapy. The main technique of engraftment for HSC and MSC cotransplantation evaluation was chimerism, followed by hematopoietic reconstitution and survival analysis. Besides the engraftment, homing and cellularity were also evaluated in some studies.
The preclinical findings validate the potential of MSCs to enable HSC engraftment in vivo in both xenogeneic and allogeneic hematopoietic cell transplantation animal models, in the absence of toxicity.
Core Tip: The systematic review provided a current view on the characteristics of mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs) cotransplantation to achieve successful engraftment and improve hematopoietic reconstitution. The studies demonstrated a diversity in experimental designs and MSC isolation and characterization protocols; however, the lack of standardization in MSC use makes translation to clinical practice more difficult.
- Citation: Garrigós MM, de Oliveira FA, Nucci MP, Nucci LP, Alves ADH, Dias OFM, Gamarra LF. How mesenchymal stem cell cotransplantation with hematopoietic stem cells can improve engraftment in animal models. World J Stem Cells 2022; 14(8): 658-679
- URL: https://www.wjgnet.com/1948-0210/full/v14/i8/658.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i8.658
Hematopoietic stem cell (HSC) transplantation has saved many lives in individuals with severe hematological diseases. However, the results need to be improved, and co-infusion of mesenchymal stem cells (MSCs) could be the key to creating this therapy as a viable alternative. Bone marrow (BM) stromal cells in adults were discovered by Friedestein in 1968, as an adherent, fibroblast-like population capable of reconstructing rudiments of bone in vivo[1]. These cells, which make up only 0.1% of mature BM cells, provide the supportive niche for hematopoietic stem cells (HSCs)[2]. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has proven a life-saving therapy for many malignant and nonmalignant hematological illnesses throughout the last few decades, allowing for complete blood cellular constituent recovery and the graft-versus-leukemia effect[3]. The HSC therapeutic impact depends on the successful engraftment of donor stem cells[4]. However, a number of significant issues might make transplantation difficult. Graft-versus-host disease (GVHD), which can be acute or chronic, as well as disease recurrence and opportunistic bacterial, viral or fungal infections, are the most serious and potentially fatal side effects of allo-HSCT. All of these conditions may cause serious morbidity and mortality in allo-HSCT recipients[3]. According to recent research[4,5], MSCs are the primary cells implicated in HSC homing. Although the molecular interaction and/or grafting cytoarchitecture are yet unclear, more research is needed to assess the true efficacy of MSC cotransplantation in allo-HSCT.
MSCs have significant immunomodulatory effects on both the adaptive and innate immune systems as a result of this. MSC-modulated lymphocyte suppression appears to be mediated by paracrine processes such as secreted mediators (e.g., transforming growth factor, hepatocyte growth factor and prostaglandin E2), participates in complex interactions with dendritic cells, B cells and T cells, including T regulatory cells, killer cells, and a variety of T helper cells and metabolic activities [e.g., indoleamine 2,3-dioxygenas (IDO)]. MSCs have been shown to induce T cells to become polarized toward a regulatory phenotype, which may contribute to the reduction of inflammation, preventing GVHD, improving the BM niche. MSCs also increase the expression of many hematopoietic factors, inhibit apoptosis, allowing HSCs to survive and proliferate in the stroma[6,7].
MSCs can directly affect HSCs by releasing soluble compounds such as IDO, prostaglandin E2, nitric oxide, transforming growth factor, interferon-, and interleukin 1, although the net interactions between cells are unknown. The other molecular process enhanced post cotransplantation (MSC/HSC) is C-X-C chemokine receptor (CXCR)4 and stromal cell-derived factor (SDF)-1, also known as CXC ligand 12, in recovery in murine models. The optimization of self-renewal and proliferation of HSCs depends on the survival and homing of HSCs into BM. The use of MSCs improves homing and hematopoietic reconstitution and produces chimerism superior to that of conventional transplantation[8], but these interactions are unclear. Several studies have been carried out to investigate the safety and/or efficacy of MSC and HSC cotransplantation, but controversy persists, probably due to heterogeneous doses, routes and sources of MSCs and HSCs. To date, a few literature reviews have summarized these conflicting results but have not yielded any encouraging findings.
As a result of the difficult in setting up the hematopoietic engraftment and MSC cotransplantation benefits, the goal of this review was to analyze HSC and MSC characteristics and their interactions through cotransplantation in animal models.
We searched for original articles that were indexed in PubMed and Scopus. The original articles found in the databases were analyzed and verified by the Reference Citation Analysis (https://www.referencecitationanalysis.com/). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed during all procedures. The following selected criteria of interest, keyword sequences [(Mesenchymal Stem Cell) AND (Hematopoietic Stem Cell) AND (Graft)], and boolean operators (DecS/MeSH) were used.
PubMed: ("Mesenchymal Stem Cells" [Title/Abstract] OR "Mesenchymal Stem Cell" [Title/Abstract] OR "Mesenchymal Stromal Cells" [Title/Abstract] OR "Mesenchymal Stromal Cell" [Title/Abstract] OR "Multipotent Stromal Cells" [Title/Abstract] OR "Multipotent Stromal Cell" [Title/Abstract] OR "Stromal Stem Cells" [Title/Abstract] OR "Stromal Stem Cell" [Title/Abstract] OR "Stromal Cells" [Title/Abstract] OR "Stromal Cell" [Title/Abstract]) AND ("Hematopoietic Stem Cells" [Title/Abstract] OR "Hematopoietic Stem Cell" [Title/Abstract] OR "Hematopoietic Progenitor Cells" [Title/Abstract] OR "Hematopoietic Progenitor Cell" [Title/Abstract] OR "Hematopoietic Cells" [Title/Abstract] OR "Hematopoietic Cell" [Title/Abstract]) AND ("Engraftment" [Title/Abstract] OR "Grafting" [Title/Abstract] OR "Graft"[Title/Abstract]).
Scopus: TITLE-ABS-KEY ("Mesenchymal Stem Cells") OR TITLE-ABS-KEY ("Mesenchymal Stem Cell") OR TITLE-ABS-KEY ("Mesenchymal Stromal Cells") OR TITLE-ABS-KEY ("Mesenchymal Stromal Cell") OR TITLE-ABS-KEY ("Multipotent Stromal Cells") OR TITLE-ABS-KEY ("Multipotent Stromal Cell") OR TITLE-ABS-KEY ("Stromal Stem Cells") OR TITLE-ABS-KEY ("Stromal Stem Cell") OR TITLE-ABS-KEY ("Stromal Cells") OR TITLE-ABS-KEY ("Stromal Cell") AND TITLE-ABS-KEY ("Hematopoietic Stem Cells") OR TITLE-ABS-KEY ("Hematopoietic Stem Cell") OR TITLE-ABS-KEY ("Hematopoietic Progenitor Cells") OR TITLE-ABS-KEY ("Hematopoietic Progenitor Cell") OR TITLE-ABS-KEY ("Hematopoietic Cells") OR TITLE-ABS-KEY ("Hematopoietic Cell") AND TITLE-ABS-KEY (engraftment) OR TITLE-ABS-KEY (grafting) OR TITLE-ABS-KEY (graft).
This systematic review included only original articles written in English, published between 2011 and 2021, that had used: (1) HSC and MSC cotransplantation; (2) in vivo BMT in animal models; and (3) engraftment evaluation, factors involved in PICO criteria: Problem: inefficiency of HSC transplantation; Intervention: MSC cotransplantation; Comparison: HSC-only transplantation and associated with MSCs; and Outcome: engraftment evaluation. Reasons for excluding studies were as follows: (1) not original articles; (2) publications in languages other than English; (3) indexed articles published in more than one database (duplicates); (4) studies involving GVHD; (5) studies involving other diseases; and (6) studies with only in vitro results.
In this systematic review, seven of the authors (M.M.G., F.A.O., M.P.N., L.P.N., A.H.A., O.F.M.D. and L.F.G.) independently and randomly selected (in pairs), revised, and evaluated the titles and abstracts of the publications identified by the search strategy in the databases cited above, and all potentially relevant publications were retrieved in full. These same reviewers evaluated the full-text articles to decide whether the eligibility criteria were met. Discrepancies in study selection and data extraction between the two reviewers were discussed with a third reviewer and resolved.
M.M.G., F.A.O., M.P.N. and L.P.N. searched for MSC characteristics; M.M.G., F.A.O., O.F.M.D., and A.H.A. searched for HSC characteristics; M.M.G., F.A.O., M.P.N. and L.F.G. searched for MSC and HSC cotransplantation parameters, M.M.G., F.A.O., M.P.N. and L.P.N. searched for therapy evaluation. The analysis process and table plots were carried out by a full consensus of peers, respecting the distribution above.
In cases of disagreement, a third, independent senior author decided to add or subtract data, decreasing the risk of bias. The final inclusion of studies into the systematic review was by agreement of all reviewers.
For all variables evaluated in the tables, the percentage distribution was used to characterize and illustrate the results.
We searched original articles published between January 2011 and December 2021, indexed in PubMed and Scopus, and a total of 2565 articles were found. Of the 555 articles identified in PubMed, 413 were excluded after screening (200 reviews, 212 studies in humans, and one published in another language), and 130 after eligibility assessment (27 in vitro studies, 42 GVHD studies, and 61 other hematological diseases); only 12 studies were included. Of the 2010 articles identified in Scopus, 1625 were excluded after screening (1140 not original articles, 390 studies in humans, three published in other languages, and 92 articles duplicated in PubMed search), and 379 after eligibility assessment (125 only in vitro study, 67 focus in GVHD study, and 187 in other hematological diseases), leaving six included studies. As a result, 18 papers[8-25] met all the criteria for inclusion and exclusion in this systematic review (Figure 1).
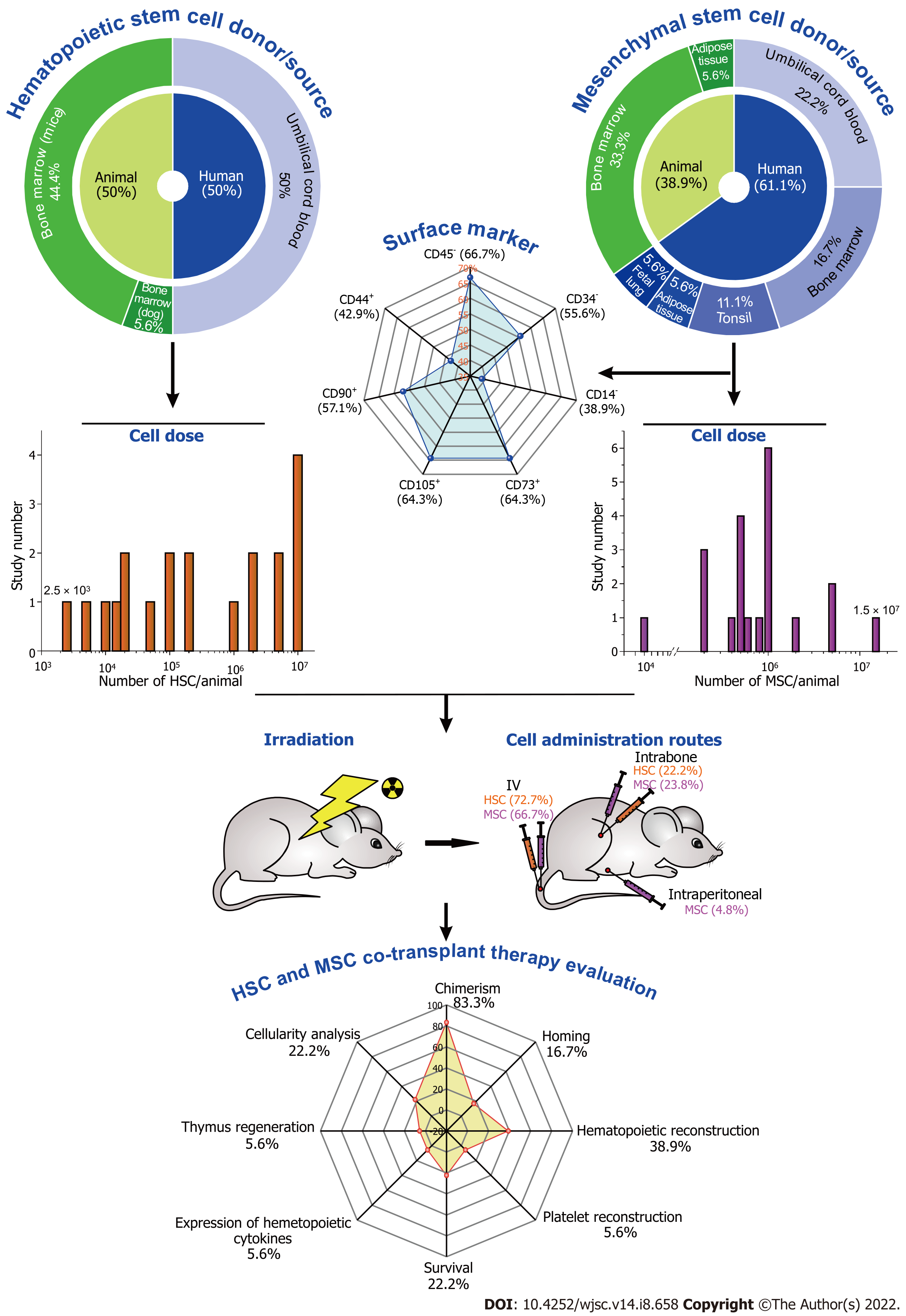
In terms of HSCs, half of the selected studies[8,10,15,16,18,20-22,25] used cells from human umbilical cord blood (UCB), while the other half[9,11-14,17,18,23,24] used cells from animal BM (Table 1). Wu et al[20] reported the transplantation of the pool of human nucleated cells. Huang et al[10] and Lim et al[21] reported the use of a mononuclear cell pool without any cell selection process, and the other 33.3% of the selected studies used human cells CD34+ before transplantation. The majority of studies[9,11-14,18,23,24] used the pool of nucleated cells (44.4%) from animals, with the exception of the study by Fernández-García et al[17] (5.6%) that used cells of lineage Sca-1+ cKit+ (LSK).
| Ref. | Year | Hematopoietic stem cells | Transplantation | ||||
| Donor | Source | Lineage | Cell dose | Route | Volume (µL) | ||
| Lee et al[9] | 2021 | Mice (C57BL/6) | BM | NC | 1 × 107 | IV | NR |
| Huang et al[10] | 2021 | Human | UCB | MNC | 1 × 107 | IV; IB | 200; NR |
| Yin et al[8] | 2020 | Human | UCB | CD34+ | 2.5 × 103; 5 × 103; 1 × 104; 2 × 104 | IV; IB | NR; 10 |
| Choi et al[11] | 2020 | Mice (C57BL/6) | BM | NC | 1 × 107 | IV | 200 |
| Trento et al[12] | 2017 | Mice (C57BL/6 CD45.1) | BM | NC | 2 × 106 | IV | NR |
| Abbuehl et al[13] | 2017 | Mice (FVB Actin-GFP) | BM | NC | 5 × 106 | IV | 100 |
| Kim et al[14] | 2016 | Mice (C57BL/6J-Pep3b-Ly5.1 Pep3b) | BM | NC | 2 × 104 | NR | NR |
| Futrega et al[15] | 2016 | Human | UCB | CD34+ | 5 × 104 | IV; IB | 100; 10 |
| van der Garde et al[16] | 2015 | Human | UCB | CD34+ | 2 × 105 | IV | NR |
| Fernández-García et al[17] | 2015 | Mice (P3D2F1 H2b/d CD45.1) | BM | LSK | 1.5 × 104 | NR | NR |
| Chen et al[18] | 2015 | Mice (C57BL/6) | BM | NC | 5 × 106 | IV | NR |
| Chen et al[19] | 2014 | Human | UCB | CD34+ | 1 × 105 | IV | NR |
| Wu et al[20] | 2013 | Human | UCB | NC | 1 × 106 | IV | 250 |
| Lim et al[21] | 2013 | Human | UCB | MNC | 1 × 107 | IV | 200 |
| Lee et al[22] | 2013 | Human | UCB | CD34+ | 2 × 105 | IV | 150-200 |
| Kornblit et al[23] | 2013 | Dog (beagle) | BM | NC | 1.8-5.3 × 108/kg | IV | 50 mL |
| Fortin et al[24] | 2013 | Mice (B6.SJL-PtrcaPep3b/BoyJ CD45.1) | BM | NC | 2 × 106 | IV | NR |
| Carrancio et al[25] | 2013 | Human | UCB | CD34+ | 1 × 105 | IV; IB | 200; 20 |
The transplantation process was reported using mainly 1 × 107 cells[9-11,21], ranging from 2.5 × 103[8] to 1 × 107 cells, with exception of the study by Kornblit et al[23] that used 1.8 × 108 to 5.3 × 108/kg cells in Beagle dogs with an administered volume of 50 mL. Four studies[8,10,15,25] compared two routes, intravenous (IV) versus intrabone (IB), but the majority of studies used the IV route (72.7%) with a volume of 100 to 250 µL, followed by 22.2% IB[8,10,15,25], being administered at a volume between 10 and 20 µL.
The MSCs used in cotransplantation with HSCs had interesting features (Table 2). The main MSC source was human (61.1%), being extracted from difference sources, BM (16.7%), UCB (22.2%)[8,10,20-22], tonsils (11.1%)[9,11], adipose tissue (5.6%)[22], or fetal lung (5.6%). The animal donor source of MSCs (38.9%)[12-14,17,18,23,24] was 33.3% from BM[12-14,18,23,24] and 5.7% from adipose tissue[17]. Among animal donors, the study by Kornblit et al[23] used Beagles as an MSC donor, extracting the cells from BM.
| Ref. | Mesenchymal stem cells | Transplantation | ||||||
| Donor | Source | Surface marker | Passage | Modification | Cell dose | Route | Volume (µL) | |
| Lee et al[9] | Human | Tonsil | Negative: CD14, CD34, CD45, HLA-DR, CD40, CD80, CD86 CD11b, CD21, CD23, CD35 and CD54 | P3-5 | MMP3-knockdown | 1 × 106 | IV | NR |
| Positive: CD73, CD95 and CD105 | ||||||||
| Huang et al[10] | Human | UCB | Negative: CD34 and CD45 | P4 | NA | 5 × 106; 1 × 106 | IV; IB | NR |
| Positive: CD90, CD105 and CD75 | ||||||||
| Yin et al[8] | Human | UCB | NR | NR | GFP; EGF; FGF2; PDGFB | 5 × 105 | IB | 10 |
| Choi et al[11] | Human | Tonsil | Negative: CD14, CD34, CD45, HLA-DR, CD40, CD80, CD86 CD11b, CD21, CD23, CD35 and CD54 | P3-5 | NA | 2 × 106 | IV | 200 |
| Positive: CD73, CD95 and CD105 | ||||||||
| Trento et al[12] | Mice (C57BL/6 CD45.2 orNos2-/-) | BM | Negative: CD45 | Max P8 | Nos2-/- | 2 × 105 | IV | NR |
| Positive: PDGFRα and Sca-1 | ||||||||
| Abbuehl et al[13] | Mice (FVB Insulin-GFP) | BM | Negative: CD105 | NR | NA | 1 × 104 | IB | 3 |
| Positive: CD73 and Sca1 | ||||||||
| Kim et al[14] | Mice (C57BL/6J-Ly 5.2 BL6) | BM | Negative: CD45 | Min P2 | NA | 2 × 105 | NR | NR |
| Futrega et al[15] | Human | BM | Negative: CD45, CD34 and HLA-DR | P3 | NA | 5 × 105 | IV; IB | 100; 10 |
| Positive: CD44, CD90, CD73, CD105 and CD146 | ||||||||
| van der Garde et al[16] | Human | FL | Negative: CD34 | NR | NA | 1 × 106 | IV | NR |
| Fernández-García et al[17] | Mice (B6D2F1, H2b/d, CD45.2) | AT | Negative: CD34, CD45.1 and CD80Low: Sca-1 | P5-8 | NA | 2 × 105; 4 × 105; 6-10 × 105 | IV | NR |
| Positive: CD29, CD44, CD73, CD90.2, CD105, CD106, CD144, and CD166 | ||||||||
| Chen et al[18] | Mice (C57BL/6) | BM | NR | P3-8 | CXCR4 | 5 × 105 | IV | NR |
| Chen et al[19] | Human | BM | Negative: CD34 and CD45 | NR | SDF1; HOXB4 | 8 × 105 | IV | NR |
| Positive: CD90 and CD44 | ||||||||
| Wu et al[20] | Human | UCB | Negative: CD14, CD31, CD34, CD45, and HLA-DR | NR | NA | 1 × 106 | IV | 250 |
| Positive: CD13, CD29, CD44, CD73, CD90, and CD105 | ||||||||
| Lim et al[21] | Human | UCB | Negative: CD14, CD34 and CD45 | P4 | NA | 5 × 106 | IV | 200 |
| Positive: CD73 and CD105 | ||||||||
| Lee et al[22] | Human | UCB; AT; BM | Negative: CD14, CD34, and CD45 | NR | NA | 1 × 106 | IV | 150-200 |
| Positive: CD73, CD90, and CD105 | ||||||||
| Kornblit et al[23] | Dog (beagle) | BM | NR | P1 | NA | 4.8-10 × 108/kg | IV | 50 mL |
| Fortin et al[24] | Mice | BM | Negative: CD45 and CD31 | NR | solG-CSFR | 1.5 × 107 | IP | NR |
| Positive: CD44 and CD90dim | ||||||||
| Carrancio et al[25] | Human | BM | Positive: CD73, CD90, CD105, CD44, and CD166 | P3 | NA | 5 × 105 | IV; IB | 200; 20 |
For MSC characterization, the selected studies reported mainly the following CD surface markers, negative expression of CD45 (66.7%)[9-12,14,15,17,18,20-24], CD34 (55.6%)[9-11,15-18,20-23], and CD14 or CD11b (38.9%)[9,11,20-22] for both human and animal source cells, and positive expression of CD73 (64.3%)[9,11,13,15,17,20-22,25], CD105 (64.3%)[9-11,15,17,20-22,25], CD90 (57.1%) and CD44 (42.9%) markers.
Most of the selected studies (61.1%)[9-12,14,15,17,18,21,23,25] reported use of cells with a passage between P1 and P8, mainly in the low passages (P3 and P4). Only 33.3% of the studies reported some type of cell modification such as the use of donors cell deficient in type 2 nitric oxide (Nos2-/-), which is related to the immunosuppressed activity of MSCs and to the differentiation and expansion of MSCs and myeloid cells[12]; metalloproteinase (MMP)3 knockdown, a metalloproteinase that degrades proteins from the extracellular matrix and activates other MMPs[9], facilitating the homing; overexpression of CXCR4, which is essential for the homing and maintenance of HSCs in BM niches; epidermal growth factor (EGF), involved in the HSC long-term recovery and improvement of the mouse survival rate after facilitating homing[8]; SDF-1, a chemokine that performs an important role in HSC homing to BM; Homeobox (HOX)B4 that is involved in HSC stimulation and self-renovation[19]; or receptor for soluble granulocyte colony-stimulating factor (G-CSFR), a cytokine known for inducing cellular mobilization that is increased in mouse BM shortly after total body irradiation (TBI)[24]. For MSC transplantation, the cell dose used was mainly around 106 (25%)[9,10,16,17,20,22], ranging from 104[13] to 1.5 × 107 cells[24]; 66.7% of the selected studies used the IV route, administering a volume ranging from 100 to 250 µL; the other 23.8%[8,10,13,15,25] were administered IB, using a volume ranging from 3 to 20 µL, and 4.8%[24] of the studies used intraperitoneal (IP) administration and did not report the volume administered. In the study by Kornblit et al[23], the cell dose (4.8 × 108 to 10 × 108/kg) was greater due to the use of dogs as a host, using 50 mL as a volume for administration.
To achieve a supported and quantitative HSC engraftment after BMT, it is necessary to condition the animal with irradiation or chemotherapy before transplantation. Bearing this in mind, 88.9% of the selected studies (Table 3) used TBI with different types of radioactive sources (18.8% by Caesium-137[15,21,25], 31.3% by Cobalt-60, and 50% did not specify the source[8,12-14,16,17,20,22]), and 11.1%[9,11] used chemotherapy with busulfan (Bu) and cyclophosphamide (Cy). The conditioning dose (intensity and frequency) varied depending on the resistance of the host animal. C57BL/6 mice: a high dose was reported (from 5[17] to 9 Gy[14]; BALB/c mice: 7.5 Gy Co60[18] or 20–25 mg/kg/d Bu associated with 100 mg/kg/d Cy[9,11]; NOD/SCID mice: low irradiation doses, ranging from 2.5[8,15,21] to 3.5 Gy[16,19,20]. 61.1% of the xenogeneic transplants were performed on NOD/SCID mice using human HSCs and MSCs, and BALB/c mice also received human MSCs[9,11]. In the other studies (38.9%)[12-14,17,18,23,24] allogeneic transplants were performed in mice (BALB/c, C57BL/6, or FVB Insulin-GFP). Kornblit et al[23] used Beagles for recipient and donor cells. The experimental groups involved different conditions of analyses, but only four studies (22.2%) included a control group (untreated condition), while in the other studies, the basal reference was the group that used only HSC transplantation to compare with other conditions.
| Ref. | HSC donor | MSC donor | Host | Conditioning | Source | Dose (Gy) | MSC immunogenicity | Groups |
| Lee et al[9] | Mice (C57BL/6) | Human | Mice (BALB/c H-2d) | Chemotherapy | Bu/Cy | 25 mg/kg/4 d | Xenogeneic | Control; HSC; HSC + T-MSC; HSC + MMP3kdT-MSC |
| 100 mg/kg/2 d | ||||||||
| Huang et al[10] | Human | Human | Mice (NOD/SCID) | TBI | 60-Co | 2.7 | Xenogeneic | Control; HSC; HSC + UCB-MSC (IV/IB) |
| Yin et al[8] | Human | Human | Mice (NOD/SCID; NOG) | TBI | NR | 2.5 | Xenogeneic | HSC; HSC + GFP-MSC; HSC + EGF-MSC; HSC + FGF2-MSC; HSC + PDGFB-MSC (IV/IB) |
| Choi et al[11] | Mice (C57BL/6) | Human | Mice (BALB/c) | Chemotherapy | Bu/Cy | 20 mg/kg/4 d | Xenogeneic | Control; HSC; HSC + T-MSC |
| 100 mg/kg/2 d | ||||||||
| Trento et al[12] | Mice (C57BL/6 CD45.1) | Mice (C57BL/6 CD45.2 orNos2-/-) | Mice (C57BL/6 CD45.2) | TBI | NR | 8 | Allogeneic | HSC; HSC + MSC; HSC + Nos2−/−MSC |
| Abbuehl et al[13] | Mice (FVB Actin-GFP) | Mice (FVB Insulin-GFP) | Mice (FVB Insulin-GFP) | TBI | NR | 2 × 4.5 | Allogeneic | HSC; HSC + MSC |
| Kim et al[14] | Mice (C57BL/6J-Pep3b-Ly5.1 Pep3b) | Mice (C57BL/6J-Ly 5.2 BL6) | Mice (C57BL/6J-Ly 5.2 BL6) | TBI | NR | 9 | Xenogeneic | HSC; HSC + MSC (NSS/SS) direct or priming |
| Futrega et al[15] | Human | Human | Mice (NSG) | TBI | 137-Cs | 2.5 | Xenogeneic | HSC (IV/IB); HSC + MSC; HSC + MSC-spheroids (IB) |
| van der Garde et al[16] | Human | Human | Mice (NOD/SCID) | TBI | NR | 3.5 | Xenogeneic | HSC; HSC + MSC; Ex/TPO-HSC; Ex/TPO-HSC + MSC |
| Fernández-García et al[17] | Mice (P3D2F1 H2b/d CD45.1) | Mice (B6D2F1,H2b/d, CD45.2) | Mice (B6D2F CD45.2) | TBI | NR | 5-7 | Allogeneic | HSC; HSC + AT-MSC |
| Chen et al[18] | Mice (C57BL/6) | Mice (C57BL/6) | Mice (BALB/c H-2d) | TBI | 60-Co | 7.5 | Allogeneic | HSC; HSC + EGFP-MSC; HSC + CXCR4-MSC |
| Chen et al[19] | Human | Human | Mice (NOD/SCID/IL2rγnull) | TBI | 60-Co | 3.5 | Xenogeneic | Control; HSC; HSC + MSC; HSC + SDF-1-MSC; HSC + HOXB4-MSC; HSC + SDF1-HOXB4-MSC |
| Wu et al[20] | Human | Human | Mice (NOD/SCID) | TBI | NR | 3.5 | Xenogeneic | HSC; HSC + UCB-MSC |
| Lim et al[21] | Human | Human | Mice (NOD/SCID) | TBI | Cs | 2.5 | Xenogeneic | HSC; HSC + hPTH; HSC + UCB-MSC + hPTH |
| Lee et al[22] | Human | Human | Mice (NOD/SCID) | TBI | NR | 3 | Xenogeneic | HSC; HSC + BM-MSC; HSC + AT-MSC; HSC + UCB-MSC |
| Kornblit et al[23] | Dog (beagle) | Dog (beagle) | Dog (Beagle) | TBI | 60-Co | 9.2 | Allogeneic | Control; HSC; HSC + MSC (unrelated or DLA-id MSC) |
| Fortin et al[24] | Mice (B6.SJL-PtrcaPep3b/BoyJ CD45.1) | Mice | Mice (C57BL/6J) | TBI | 60-Co | 8 | Allogeneic | HSC; HSC + MSC; HSC + solG-CSFR-MSC |
| Carrancio et al[25] | Human | Human | Mice (NOD/SCID) | TBI | Cs | 3 | Xenogeneic | HSC (IV/IB); HSC IV + MSC (IV/IB) |
The primary goal of the studies included in this review was to assess the therapeutic efficacy of HSC and MSC cotransplantation using various MSC sources, cell alterations, and niche environment as described in Table 4. The chimerism analysis by flow cytometry was the main approach (83.3%)[8-10,12-20,22,24,25] for this evaluation, followed by 44.4% hematopoietic reconstruction analysis by blood count or flow cytometry or immunohistochemistry[9,12-14,18,19,21,23], 22.2% homing analysis by flow cytometry or images[9,10,17,24], survival (Kaplan–Meier estimator)[9-11,19], and cellularity analyses (HE and Wright staining)[9,18,19,21], and in low frequency (5.6%) platelet reconstruction by flow cytometry[16], expression of hematopoietic cytokines by immunohistochemistry[10], and thymus regeneration by different techniques (volumetry, histological and immunohistochemistry analyses)[11].
| Ref. | Objective | Therapy evaluation | Evaluation technique | Evaluation time (d) | Outcome | Conclusion |
| Lee et al[9] | Evaluate cotransplantation of HSCs and T-MSCs and influence of MMP3 expression | Chimerism | FC | 10 | Only the HSC + T-MSC group had a significant increase in frequency of H-2d cells in PB receptor | Cotransplantation of T-MSCs with intact expression of MMP3 increased homing and engraftment of HSCs, as well as blood cell recovery and survival |
| The HSC + MMP3kdT-MSC group did not present any alteration | ||||||
| Hematopoietic reconstitution | BC | 24 | The HSC + T-MSC group had the highest number of circulating WBCs and RBCs and similar level to the control group | |||
| Homing | FC | 1 | The groups that received T-MSCs presented higher homing independently of expression of MMP3 | |||
| Cellularity | H&E | 24 | The cellularity of the BM only was significantly increased in the HSC + T-MSC group | |||
| Survival | Kaplan-Meier estimator | 24 | The HSC + T-MSC group had a higher survival rate (71%) in comparison to the HSC + MMP3kdT-MSC group (38%) | |||
| Huang et al[10] | Evaluate the cotransplantation of HSCs and UCB-MSCs in an iron overload model | Chimerism | FC | 42 | Cotransplantation of HSCs with UCB-MSCs increased the frequency of CD45+ cells in BM, independently of route, and presented a higher frequency of CD34+ only with IB route | Cotransplantation of HSCs with UCB-MSCs increased the engraftment and the proliferation of UCB-MNCs, improving their differentiation in the iron overload model independently of the administration rote |
| Distribution | Fluorescence | 42 | HSCs in the IV group accumulated in the spleen but not BM, and in the IB group, accumulation was mainly in BM | |||
| Expression of hematopoietic cytokines | IHC | 42 | Cotransplantation of HSCs with UCB-MSCs increased expression of VEGF-A, OPN, and SDF-1 independently of route | |||
| Survival | Kaplan-Meier estimator | 42 | The HSC + UCB-MSC group via IB had a higher survival rate | |||
| Yin et al[8] | Evaluate the cotransplantation of HSC and MSC expressing EGF, FGF2 or PDGFB | Chimerism | FC | 84 | There was no difference in the frequency of CD34+ and CD45+ between the HSC + MSC and HSC groups | BM treated with PDGFB-MSCs improved the self-renewal of human HSCs in primary recipients, leading to superior engraftment in secondary transplantation |
| PDGFB-MSC significantly increased the frequency of CD45+ and CD34+ human cells in comparison with HSC group, except CD34+ IV | ||||||
| The FGF2-MSC group had a significant increase in CD45+ by IB route compared with the HSC group | ||||||
| PDGFB-MSC promoted a higher frequency of CD45+ in secondary transplantations | ||||||
| Choi et al[11] | Evaluate the cotransplantation of HSCs and T-MSCs in thymus regeneration | Survival | Kaplan–Meier estimator | 40 | The HSC + T-MSC group had a higher survival rate | Cotransplantation of HSCs and T-MSCs improved survival rate and restored the thymus structure and increased the diversity of thymus-derived T cells |
| Thymus regeneration | Tissue volumetry | 3, 10 and 40 | In 10 d, the thymuses of the HSC + T-MSC group were larger | |||
| In 40 d, the thymuses of all groups returned to a size similar to the control thymus | ||||||
| Histology | Highest cellularity and better-defined structures in the HSC + T-MSC group | |||||
| IHC | The HSC + T-MSC group presented more CD3+ cells | |||||
| Trento et al[12] | Evaluate the cotransplantation of HSC and Nos2−/− MSC in the differentiation of myeloid cells | Chimerism | FC | 13 | There was no difference in the frequency of CD45.1+ myeloid cells in the BM and in the spleen of the recipient animals | There was no difference in the frequency of neutrophils and eosinophil between the groups, macrophages and monocytes were more numerous in the HSC + MSC group |
| Hematopoietic reconstitution | FC | 13 | There was an increase of the frequency of macrophages and monocytes in the HSC + MSC group compared to the HSC and HSC + Nos2−/− MSC group | |||
| Abbuehl et al[13] | Evaluate the cotransplantation of HSCs and MSCs | Chimerism | FC | 112 | Cotransplantation of HSCs and MSCs increased the frequency and number of GFP+ LSK, HSCs (LSK CD48 CD150+) and LT- HSCs (LSK CD48- CD150+ CD34) cells in secondary receptors of HSCs derived from the HSC + MSC group | Cotransplantation of HSCs with MSCs significantly increased number of functional HSCs derived from donors |
| Hematopoietic reconstitution | BC | 7 and 14 | Highest number of lymphocytes and neutrophils in 14 d in the HSC + MSC group | |||
| Kim et al[14] | Evaluate the cotransplantation of HSCs and stimulated MSCs | Chimerism | FC | 64 to 84 | Percentage of CD45.1+ and number of LSK CD45.1 cells increased in the HSC + MSC-SS group | Cotransplantation of HSCs with MSCs under stimulatory condition increased HSC engraftment |
| Percentage of CD45.1+ lymphoid cells was equal in the HSC and HSC + MSC-NSS groups, however there was a reduction in the HSC + MSC-SS group IB route, the reverse was observed in myeloid cells | ||||||
| Hematopoietic reconstitution | IHC | 64 to 84 | Only observed in the HSC + MSC-SS group | |||
| Futrega et al[15] | Evaluate the cotransplantation of HSCs and MSC-spheroids | Chimerism | FC | 56 (weekly) | Reduction of CD45+ in the HSC + MSC group in spleen comparing IB to IV route | HSC transplantation by IB route improved IB engraftment, but did not contribute to high levels of systemic engraftment in xenogeneic animal models and cotransplantation with MSC-spheroids enhanced supportive environment to retention of HSC in IB route |
| Significant reduction of CD34+ in the MSC-spheroids group in PB and spleen in IB route | ||||||
| Increase in engraftment of CD45+ and CD34+ in IB administration of the HSCs with MSCs or without MSCs in comparison to distal bone | ||||||
| van der Garde et al[16] | Evaluate the cotransplantation of HSCs expanded with TPO and MSCs | Chimerism | FC | 42 | The HSC + MSC group had significantly increased CD45+ in the receptors while TPO only induced engraftment | Cotransplantation of MSCs can improve engraftment after 6 wk, whereas TPO expansion improves early platelet recovery |
| Platelet recovery | FC | 14 and 42 | In short term, use of Ex/TPO-HSCs with or without MSCs increased platelet number, in long term, only the presence of MSCs with HSCs had an effect on platelet formation | |||
| Fernández-García et al[17] | Evaluate the cotransplantation of HSCs and AT-MSCs | Chimerism | FC | 28, 56, and 84 | Cotransplantation of HSCs and MSCs resulted in an increase of CD45.1+ in the receptor dose-dependently in the mild conditioning (5 Gy) | Cotransplantation with low doses of AT-MSCs accelerated early HSC engraft, but only higher dose of MSCs improved later HSC engraftment, as also long-term repopulating HSCs, and homing of HSCs, facilitating hematopoietic reconstitution |
| Highest frequency of CD45.1+ in secondary and tertiary receptors, using HSCs + higher doses of AT-MSCs | ||||||
| Homing | FC | 2, 4, and 24 h | Co-infusion of AT-MSCs increased homing of LSK CD45.1+ cells in BM | |||
| Chen et al[18] | Evaluate the cotransplantation of HSCs and MSCs overexpressing CXCR4 | Chimerism | FC | 7 and 14 | At 7 d, frequency of H-2b cells in the receptors was lower in the HSC + CXCR4-MSC group, increasing equally in all groups at 14 d | Cotransplantation of HSCs with CXCR4-MSCs accelerates hematopoietic reconstitution, promotes HSC engraftment, PB cell recovery, and BM hyperplasia |
| Hematopoietic reconstitution | BC | 7 to 21 | The HSC + MSC group increased reconstitution of leukocytes and platelets, and HSC + CXCR4-MSC group had more rapid effect | |||
| Cellularity | HE | 7 and 14 | Highest cellularity in the BM and in the spleen of the receptors of CXCR4-MSC, predominantly myeloid in BM | |||
| Chen et al[19] | Evaluate the cotransplantation of HSCs and MSCs modified to express SDF-1/HOXB4 | Chimerism | FC | 28 | The presence of CD45+ was higher in the groups that received MSCs, with emphasis in HSC + SDF1-HOXB4-MSC group | HSC + SDF1-HOXB4-MSC group significantly increased engraftment of HSCs, hematopoietic recovery, and rapid recovery of BM cellularity |
| Hematopoietic reconstitution | BC | 7, 14, 21, and 28 | In the HSC + SDF1-HOXB4-MSC group, the WBCs, PLT and HGB levels returned to normal | |||
| The HSC + SDF-1-MSC group did not present total recovery, although the WBC, PLT and HGB levels recovered more quickly than in other groups | ||||||
| Cellularity | Wright staining | 14 and 28 | The cellularity was significantly higher in the HSC + SDF-1-MSC and HSC + HOXB4-MSC groups | |||
| Survival | Kaplan–Meier estimator | 14 and 28 | The HSC + SDF1-HOXB4-MSC group had higher survival rate than other groups | |||
| Wu et al[20] | Evaluate the cotransplantation of HSCs and MSCs | Chimerism | FC | 56 to 77 | The HSC + UCB-MSC group had higher frequency of CD45+ in the PB and BM | The use of UCB-MSCs in cotransplantation resulted in better engraftment of HSCs |
| Lim et al[21] | Evaluate cotransplantation of HSCs treated with hPTH and MSCs | Hematopoietic reconstitution | BC | 28, 42 and 49 | There was no difference in the number of WBCs, RBCs and PLTs in the groups over time | Cotransplantation of HSCs with MSCs could lead to an increase of hematopoietic reconstitution and may be a synergistic effect between MSCs and hPTH |
| Cellularity | FC | 49 | There was difference only in the HSC and HSC + UCB-MSC groups treated with hPTH | |||
| CD34+ did not differ between the groups, but myeloid and lymphoid lineages were markedly higher in HSC + UCB-MSC + hPTH group | ||||||
| HE | 56 | Highest cellularity of the BM in the groups that received hPTH with or without MSCs | ||||
| Lee et al[22] | Evaluate the cotransplantation of HSCs and AT-MSCs, UCB-MSCs or BM-MSCs | Chimerism | FC | 42 or 70 | The groups that received MSCs, independently of the source, had an increase of the frequency of CD45+ cells | Cotransplantation of HSCs with BM-MSCs, AT-MSCs or UCB-MSCs increased engraftment, and UCB-MSCs had higher proliferation rates |
| Kornblit et al[23] | Evaluate the cotransplantation of HSCs and MSCs with identical DLA or not | Hematopoietic reconstitution | BC | 100 | There was no difference in the number of PLTs and granulocytes between the groups | Cotransplantation of HSCs with MSCs did not increase engraftment of HSCs, and the MSCs with identical DLA or not was safe |
| Fortin et al[24] | Evaluate the cotransplantation of HSCs and MSCs expressing solG-CSFR | Chimerism | FC | 13 and 45 | In the HSC + MSC group, there was a higher number of CD45+ compared to other groups | In the cotransplantation of HSCs with MSCs, the presence of solG-CSFR increased the homing and accelerated hematopoietic reconstitution |
| Homing | FC | 18 h | The homing was significantly higher in the HSC + solG-CSFR-MSC group than the HSC + MSC group, and in this last group the increase did not differ from the control group | |||
| Carrancio et al[25] | Evaluate the cotransplantation of HSCs and MSCs according to the routes (IB and IV) | Chimerism | FC | 21 and 42 | The number of CD45+ was significantly higher in the HSC + MSC s by IV route (at 21 d), but at 42 d, this increase occurred in the HSC + MSC group by IB route in the local area of administration, followed by the HSC + MSC group by IV | MSCs increased hematopoietic engraftment when cotransplanted by both routes (IV/IB) |
The chimerism was assessed throughout a period of time extending from 7 to 112 d, with the analysis becoming more visible around 12 and 42, d and showed that the number of donor cells in the recipients was higher in cotransplantation with MSCs than in HSC-only transplantation, and the cotransplantation had a lower chimerism than the control group[15]. However, three studies[12,18,23] did not show any difference in the use of cotransplantation, comparing the groups HSC versus HSC + MSC. The most used marker for chimerism analysis was human CD45+ (60%)[8,10,15,16,19,20,22,24,25], while other studies used murine cell markers such as CD45.1+, H-2b and H-2d (33,3%)[9,12,14,17,18] and a single study used green fluorescent protein expression by HSCs for flow cytometry analysis[13].
The hematopoietic reconstruction was evaluated up to 49 d after cotransplantation, with the exception of one study[23] that evaluated at 100 d. However, significant results were found between 7 and 14 d after transplantation. After this period the results did not show relevant differences in the group comparisons about the number of circulating white blood cells, except for the study by Kim et al[14] that reported differences only in the group with cotransplantation of HSCs and MSCs under stimulatory conditions.
The cell homing was evaluated within 24 h after transplantation (2, 4, 18 and 24 h) and the MSCs, modified or not, showed an increase and improved the cell homing, facilitating hematopoietic reconstitution[9,10,17,24].
The survival analysis was performed mainly around 24 and 40 d, showing that the HSC and MSC cotransplantation group had higher survival in comparison with the HSC group, and in some specific studies[9,19] that used the MSC modification (for example, HSC + SDF1-HOXB4-MSC), the survival improved even more during cotransplantation, in comparison to other groups.
The cellularity analysis was performed between 7 and 56 d (mainly 14 and 28 d), and showed a significant increase in the groups that used MSC and HSC cotransplantation, with or without MSC modifications[9,18,19,21].
Some of the specific analyses provided by the selected studies revealed, for example, that the use of HSC expanded with thrombopoietin (Ex/TPO-HSC) resulted in an increased platelet count only within a short time (14 d after transplantation), but the use of MSCs had an influence on platelet production in the short and long term (14 and 42 d)[16]. Other selected studies[11] revealed, using different techniques of evaluation (3, 10 and 40 d after cell transplantation), that cotransplantation improved thymus regeneration, and increased the expression of hematopoietic cytokines such as vascular endothelial growth factor (VEGF-A), osteopontin (OPN) and SDF-1 independently of administration route (at 42 d after UCB-MSC and HSC transplantation). Overall, 72.2% of the selected studies[8-10,13-17,19,20,22,24,25] reported an improvement when HSCs and MSCs were cotransplanted. When MSCs expressing platelet-derived growth factor subunit B (PDGFB)[8], SDF1-HOXB4, CXCR4[18] are cotransplanted, or coadministered with human parathyroid hormone (hPTH)[21] in HSC and MSC cotransplantation, a meaningful improvement can be seen. In the study by Kim et al[14] there was only improvement in the engraftment when the MSCs were previously cultured with a stimulating serum. In the study by Fortin et al[24], solG-CSFR-MSC cotransplantation improved homing and accelerated hematopoietic reconstitution, but not engraftment, when compared with HSC + MSC cotransplantation.
Figure 2 summarizes the key findings of this systematic review on the characterization of HSCs and MSCs (donor and source percentile distribution); the importance of evaluating MSCs before their administration using surface markers (positive and negative expression); the differences in doses (HSCs, orange bars and MSCs, pink bars in Figure 2); administration routes of each cell in cotransplantation (IV, IB and IP); and the main techniques for evaluating therapeutic success and improvement in the grafting process.
Many hematological diseases can be cured with allo-HSCT and optimizing the homing and overall survival process is a critical step. The main goal of this systematic review was to search for preclinical studies of MSC and HSC cotransplantation, demonstrating several aspects of the MSC and HSC characteristics and transplantation process, as well as showing molecular and/or structural synergistic aspects of cotransplantation that result in complete successful engraftment.
After searching original articles published between January 2011 and December 2021, indexed in PubMed and Scopus, the current systematic review examined 2565 preclinical studies of evaluation of the use of MSCs in HSC engraftment in animal models and included only 18 studies. Most of the papers were produced by Asian researchers and published between 2013 and 2016, with assistance from a number of countries, most notably the USA. A recent systematic review that included meta-analysis of clinical trials on the same topic found 19 studies (searching in six databases), 10 of which (52%) were conducted in China, with some of them being published in Chinese and the other 13 in English[4]. According to this evaluation and previous investigations by our group[4,26,27], there is a considerable concentration of evidence generation in this field of knowledge by Chinese researchers, with the most recent studies on HSC and MSC cotransplantation focusing primarily on models of hematological diseases, particularly GVHD, with some meta-analyses of clinical trials published on the topic[28-31]; approaches that were excluded from our review based on predetermined criteria.
Most of the studies found that the graft improved when comparing HSC and MSC cotransplantation to HSC-only transplantation. HSC and MSC cotransplantation has previously been shown to promote engraftment, chimerism and homing in a variety of species including monkeys[32], sheep[33], mice[34] and humans[35]. Despite the fact that the majority of studies have yielded excellent results, there are still challenges to be solved, such as the heterogeneity of MSC sources, the volume of cells administered, the optimum route of administration, and the safety of MSC transplantation[4]. The improvement of the self-renovation and proliferation of HSCs in BM is a critical step for the success of engraftment or transplantation as therapy for many hematopoietic and immune system disorders[5]. This condition is influenced by several factors; the most important of them are the characteristics of the engrafted HSCs[27]. HSCs from human UCB were used in half of the studies in this review, while HSCs from animal BM were used in the other half. Two recent reviews found the same aspect and percentage of HSC source distribution[36,37], showing that the source of human HSCs has an impact on a variety of clinical outcomes, particularly survival and homing, and the BM HSCs have better survival and homing than UCB HSCs[38]. A recent study found that the cell source chosen is influenced by a variety of patient- and diagnosis-related characteristics, as well as the availability of appropriately matched donors, leaving the question of which cell source is superior or has more benefits unanswered[39]. The recent systematic review of clinical trials[40] also reported the use of the BM HSCs as the main source by IV transplantation. Wu et al[20] reported transplantation of a pool of human nucleated cells, Huang et al[10] and Lim et al[21] reported the use of a mononuclear cell pool without any cell selection process, and Fernández-García et al[17] used LSK cells.
Most of the selected studies[8-16,18-22,24,25] showed success in the ability of myeloid cells to expand and MSCs to accelerate hematopoietic regeneration or self-renovation independently of the dose used, ranging from 2.5 × 103 to 1 × 107 cells, but the majority of studies used 1 × 107 cells in around 200 µL volume of administration, with the exception of Kornblit et al[23], who used 1.8 to 5.3 × 108/kg in the Beagle dogs in 50 mL volume. Fernández-García et al[17] showed that the improvement of HSC engraftment is MSC dose-dependent, mainly in the later stage. However, Park’s study found an adipose-tissue-derived MSC (AT-MSC) dose-dependent hematopoietic engraftment effect, which we also could see in our autologous transplantation model. Fernández-García et al[17] used one of the lowest doses reported by selected studies in our review to confirm an efficient immunomodulatory effect of MSCs in the BM niche. A recent systematic review of clinical trials[4] found no association between allo-HSC dose and better outcomes, but another review found that patients who were infused with a higher dose of HSCs had better survival rates than those who were infused with a lower dose. These studies also found a stronger link between the administration route, dose and other MSC features.
The variety of HSC delivery modes investigated (IV and IB) aimed to improve therapeutic outcomes by enhancing homing and engraftment. Only 22.2% of studies[8,10,15,25] compared the HSC administration routes (IV vs IB) in HSC cotransplantation with MSCs; however, the IV route was the most common in all selected studies (72.7%), and in the comparison between routes, IB administration showed better specific HSC graft results when associated with some MSC modifications[8,10,15]. Curiously, the studies normally used the same route for HSC and MSC administration, but the study of Abbuehl et al[13] adopted IB administration for MSCs and IV administration for HSCs. Some preclinical studies have shown that direct BM injection of MSCs can enhance the engraftment of cord blood cells more than the IV injection. However, the MSCs administered by the IV route were retained, mainly in the lungs. In clinical studies, BM injection of MSCs was safe.
The MSCs were administered mainly by the IV route (66.7%), but the studies that compared the IV and IB routes[10,15,25] showed that there was an improvement in the HSC outcomes with the IB route[8]. Futrega et al[15] reported better results when administration of both cells was by the IB route, increasing the number of HSCs in the local administration area; however, it did not improve the systemic engraftment. Huang et al[10] also reported improvement of survival when the cells were administered by the IB route, which was higher in comparison with the IV route. A single study[24] also used the IP route, with 1.5 × 107 cells administered per animal. However, it has already been demonstrated that MSCs injected into the peritoneum aggregate with macrophages in the peritoneal cavity, limiting the number of viable MSCs available for therapy[41]. The average number of cells given to the animals that received IV MSCs was higher than that given to animals who received IB MSCs. This is probably due to the medullary cavity’s small capacity, which allows a greater number of MSCs to be delivered systemically. Despite the fact that the IV route does not have the same spatial limitations as the IB route, it is still vital to pay attention to the number of cells that are infused, since large doses can cause thrombolysis and threaten the animal’s survival[42]. The study by van der Garde et al[16] reported deaths at the time of administration, probably due to the increased size of MSCs, which ended up being held in the lungs due to a phenomenon known as the lung barrier. Pneumopulmonary edema was observed 9 d following MSC injection in dogs by Kornblit et al[23].
Before analyzing the routes and doses for MSC transplantation, it is important to characterize these cells, and this was carried out in 78% of studies, and only two studies did not report this analysis, using MSCs from animal BM[18], and human UCB[8], and the study by Kornblit et al[23] used the PCR technique for this goal. Among the negatively expressed markers, the most used for MSC characterization were CD34, CD45, CD14 or CD11b, and HLA-DR, and the common markers for human and animal MSCs were CD34, CD45, CD80 and CD31. The human MSCs used a greater number of negative markers in this characterization, mainly for tonsillar and UCB cell sources. As reported in studies that analyzed mouse MSC characterization and others that focused on human MSC characterization, as well as the minimal criteria for defining multipotent MSCs[43,44], the following positive markers, CD105, CD90, CD44, CD73 and CD29, were common for human and animal MSCs, with the exception of human MSC CD95 and CD75, and animal MSC Sca-1, PDGFR, CD106, CD144, CD146, and CD13. As a result, MSCs from animals had more positive indicators than human MSCs, primarily for adipose tissue. Therefore, MSC from animals have more positive markers than human MSCs have.
Besides the characterization of MSC, most studies[8,10,15,16,19-22,25] that aimed to evaluate the efficacy of HSC and MSC cotransplantation used the humanized mouse model. This model involves the transplantation of human cells into an immunodeficient mouse. This technique allows for the examination of a variety of disorders that would not be viable in humans, as well as a step forward in the development of clinical trials[45,46]. Humanized mice have been used for decades to better understand the mechanics of BMT, including HSC homing and grafting[47-50]. Despite the increased usage of MSCs in clinical trials in recent research, the findings are still inconsistent.
Chimerism analysis in recipient animals, which is the assessment of the frequency of donor cells in recipients achieved by particular biomarkers of human blood cells such as CD45+[8,10,15,16,19,20,22,24,25], was the main method of evaluating the graft found in our study. The chimerism was assessed throughout a period of time extending from 7 to 112 d and three of the 15 studies looking at chimerism indicated that cotransplanting HSCs with MSCs alone did not benefit the graft[12,18,23], whereas the others found that when recipient mice were cotransplanted with MSCs, the frequency of donor cells increased. It is also interesting to note that several studies modified the MSCs to see how protein expression in the transplant affected the results.
In the cell homing analysis[9,10,17,24] within 24 h after cell transplantation (2, 4, 18 and 24 h), the cotransplantation of HSCs and MSCs increased cell homing, facilitating hematopoietic reconstitution after HSC engraftment. However, Fernández-García et al[17] showed that HSC and MSC co-infusion of not only BM-derived, but also AT-MSCs with low numbers of HSC significantly enhanced short- and long-term hematopoietic reconstitution in an autologous transplant setting in mice. Lee et al[9] observed a higher homing independently of the expression of MMP3. In Fortin et al’s study[24] only MSCs with the presence of solG-CSFR increased the homing. The ability of MSCs to home to targets after infusion is one of the most essential characteristics of their efficacy in tissue regeneration[51]. Through the production of paracrine mediators, it may be possible to re-establish the BM microenvironment that has been disrupted by the conditioning regimen, resulting in enhanced homing[4]. MSCs improved hematopoiesis by increasing CD123+ HSC expression, implying myeloid differentiation[52].
Hematopoietic reconstitution is the most important outcome after allo-HSCT, with most studies[9,12-14,18,19,21,23] showing a fast increase in blood cells or a high number of leukocytes after 14 d of coengraftment with MSCs and HSCs. The co-infusion of HSCs with MSCs overexpressing CXCR4 or SDF-1/HOXB4 enhanced post-transplant hematopoietic recovery in murine models[52]. Extracellular vesicles, including microvesicles and exosomes, have been proven to represent a key conduit of intercellular communication between MSCs and HSCs, leading to improved hematological recovery. Furthermore, the hematopoietic system’s regenerative properties may apply to other tissues. MSC-educated myeloid cells exhibit a molecular and functional profile that is similar to that of resident macrophages, which have been implicated in tissue healing in other organs[53].
In addition to HSC and MSC cotransplantation to increase engraftment, MSCs have been manipulated in some experiments to express chemicals, promote migration, or improve the hema
MSC modifications used in a few studies[8,9,12,18,19,24] verified that MSCs genetically modified for Nos2–/– did not show the ability to differentiate and expand myeloid cells and macrophages when compared to the HSC and MSC cotransplantation group[12]. BM-MSCs with recombinant adenovirus expressing an SDF-1/HOXB4 fusion gene cotransplanted with human cord blood CD34+ HSCs showed beneficial effects on hematopoietic recovery and survival in lethally irradiated mice, and a significant increase in HSC growth in vitro and engraftment in vivo. MSCs overexpressing solG-CSFR had improved homing, but did not accelerate hematopoietic reconstitution in mice. The increase in the level of G-CSF post-irradiation can have a long-lasting impact on homing, possibly through the effects of G-CSF on osteoblast homeostasis and the SDF-1a/CXCR4 axis[24]. In the study by Yin et al[8], only the PDGFB-MSCs showed significant results in comparison to other cell modifications (EGF, and FGF2), enhancing MSC survival and expansion after transplantation, improving human HSC engraftment in immunodeficient mice, and transplanted human HSC self-renewal in secondary transplantation. Knockdown by siRNA of MMP3 in MSCs can influence negatively the engraftment and the homing of MSCs and HSCs[9].
The main limitation of this study was that few studies have looked at how MSCs enhance HSC engraftment, such as the evaluation of homing promotion by mesenchymal secretion of MMP3, PDGFB and solG-CSFR, as well as the interaction of the SDF-1/CXCR4 axis and the binding of the HOXB4 in HSC self-renewal. Only one study[10] looked at variables like VEGF-A, which acts as an antiapoptotic agent, and OPN, which is linked to the ability of HSCs to regenerate and the pool of progenitors in BM. The mechanisms involved in cotransplantation have been largely ignored in most investigations, and although it is widely assumed that both cell-to-cell interaction and release of soluble substances play a role, the mechanisms by which MSCs perform their roles have not been explained clearly, being a potential source of bias in the interpretations of outcomes included in this review. Another limiting factor that prevented more conclusive results on cotransplantation from being found was the use of a wide variety of experimental designs in the included studies, primarily regarding the source of the cells, the dose administered, and the heterogeneity of the protocols used for isolation and characterization of MSCs. Some studies[54-56] that reported the cotransplantation performed a coculture of both MSCs and HSCs and administered only HSCs in the host animals. This methodology bias was excluded during the selection process. The standardization of this, particularly with regard to MSCs, is a critical step toward the clinical adoption of cotransplantation[57]. This systematic review did not include the cotransplantation of MSCs and HSCs in hematological disease models to assess the immunosuppressive role they play, particularly in the control of GVHD, which is one of the primary applications of MSCs in BMT.
Notwithstanding these methodological limitations in the preclinical research, clinical trials have reported significant results related to the increase in engraftment and survival rate through HSC and MSC cotransplantation. The recent systematic reviews of clinical trials[4,40,58] have shown that the more homogeneous the MSCs are in terms of the donor, source, extraction method, culture, and other aspects, the better is their efficacy and potentially, the less treatment dose required, and therefore the less likely they are cause adverse events. These clinical trial reviews showed that use of allogeneic MSC cotransplantation in allo-HSC, in phase II, or autogenic sources of MSC in phases I and II during cotransplantation of HSC and MSC increased the survival rate in clinical trials compared with other animal studies. However, many of these studies also reported some difficulties like the ones found in this review, such as the wide variety of biological characteristics of stem cells and the MSC source[38], which makes it difficult to understand the real mechanism responsible for improving the engraftment and decreasing the self-rejection; factors that should be initially elucidated in preclinical analysis to facilitate the prospective clinical results.
Despite the difficulties mentioned above in the development of MSCs as a therapy, recent discoveries highlight the unrealized therapeutic potential of MSCs and suggest that they will become a key component of the hematological therapy armamentarium[3]. The first step for this is to elucidate the complete mechanism of specific therapeutic activity. MSCs have a wide range of immunomodulating properties, but it is unclear whether they use all of them in all circumstances. Such understanding will help in the creation of much-needed clinically applicable potency assays, as well as tactics to boost MSC potency, such as genetically editing[5] MSCs, and aims to improve manufacturing protocols; all of which are key components of long-term success. The second hurdle is that investigators must comprehend better the timing of MSC activity in phase II and III clinical trials[4,40].
The preclinical findings reported in this systematic review validate the potential of MSCs to enable HSC engraftment in vivo in both xenogeneic and allogeneic hematopoietic cell transplantation animal models, without toxicity. Some MSC modifications used in cotransplantation showed a greater benefit of HSC engraftment, and in accelerating hematopoietic reconstruction in preclinical studies. However, the best cell characteristics for this application are still inconclusive due to the diversity and heterogeneity of the studies, but their potential can be seen in malignant hematological conditions such as leukemias and nonmalignant conditions such as anemia and other hemoglobinopathies.
Although bone marrow transplantation (BMT) may be applied to the treatment of hematological and nonhematological diseases, this treatment still presents a series of difficulties and obstacles that corroborate to the treatment failure.
The motivation to study the use of mesenchymal stem cells (MSCs) in hematopoietic stem cell (HSC) transplantation is that the use of both cells at once may increase the success rate of BMT.
The purpose of this systematic review was to investigate the characteristics of HSCs and MSCs, as well as their various interactions in murine models of cotransplantation.
A systematic review was conducted in the PubMed and Scopus databases, looking for original articles from the last decade that used HSC and MSC cotransplantation, as well as in vivo BMT in animal models, excluding studies involving graft-versus-host disease or other diseases.
Only 18 of 2565 articles found in the databases met the eligibility criteria. Regarding the cell characteristics used in the selected studies, most used MSCs from different human sources, characterized before administration, using a lower dose than HSCs, but by similar routes. HSCs were from human umbilical cord blood or animal BM and the recipients were mainly irradiated immunodeficient mouse. The cotransplantation was evaluated mainly by chimerism followed by hematopoietic reconstruction, showing HSC engraft improvement with concomitant MSC implantation.
The preclinical findings in this systematic review validate the potential of MSCs to enable HSC engraftment in vivo in both xenogeneic and allogeneic hematopoietic cell transplantation animal models.
The use of HSCs in BMT shows promise for improvement of engraftment in animal models; however, there is still a need for MSC standardization to evaluate the real potential of the therapy in humans.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Gong N, China; Liu L, China; Maurya DK, India; Prasetyo EP, Indonesia; Zhu L, China S-Editor: Gong ZM L-Editor: Kerr C P-Editor: Gong ZM
| 1. | Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247. [PubMed] |
| 2. | Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009;24:759-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Copelan EA, Chojecki A, Lazarus HM, Avalos BR. Allogeneic hematopoietic cell transplantation; the current renaissance. Blood Rev. 2019;34:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Li T, Luo C, Zhang J, Wei L, Sun W, Xie Q, Liu Y, Zhao Y, Xu S, Wang L. Efficacy and safety of mesenchymal stem cells co-infusion in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. Stem Cell Res Ther. 2021;12:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Burnham AJ, Daley-Bauer LP, Horwitz EM. Mesenchymal stromal cells in hematopoietic cell transplantation. Blood Adv. 2020;4:5877-5887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Diehl R, Ferrara F, Müller C, Dreyer AY, McLeod DD, Fricke S, Boltze J. Immunosuppression for in vivo research: state-of-the-art protocols and experimental approaches. Cell Mol Immunol. 2017;14:146-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 7. | Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Yin X, Hu L, Zhang Y, Zhu C, Cheng H, Xie X, Shi M, Zhu P, Zhao X, Chen W, Zhang L, Arakaki C, Hao S, Wang M, Cao W, Ma S, Zhang XB, Cheng T. PDGFB-expressing mesenchymal stem cells improve human hematopoietic stem cell engraftment in immunodeficient mice. Bone Marrow Transplant. 2020;55:1029-1040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Lee HJ, Kim YH, Choi DW, Cho KA, Park JW, Shin SJ, Jo I, Woo SY, Ryu KH. Tonsil-derived mesenchymal stem cells enhance allogeneic bone marrow engraftment via collagen IV degradation. Stem Cell Res Ther. 2021;12:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Huang Z, Xiao Y, Chen X, Li H, Gao J, Wei W, Zhang X, Feng X. Cotransplantation of Umbilical Cord Mesenchymal Stem Cells Promotes the Engraftment of Umbilical Cord Blood Stem Cells in Iron Overload NOD/SCID Mice. Transplant Cell Ther. 2021;27:230.e1-230.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Choi DW, Cho KA, Lee HJ, Kim YH, Woo KJ, Park JW, Ryu KH, Woo SY. Cotransplantation of tonsilderived mesenchymal stromal cells in bone marrow transplantation promotes thymus regeneration and T cell diversity following cytotoxic conditioning. Int J Mol Med. 2020;46:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Trento C, Marigo I, Pievani A, Galleu A, Dolcetti L, Wang CY, Serafini M, Bronte V, Dazzi F. Bone marrow mesenchymal stromal cells induce nitric oxide synthase-dependent differentiation of CD11b+ cells that expedite hematopoietic recovery. Haematologica. 2017;102:818-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Abbuehl JP, Tatarova Z, Held W, Huelsken J. Long-Term Engraftment of Primary Bone Marrow Stromal Cells Repairs Niche Damage and Improves Hematopoietic Stem Cell Transplantation. Cell Stem Cell. 2017;21:241-255.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 14. | Kim JH, Lee HS, Choi HK, Kim JA, Chu IS, Leem SH, Oh IH. Heterogeneous Niche Activity of Ex-Vivo Expanded MSCs as Factor for Variable Outcomes in Hematopoietic Recovery. PLoS One. 2016;11:e0168036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Futrega K, Lott WB, Doran MR. Direct bone marrow HSC transplantation enhances local engraftment at the expense of systemic engraftment in NSG mice. Sci Rep. 2016;6:23886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | van der Garde M, Brand A, Slot MC, de Graaf-Dijkstra A, Zwaginga JJ, van Hensbergen Y. No Synergistic Effect of Cotransplantation of MSC and Ex Vivo TPO-Expanded CD34(+) Cord Blood Cells on Platelet Recovery and Bone Marrow Engraftment in NOD SCID Mice. Stem Cells Dev. 2015;24:1448-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Fernández-García M, Yañez RM, Sánchez-Domínguez R, Hernando-Rodriguez M, Peces-Barba M, Herrera G, O'Connor JE, Segovia JC, Bueren JA, Lamana ML. Mesenchymal stromal cells enhance the engraftment of hematopoietic stem cells in an autologous mouse transplantation model. Stem Cell Res Ther. 2015;6:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Chen W, Li M, Su G, Zang Y, Yan Z, Cheng H, Pan B, Cao J, Wu Q, Zhao K, Zhu F, Zeng L, Li Z, Xu K. Co-transplantation of Hematopoietic Stem Cells and Cxcr4 Gene-Transduced Mesenchymal Stem Cells Promotes Hematopoiesis. Cell Biochem Biophys. 2015;71:1579-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Chen T, Zhang P, Fan W, Qian F, Pei L, Xu S, Zou Z, Ni B, Zhang Y. Co-transplantation with mesenchymal stem cells expressing a SDF-1/HOXB4 fusion protein markedly improves hematopoietic stem cell engraftment and hematogenesis in irradiated mice. Am J Transl Res. 2014;6:691-702. [PubMed] |
| 20. | Wu KH, Tsai C, Wu HP, Sieber M, Peng CT, Chao YH. Human application of ex vivo expanded umbilical cord-derived mesenchymal stem cells: enhance hematopoiesis after cord blood transplantation. Cell Transplant. 2013;22:2041-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Lim YJ, Hwang K, Kim M, Cho YH, Lee JH, Leelee YH, Seo JJ. Effect of human parathyroid hormone on hematopoietic progenitor cells in NOD/SCID mice co-transplanted with human cord blood mononuclear cells and mesenchymal stem cells. Yonsei Med J. 2013;54:238-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Lee SH, Kim DS, Lee MW, Noh YH, Jang IK, Kim DH, Yang HM, Kim SJ, Choi SJ, Oh W, Yang YS, Chueh HW, Son MH, Jung HL, Yoo KH, Sung KW, Koo HH. A strategy for enhancing the engraftment of human hematopoietic stem cells in NOD/SCID mice. Ann Hematol. 2013;92:1595-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Kornblit B, Leisenring WM, Santos EB, Storb R, Sandmaier BM. Safety of treatment with DLA-identical or unrelated mesenchymal stromal cells in DLA-identical canine bone marrow transplantation. Chimerism. 2013;4:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Fortin A, Benabdallah B, Palacio L, Carbonneau CL, Le ON, Haddad E, Beauséjour CM. A soluble granulocyte colony stimulating factor decoy receptor as a novel tool to increase hematopoietic cell homing and reconstitution in mice. Stem Cells Dev. 2013;22:975-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Carrancio S, Romo C, Ramos T, Lopez-Holgado N, Muntion S, Prins HJ, Martens AC, Briñón JG, San Miguel JF, Del Cañizo MC, Sanchez-Guijo F. Effects of MSC coadministration and route of delivery on cord blood hematopoietic stem cell engraftment. Cell Transplant. 2013;22:1171-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Nucci MP, Filgueiras IS, Ferreira JM, de Oliveira FA, Nucci LP, Mamani JB, Rego GNA, Gamarra LF. Stem cell homing, tracking and therapeutic efficiency evaluation for stroke treatment using nanoparticles: A systematic review. World J Stem Cells. 2020;12:381-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Oliveira FA, Nucci MP, Filgueiras IS, Ferreira JM, Nucci LP, Mamani JB, Alvieri F, Souza LEB, Rego GNA, Kondo AT, Hamerschlak N, Gamarra LF. Noninvasive Tracking of Hematopoietic Stem Cells in a Bone Marrow Transplant Model. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Zhou X, Jin N, Wang F, Chen B. Mesenchymal stem cells: a promising way in therapies of graft-versus-host disease. Cancer Cell Int. 2020;20:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Morata-Tarifa C, Macías-Sánchez MDM, Gutiérrez-Pizarraya A, Sanchez-Pernaute R. Mesenchymal stromal cells for the prophylaxis and treatment of graft-versus-host disease-a meta-analysis. Stem Cell Res Ther. 2020;11:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Li R, Tu J, Zhao J, Pan H, Fang L, Shi J. Mesenchymal stromal cells as prophylaxis for graft-versus-host disease in haplo-identical hematopoietic stem cell transplantation recipients with severe aplastic anemia? Stem Cell Res Ther. 2021;12:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Yang S, Wei Y, Sun R, Lu W, Lv H, Xiao X, Cao Y, Jin X, Zhao M. Umbilical cord blood-derived mesenchymal stromal cells promote myeloid-derived suppressor cell proliferation by secreting HLA-G to reduce acute graft-versus-host disease after hematopoietic stem cell transplantation. Cytotherapy. 2020;22:718-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Masuda S, Ageyama N, Shibata H, Obara Y, Ikeda T, Takeuchi K, Ueda Y, Ozawa K, Hanazono Y. Cotransplantation with MSCs improves engraftment of HSCs after autologous intra-bone marrow transplantation in nonhuman primates. Exp Hematol. 2009;37:1250-1257.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Almeida-Porada G, Porada CD, Tran N, Zanjani ED. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95:3620-3627. [PubMed] |
| 34. | Noort WA, Kruisselbrink AB, in't Anker PS, Kruger M, van Bezooijen RL, de Paus RA, Heemskerk MH, Löwik CW, Falkenburg JH, Willemze R, Fibbe WE. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 370] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 35. | Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J, Ljungman P, Lönnies H, Nava S, Ringdén O. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 316] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 36. | Kale VP. Application of "Primed" Mesenchymal Stromal Cells in Hematopoietic Stem Cell Transplantation: Current Status and Future Prospects. Stem Cells Dev. 2019;28:1473-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Kallekleiv M, Larun L, Bruserud Ø, Hatfield KJ. Co-transplantation of multipotent mesenchymal stromal cells in allogeneic hematopoietic stem cell transplantation: A systematic review and meta-analysis. Cytotherapy. 2016;18:172-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Liu Z, Wu X, Wang S, Xia L, Xiao H, Li Y, Li H, Zhang Y, Xu D, Nie D, Lai Y, Wu B, Lin D, Du X, Jiang Z, Gao Y, Gu X, Xiao Y. Co-transplantation of mesenchymal stem cells makes haploidentical HSCT a potential comparable therapy with matched sibling donor HSCT for patients with severe aplastic anemia. Ther Adv Hematol. 2020;11:2040620720965411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Fraint E, Ulloa BA, Feliz Norberto M, Potts KS, Bowman TV. Advances in preclinical hematopoietic stem cell models and possible implications for improving therapeutic transplantation. Stem Cells Transl Med. 2021;10:337-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Li Y, Hao J, Hu Z, Yang YG, Zhou Q, Sun L, Wu J. Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: a systematic review. Stem Cell Res Ther. 2022;13:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 41. | Bazhanov N, Ylostalo JH, Bartosh TJ, Tiblow A, Mohammadipoor A, Foskett A, Prockop DJ. Intraperitoneally infused human mesenchymal stem cells form aggregates with mouse immune cells and attach to peritoneal organs. Stem Cell Res Ther. 2016;7:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Tatsumi K, Ohashi K, Matsubara Y, Kohori A, Ohno T, Kakidachi H, Horii A, Kanegae K, Utoh R, Iwata T, Okano T. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Commun. 2013;431:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 43. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12645] [Article Influence: 702.5] [Reference Citation Analysis (2)] |
| 44. | Maleki M, Ghanbarvand F, Reza Behvarz M, Ejtemaei M, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells. 2014;7:118-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 45. | Fujiwara S. Humanized mice: A brief overview on their diverse applications in biomedical research. J Cell Physiol. 2018;233:2889-2901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Brendel C, Rio P, Verhoeyen E. Humanized mice are precious tools for evaluation of hematopoietic gene therapies and preclinical modeling to move towards a clinical trial. Biochem Pharmacol. 2020;174:113711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Kang YK, Ko Y, Choi A, Choi HJ, Seo JH, Lee M, Lee JA. Humanizing NOD/SCID/IL-2Rγnull (NSG) mice using busulfan and retro-orbital injection of umbilical cord blood-derived CD34(+) cells. Blood Res. 2016;51:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Blümich S, Zdimerova H, Münz C, Kipar A, Pellegrini G. Human CD34+ Hematopoietic Stem Cell-Engrafted NSG Mice: Morphological and Immunophenotypic Features. Vet Pathol. 2021;58:161-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Wang X, Rosol M, Ge S, Peterson D, McNamara G, Pollack H, Kohn DB, Nelson MD, Crooks GM. Dynamic tracking of human hematopoietic stem cell engraftment using in vivo bioluminescence imaging. Blood. 2003;102:3478-3482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Andrade J, Ge S, Symbatyan G, Rosol MS, Olch AJ, Crooks GM. Effects of sublethal irradiation on patterns of engraftment after murine bone marrow transplantation. Biol Blood Marrow Transplant. 2011;17:608-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 910] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 52. | Liu FD, Tam K, Pishesha N, Poon Z, Van Vliet KJ. Improving hematopoietic recovery through modeling and modulation of the mesenchymal stromal cell secretome. Stem Cell Res Ther. 2018;9:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Eggenhofer E, Hoogduijn MJ. Mesenchymal stem cell-educated macrophages. Transplant Res. 2012;1:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 54. | Futrega K, Atkinson K, Lott WB, Doran MR. Spheroid Coculture of Hematopoietic Stem/Progenitor Cells and Monolayer Expanded Mesenchymal Stem/Stromal Cells in Polydimethylsiloxane Microwells Modestly Improves In Vitro Hematopoietic Stem/Progenitor Cell Expansion. Tissue Eng Part C Methods. 2017;23:200-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Corselli M, Chin CJ, Parekh C, Sahaghian A, Wang W, Ge S, Evseenko D, Wang X, Montelatici E, Lazzari L, Crooks GM, Péault B. Perivascular support of human hematopoietic stem/progenitor cells. Blood. 2013;121:2891-2901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 56. | Huang GP, Pan ZJ, Jia BB, Zheng Q, Xie CG, Gu JH, McNiece IK, Wang JF. Ex vivo expansion and transplantation of hematopoietic stem/progenitor cells supported by mesenchymal stem cells from human umbilical cord blood. Cell Transplant. 2007;16:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Trento C, Bernardo ME, Nagler A, Kuçi S, Bornhäuser M, Köhl U, Strunk D, Galleu A, Sanchez-Guijo F, Gaipa G, Introna M, Bukauskas A, Le Blanc K, Apperley J, Roelofs H, Van Campenhout A, Beguin Y, Kuball J, Lazzari L, Avanzini MA, Fibbe W, Chabannon C, Bonini C, Dazzi F. Manufacturing Mesenchymal Stromal Cells for the Treatment of Graft-versus-Host Disease: A Survey among Centers Affiliated with the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018;24:2365-2370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 58. | Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, Alturki M, Fallatah M, Almalik A, Alhasan AH, Shah K, Karp JM. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6:eaba6884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 429] [Article Influence: 85.8] [Reference Citation Analysis (1)] |