Published online Nov 26, 2022. doi: 10.4252/wjsc.v14.i11.798
Peer-review started: July 20, 2022
First decision: August 8, 2022
Revised: October 5, 2022
Accepted: November 22, 2022
Article in press: November 22, 2022
Published online: November 26, 2022
Processing time: 128 Days and 2.3 Hours
Cartilage tissue engineering is a promising strategy for treating cartilage damage. Matrix formation by adipose-derived stem cells (ADSCs), which are one type of seed cell used for cartilage tissue engineering, decreases in the late stage of induced chondrogenic differentiation in vitro, which seriously limits research on ADSCs and their application.
To improve the chondrogenic differentiation efficiency of ADSCs in vitro, and optimize the existing chondrogenic induction protocol.
Tumor necrosis factor-alpha (TNF-α) inhibitor was added to chondrogenic culture medium, and then Western blotting, enzyme linked immunosorbent assay, immunofluorescence and toluidine blue staining were used to detect the cartilage matrix secretion and the expression of key proteins of nuclear factor kappa-B (NF-κB) signaling pathway.
In this study, we found that the levels of TNF-α and matrix metalloproteinase 3 were increased during the chondrogenic differentiation of ADSCs. TNF-α then bound to its receptor and activated the NF-κB pathway, leading to a decrease in cartilage matrix synthesis and secretion. Blocking TNF-α with its inhibitors etanercept (1 μg/mL) or infliximab (10 μg/mL) significantly restored matrix formation.
Therefore, this study developed a combination of ADSC therapy and targeted anti-inflammatory drugs to optimize the chondrogenesis of ADSCs, and this approach could be very beneficial for translating ADSC-based approaches to treat cartilage damage.
Core Tip: Adipose stem cells are important seed cells that are used in cartilage tissue engineering. However, at present, cartilage matrix secretion by adipose-derived stem cells (ADSCs) inevitably decreases during the late stage of induced chondrogenic differentiation in vitro, which seriously limits the further application of ADSCs. Our team found that the level of inflammation in the culture system, mainly the levels of tumor necrosis factor-alpha (TNF-α) and matrix metalloproteinase 3, continuously increased during the chondrogenic differentiation of ADSCs. To address this issue, our team added etanercept or infliximab, which are targeted inhibitors of TNF-α, to the chondrogenic differentiation induction medium and successfully restored matrix formation by human ADSCs in the late stage of chondrogenic differentiation. Further studies found that these effects were achieved by reducing NF-κB pathway activation.
- Citation: Wan JT, Qiu XS, Fu ZH, Huang YC, Min SX. Tumor necrosis factor-α inhibition restores matrix formation by human adipose-derived stem cells in the late stage of chondrogenic differentiation. World J Stem Cells 2022; 14(11): 798-814
- URL: https://www.wjgnet.com/1948-0210/full/v14/i11/798.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i11.798
Adipose-derived stem cells (ADSCs) are mesenchymal stem cells with multidirectional differentiation potential that are isolated from autologous adipose tissue[1]. After years of research and application since the first successful isolation of ADSCs in 2001, they have become one of the most widely used types of adult stem cells in the field of tissue regeneration. In terms of their multidifferentiation ability, a large number of reported studies have shown that these cells can differentiate into a variety of cell types, such as bone cells, cartilage cells, and muscle cells of the motor system; myocardium cells, and vascular endothelial cells of the circulatory system; and nerve cells of the nervous system[2,3]. Compared with bone marrow mesenchymal stem cells, adipose stem cells are abundant in tissue sources, are easy to extract, and exhibit higher proliferation[4]. However, similar to other tissue-derived adult stem cells, the capacity of ADSCs to synthesize and secrete cartilage matrix decreases during chondrogenic differentiation in vitro. There are several possible explanations for this phenomenon, including cell senescence, apoptosis[5], autophagy[6], oxidative stress[7], epigenetic inheritance[8], and abnormal cell adhesion[9]. Researchers have also proposed some signaling pathways that may participate in the process mentioned above, such as PI3K/Akt, and TGFβ/Smad3 signaling pathways. However, the specific mechanism remains to be further studied.
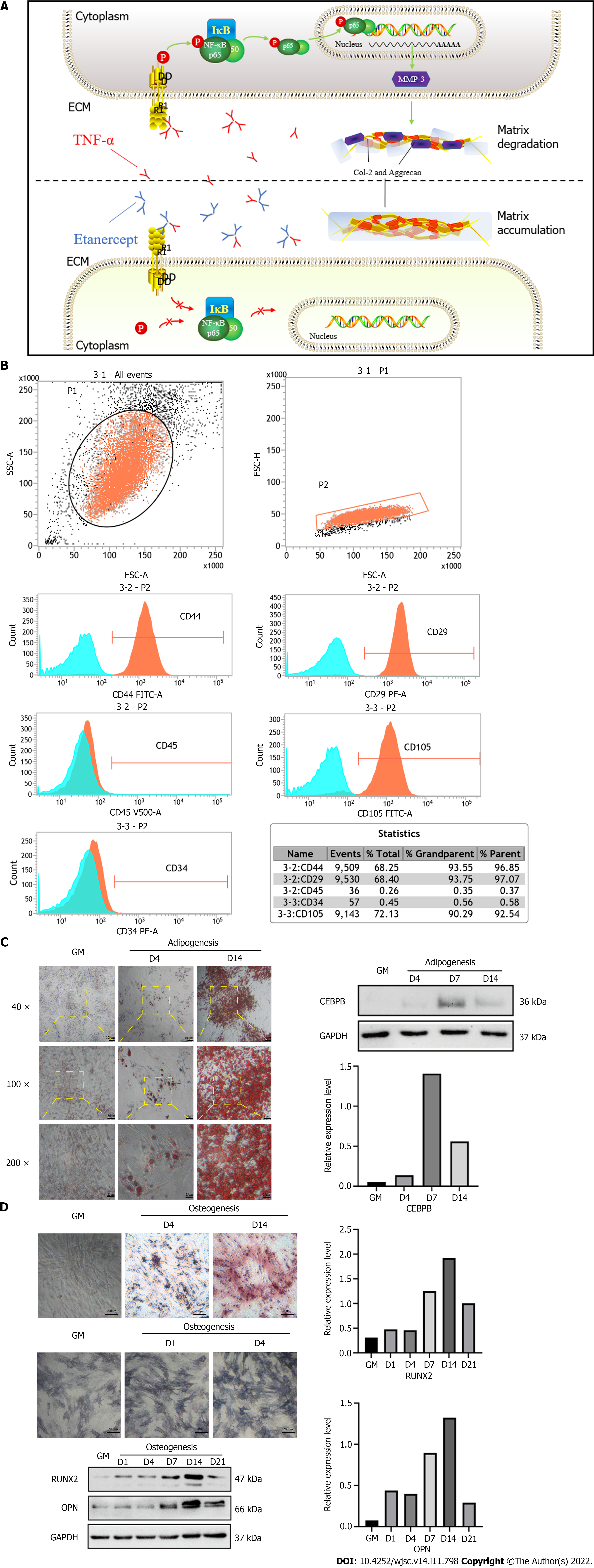
Tumor necrosis factor (TNF) is a serum glycoprotein that is produced by activated macrophages and other monocytes in mammals. Its functional unit is a homologous trimer that is composed of three subunits and 157 amino acids[10]. TNF, which is also known as TNF-alpha (TNF-α), exerts necrotizing effects on tumor cell lines and increases tumor transplant rejection. TNF-α is only 30% homologous to TNF-beta (lymphotoxin). Nevertheless, they share the same TNF receptors, namely, TNF-R1 and TNF-R2.11 Many studies have shown that TNF-R1 mediates most of the biological activity of TNF. The combination of these receptors triggers a series of intracellular events that ultimately lead to the activation of two major transcription factors, namely, nuclear factor kappa-B (NF-κB) and C-Jun[11]. Through these transcription factors, TNF-α induces the expression of genes that are essential for various biological processes, including cell growth and death, development, carcinogenesis, immunity, inflammation, and stress responses.
TNF-α inhibitors that are commonly used in clinical practice include etanercept, infliximab, and adalimumab. Etanercept is increasingly used because it is effective and affordable. Etanercept is a dimeric fusion protein that binds to TNF. Etanercept has been widely used in treating cartilage-related diseases, such as osteoarthritis, ankylosing spondylitis, and rheumatoid arthritis. Infliximab is a chimeric monoclonal IgG1 antibody that specifically binds to TNF-α, and it is mainly used in the study of autoimmune diseases, such as Crohn's disease, chronic inflammation and diabetic neuropathy. Both of these drugs inhibit the binding of TNF-α to its receptors on the cell surface, resulting in the biological inactivation of TNF-α, and their application is associated with few adverse reactions. In recent years, with the development of molecular biology, drugs with structures that are similar to etanercept, such as GP2015, LBEC0101, and Chs-0214, have been developed and have passed the assessments of a series of noninferiority studies[12]. In addition, some plant extracts, including saffron extract[13], resveratrol[14], nobiletin[15], etc., have also been reported to exert broad anti-inflammatory effects, and their effects are partly dependent on their ability to inhibit TNF-α.
In this study, we found that the expression of TNF-α increased during the early stage of the induced chondrogenic differentiation of hADSCs and resulted in a decrease in cartilage matrix secretion by hADSCs during late stages of chondrogenic differentiation; these effects could be delayed or eliminated by treatment with etanercept and Infliximab, which are TNF-α inhibitors (Figure 1A).
Human ADSCs (hADSCs) were isolated from human fat tissue using the type I collagenase digestion method. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; CA, United States) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (Gibco) at 37 °C and a humidified 5% CO2 atmosphere. The growth medium was changed every two days; third-generation cells were used for the experiments. The cells were passaged when they reached 90% confluence. Third-generation cells were used for the experiments below.
Four groups were established as follows: (1) hADSCs grown in growth medium alone (GM group); (2) hADSCs treated with chondrogenic differentiation medium (CH group); (3) hADSCs treated with chondrogenic differentiation medium and 1 μg/mL etanercept (CHE group); and (4) hADSCs treated with chondrogenic differentiation medium and 10 μg/mL infliximab (CH+Inf group).
The chondrogenic differentiation medium was composed of basic high-glucose DMEM (1X), 5% FBS, 1% penicillin/streptomycin, 1% insulin-transferrin-selenium-sodium-pyruvate solution (ITS-A; Gibco), 100 nmol/L dexamethasone (Theremofisher; Massachusetts, USA) 50 μg/mL, 40 mg/mL L-proline (Macklin; Shanghai, China), and 10 ng/mL TGF-β3 (Peprotech; NJ, United States). For the CHE and CH+Inf groups, 1 μg/mL etanercept (MCE, NJ, United States) and 10 μg/mL infliximab (MCE) were added to the chondrogenic differentiation medium to inhibit the bioactivity of TNF-α. For every group, the GM was changed every other day, and all the treatments were applied when the cells reached confluence in the culture dishes.
The cells were harvested and assessed on days 7, 14, 21, and 28. The results presented are mainly those of Western blotting analysis, enzyme linked immunosorbent assay (ELISA), fluorescence imaging, and toluidine blue staining.
To identify the types of cells used in the experiment, passage 3 hADSCs were removed from the culture dish by digestion with 0.1% trypsin and centrifuged. The cells were suspended (106/mL), and the solution was aliquoted into EP tubes after washing twice with phosphate-buffered saline (PBS). Diluted antibodies, namely, FITC-labeled CD29, CD34, CD44, CD45, and CD105 antibodies, were added according to the antibody instructions. The mixtures were incubated at 4 °C for 30 min, and then, the supernatants were discarded after centrifugation. The remaining antibodies that had bound to cell surface molecules were removed by washing with PBS. The cells in each tube were suspended in 400 μL of PBS and placed into the flow tube. The processed cells were stored at 4°C in the dark or analyzed by flow cytometry.
Passage 3 hADSCs were harvested and seeded in a 96-well culture plate. For the experimental group, on the second day, the GM was removed and replaced with chondrogenic differentiation medium containing etanercept. For the control group, the hADSCs were cultured in GM. Every 12 h, the CCK8 kit was used to measure the proliferation of the cells in these groups.
A premixed CCK-8 detection solution was added to the samples and incubated for 2 h. Finally, the absorbance of the samples was measured at 450 nm by a spectrophotometer.
Cell migration ability was test through scratch test. The tip of a 200 μL pipette was used to create several scratches of the same width in the Petri dishes. The cells in the dishes were cultured with GM and chondrogenic differentiation medium without FBS. The width of the scratches was observed and recorded under a microscope every 12 h.
Collagen type II (Col-2) and Aggrecan (ACAN) expression was measured with the Col-2 antibody (NB600-844, Novus, US.) and ACAN antibody (DF7561, Affinity, China) to prove chondrogenic differentiation. An anti-TNF-α antibody (AF7014, Affinity, China) was used to measure TNF-α expression. TNF-α functions mainly by activating the NF-κB pathway and upregulating the expression level of matrix metalloproteinase 3 (MMP-3). Therefore, we measured the expression of MMP-3, NF-κB p65, and pNF-κB p65 (phosphorylated NF-κB p65) with the corresponding antibodies (AF0217, AF5006, AF2006, respectively, Affinity, China).
The cells were lysed and the proteins were extracted with a protein extraction kit (KeyGEN BioTECH; Nanjing, China), and the lysis buffer was prepared according to the instructions. A spatula was used to scrape the cells off the bottom of the dish, and the cell fragments were mixed with lysis buffer. The mixtures were placed on ice, and the lysates were incubated for 30 min. The samples were centrifuged at 14,000 rpm at 4 °C for 15 min and boiled at 99.8 °C for 5 min.
The protein samples were loaded on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, and the separated proteins were transferred to polyvinylidene fluoride (PVDF) (Millipore, United States) membranes. Nonspecific binding sites were blocked with 5% bovine serum albumin (BSA) dissolved in Tris-buffered saline with Tween 20 (TBST). The membranes were then incubated with the antibodies mentioned above overnight at 4°C. The antibodies were then diluted strictly according to the instructions. The secondary antibody [Goat Anti-rabbit IgG (H+L) HRP, S0001, Affinity, China; Goat Anti-Mouse IgG (H+L) HRP, S0002, Affinity, China] was selected according to the species and origin of the primary antibody; the secondary antibody was added and incubated with the membranes at room temperature for 1 h. Signal detection was performed using the FDbio-Dura ECL kit (fdbio science, Hangzhou, China). ImageJ (software) was used to perform a semiquantitative analysis of the Western blotting bands.
The cells were seeded on cell slides and fixed with 4% paraformaldehyde for 10 min. The membranes of the cells were disrupted by incubation with 0.1% Triton-X 100 for 5 min. The samples were then blocked with 2% BSA dissolved in PBS for 1 h at room temperature. The samples were stained with the following primary antibodies: anti-MMP-3 antibody (1:200, AF0217) and anti-TNFα antibody (1:200, AF7014). Actin was stained with Phalloidin-iFluor 594 Reagent (ab176757). A goat anti-rabbit IgG/Alexa Fluor 555 antibody (Bioss, Beijing, China) was used as the secondary antibody. After these steps were complete, DAPI (4',6-diamidino-2-phenylindole) was used to label the nuclear DNA.
The cell supernatants were collected when the culture medium was changed on days 7, 14, 21, and 28. A proteinase inhibitor was added (1 μg/mL) to the samples, and the samples were stored at -80 °C. The cells were starved for 24 h in medium without FBS before the cell supernatants were collected. The concentrations of TNF-α and MMP-3 in the supernatants were measured using specific ELISA Kits (SEA133 Hu, SEA101Hu, Clone Cloud, Wuhan, China) according to the instructions.
Toluidine blue (0.1 g) was dissolved in 100 mL of 0.2 mol/L acetate buffer and stirred with magnetic stirrers for 1 h. The solution was then filtered; the pondus hydrogenii (pH) of the final solution was approximately 3.72 to 4.25. The hADSCs were fixed with 4% paraformaldehyde for 15 min. Then, toluidine blue and stain were added to the shaker for 30 min. Finally, cytoplasmic staining was observed under a microscope.
All the experiments were repeated more than three times. A one-way analysis of variance (ANOVA) or t test (GraphPad Prism 9.0 software, La Jolla, CA, United States) was used to identify significant differences. P < 0.05 was considered statistically significant.
Flow cytometry showed that CD44, CD29, and CD105 were highly expressed, while CD34 and CD45 were hardly expressed, on the cell surfaces (Figure 1B), which was consistent with the reported molecular expression pattern of mesenchymal stem cells. The cells were treated with adipogenic induction medium and osteogenic induction medium. The expression of marker proteins of differentiation was measured, and characteristic staining was performed. The results showed that the expression of CCAAT-enhancer-binding protein-beta (CEBPB), which is the transcription factor that is characteristic of adipogenic differentiation, increased after 7 d of treatment with adipogenic induction medium. The cells were also positive for Oil red O staining (Figure 1C). In addition, the expression of Runt-related transcription factor 2 (RUNX2) and osteopontin (OPN), which are markers of osteogenic differentiation, increased and peaked on day 14 after treatment with osteogenic induction medium; alkaline phosphatase staining and alizarin red staining were also positive (Figure 1D).
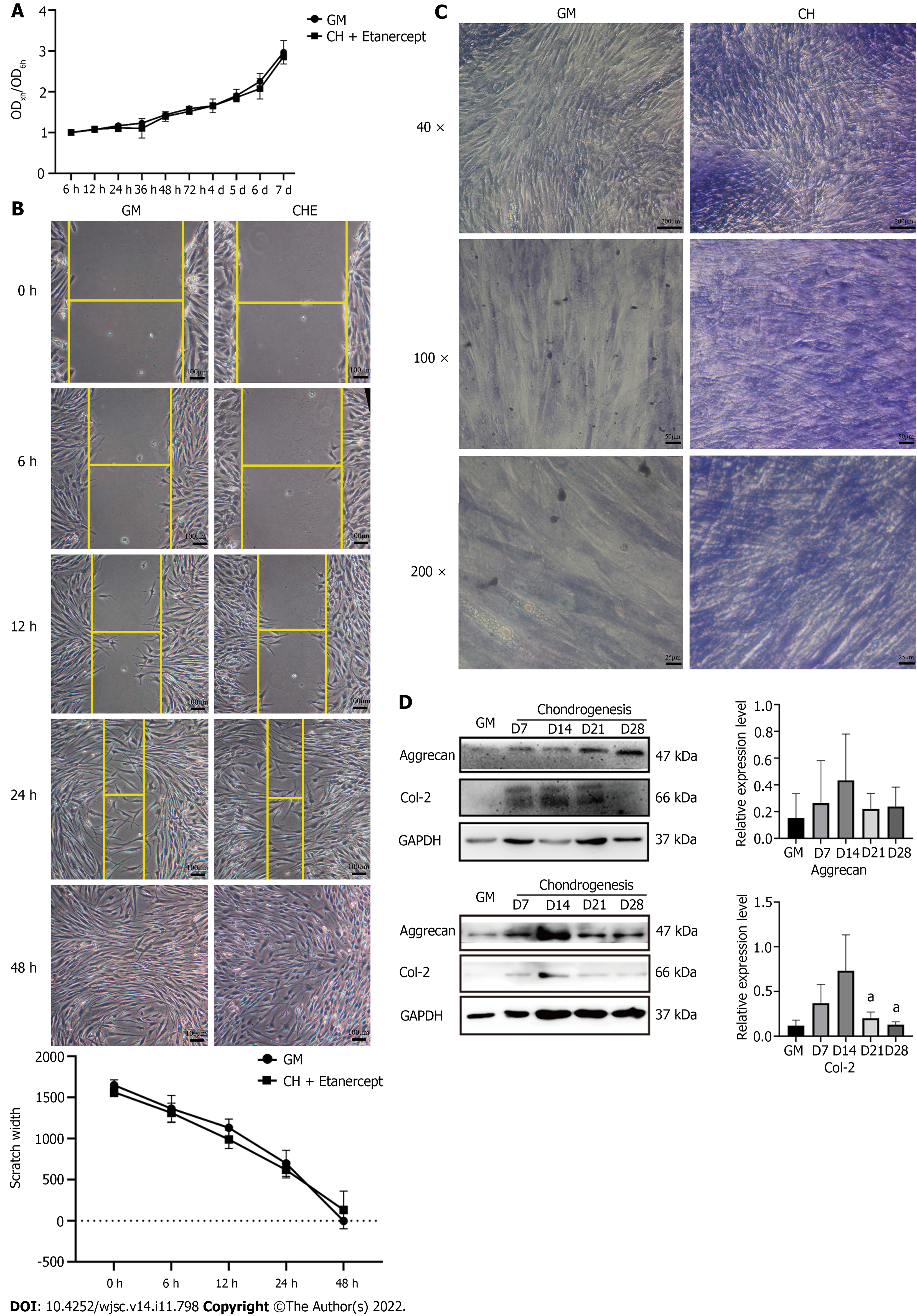
In the CCK-8 experiments, hADSCs that were treated with chondrogenic differentiation medium containing 1 μg/mL etanercept showed similar growth curves to those that were treated with GM (Figure 2A). In addition, the migration ability of the hADSCs that were grown in these two culture media was assessed with a scratch experiment. The curve of scratch width with time showed that there was no significant difference in the migration ability of hADSCs grown in GM and CHE medium (Figure 2B). After being cultured for 48 h, the scratches disappeared in both groups.
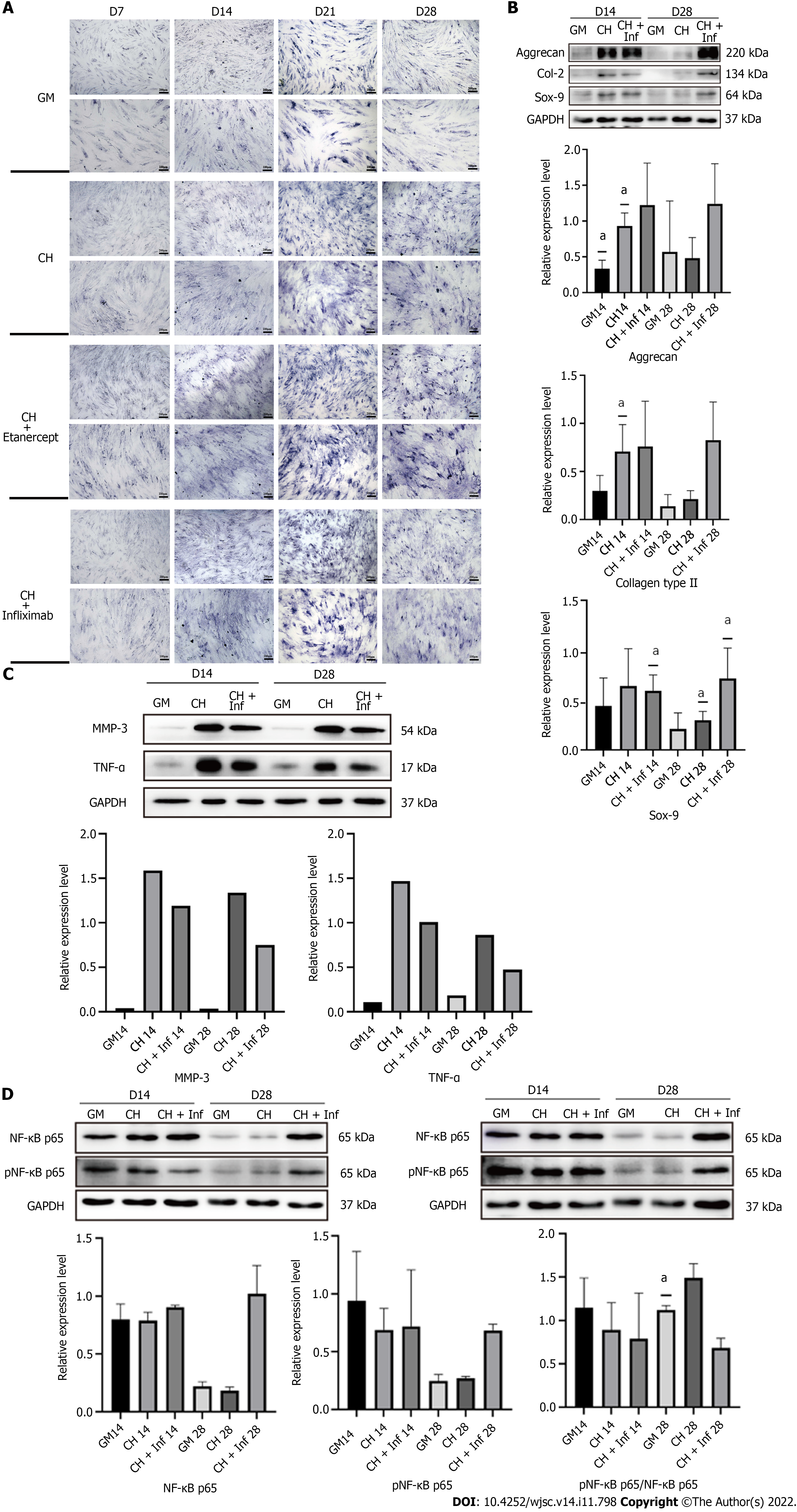
One week after the induction of chondrogenic differentiation, toluidine blue staining showed darker blue staining in the cytoplasm and the surrounding region of the cells (Figure 2D), suggesting the accumulation of cartilage matrix and the differentiation of hADSCs into chondrocytes. Western blotting analysis showed that compared with GM, chondrogenic differentiation medium increased the expression of Col-2 and Aggrecan by hADSCs from weeks 1 to 4, and the expression peaked from days 14 to 21 (Figure 2D). The expression of the chondrogenic marker proteins showed a declining trend on day 28 (Figure 2D).
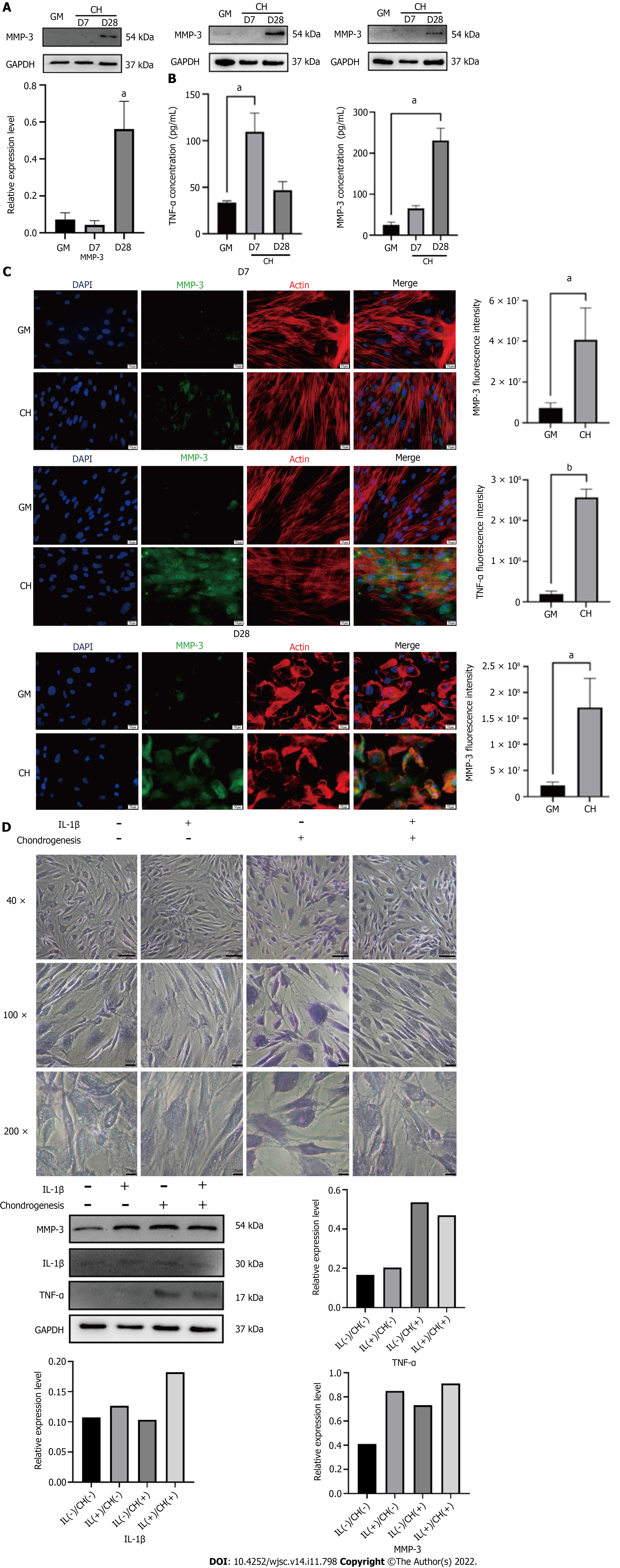
The WB results showed that TNF-α expression was increased in the early stage of chondrogenic differentiation (specifically, it increased by 3.7 times on day 7, P < 0.05), and its expression level was not significantly affected by the presence of interleukin (IL)-1β. Compared with the control group, the expression level of MMP-3 was not significantly increased at week 1 of hADSC chondrogenic differentiation, but it was significantly increased at week 4 (it increased by nearly 15 times on day 28, P < 0.1) (Figure 3A). The ELISA results were consistent with the Western blotting results. The TNF-α concentration in the cell supernatants increased by 2.4 times on day 7 (P < 0.1), and the concentration of MMP-3 in the cell supernatants increased by 10.8 times on day 28 (P < 0.1) (Figure 3B).
At 1 and 4 wk of chondrogenic differentiation, the cellular localization of TNF-α and MMP-3 in hADSCs was analyzed, and their concentrations were semiquantitatively measured, using immunofluorescence staining. The results were consistent with the Western blotting results. TNF-α expression was increased in the early stage of chondrogenic induction, while MMP-3 expression was significantly increased in the late stage. The fluorescence of these two proteins was primarily concentrated in the cytoplasmic region, which was identified by the red fluorescence of the microfilaments (Figure 3C).
IL-1β (10 ng/mL) was added to the culture medium, and chondrogenic differentiation of hADSCs was observed. Western blotting analysis showed that treatment with IL-1β alone did not affect the expression of TNF-α or MMP-3 in hADSCs. However, it upregulated the expression of MMP-3 in the presence of chondrogenic differentiation medium. Surprisingly, the expression level of TNF-α was slightly downregulated by chondrogenic differentiation medium containing IL-1β. Toluidine blue staining showed that the staining was lighter in the group treated with IL-1β and chondrogenic differentiation medium than in the group treated with chondrogenic differentiation medium alone on day 7. The staining of hADSCs with IL-1β alone without chondrogenic differentiation medium showed no significant difference from the control group (Figure 3D). As the viability of ADSCs was affected by IL-1β, a large number of ADSCs died after 7 d of treatment, so the long-term effects of IL-1β could not be observed. These research results need to be further explored.
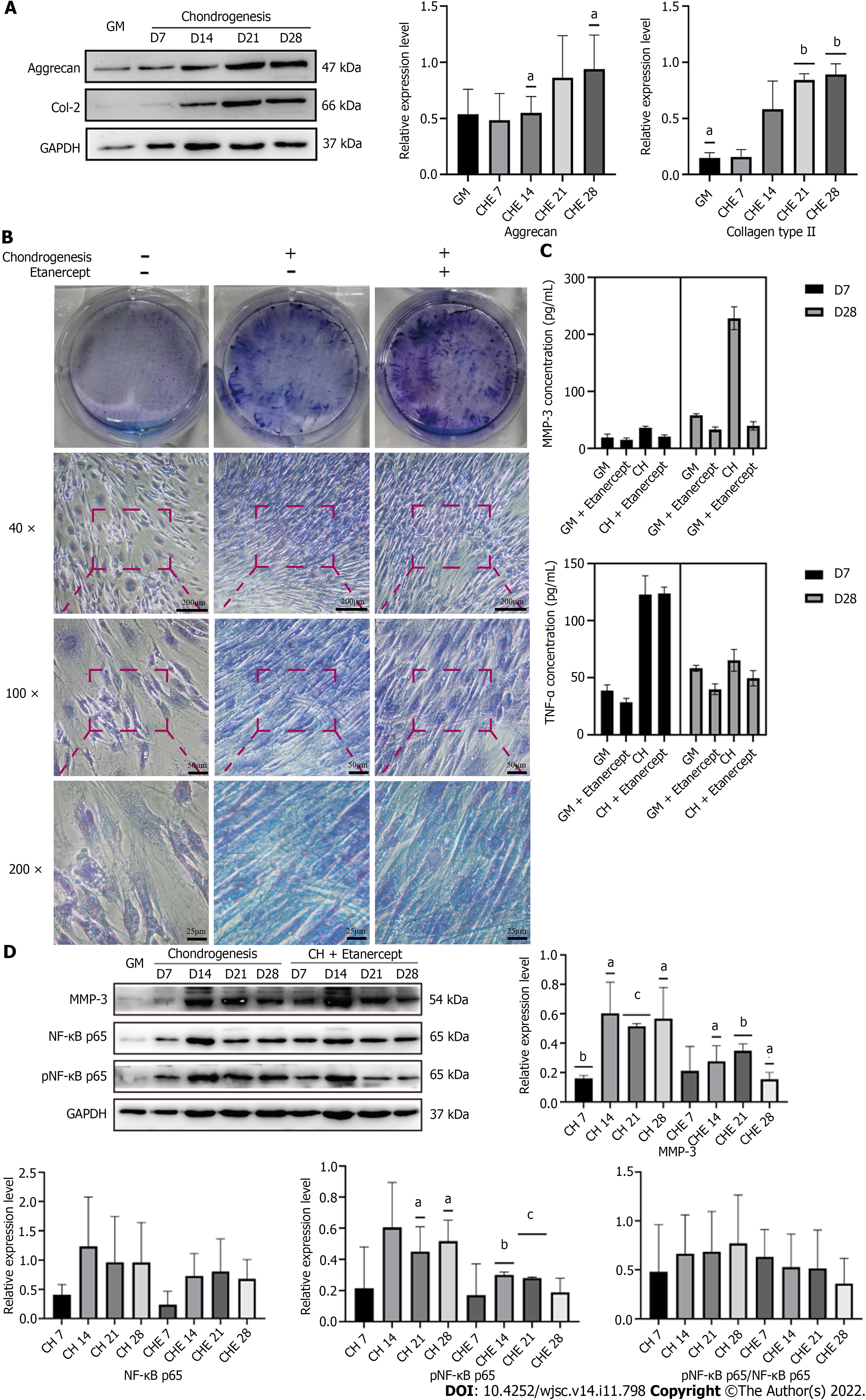
hADSCs treated with chondrogenic differentiation medium containing 1 μg/mL etanercept maintained their ability to secrete cartilage matrix for 28 d (Figure 4A, Supplementary Figure 1A). Toluidine blue staining showed that after 28 d of treatment with etanercept combined with chondrogenic induction medium, the staining intensity of the group treated with etanercept combined with chondrogenic induction medium was higher than that of the group treated with chondrogenic differentiation medium alone, and the intensities of both groups were higher than that of the control group (Figure 4B). ELISA showed that the concentrations of TNF-α and MMP-3 in the cell supernatants decreased after etanercept treatment (Figure 4C).
Toluidine blue staining showed that the cartilage matrix accumulation of CH+Inf group was more than that of CH group and GM group but not as much as CHE group (Figure 5A). hADSCs treated with chondrogenic differentiation medium containing 10 μg/mL infliximab maintained their ability to secret cartilage matrix capacity 28 d (Figure 5B and Supplementary Figure 1B). Western blot showed that the expression of TNF-α and MMP-3 in CH+Inf group was lower than CH group on both Day 14 and Day 28 (Figure 5C).
The levels of total and phosphorylated NF-κB p65 in hADSCs were increased during the induction of chondrogenic differentiation. After treatment with 1 μg/mL etanercept and 10 μg/mL infliximab, the expression level of NF-κB p65 did not significantly change, but the level of phosphorylated NF-κB p65 decreased on day 28 (Figures 4D, 5D and Supplementary Figure 1C). In addition, the expression level of MMP-3 decreased on day 28 in the CHE group.
In this study, we explored the possibility that the inflammatory environment caused by the elevated levels of TNF-α in the ADSC culture microenvironment at the early stage of chondrogenic differentiation induction affected the synthesis and secretion of cartilage matrix at the late stage of chondrogenic differentiation. Therefore, we used etanercept to inhibit TNF-α throughout the entire process of chondrogenic differentiation in vitro and observed changes in the cartilage matrix synthesis and secretion of ADSCs.
The induction of hADSCs to form cell lines that are capable of continuously secreting cartilage matrix in vitro is one approach for treating diseases that involve damaged cartilage. However, the decrease in cartilage matrix secretion in the late stage of chondrogenic differentiation is a major limitation to this research. Researchers have tried to solve this problem in several ways. Examples include: (1) 5-Aza cytidine (5-AZAC) was used to reduce the DNA methylation level[8]; (2) The expression level of cartilage matrix proteins was upregulated using noncoding RNA[16]; (3) A variety of cytokines, such as BMP and TGF-β, were added to culture[17]; and (4) A three-dimensional culture system was generated based on biomaterial scaffolds[18,19].
However, none of these methods could completely solve the problem that the production of cartilage matrix decreases during the late stage of the induced chondrogenic differentiation of stem cells in vitro. In this study, during the induced chondrogenic differentiation of hADSCs in vitro, the inflammatory factors TNF-α and MMP-3 accumulated in the culture system. Moreover, the ability of the cells to secrete cartilage matrix decreased. Therefore, we conducted a series of studies to determine whether there was a link between these two phenomena.
Long-term cartilage damage, which is characterized by local damage that is caused by the implant surgery, the immune reaction to implants, and the inflammatory factors that are inherent to the implants, can create an inflammatory microenvironment that is not conducive to stem cell therapy[20]. Improving the inflammatory state of the microenvironment can enhance many therapeutic responses, as has been reported in stem cell transplantation therapy, rheumatoid arthritis and psoriasis treatment, and even depression treatment[21]. In a study on hematopoietic stem cell transplantation, the direct binding of donor-derived TNF-α to TNF-R1 impaired the survival and division of transplanted hematopoietic stem cells and progenitor cells[22]. Anti-TNF therapy has also been shown to increase the success of hematopoietic stem cell therapy in treating human adenosine deaminase deficiency. The response to anti-TNF therapy can be considered one of the indications of whether the patient is a candidate HSCT[23]. Under physiological conditions, nearby stem cells are the first to differentiate and repair the damage when the body is injured[24,25]. Therefore, a microenvironment that is similar to that of the target tissue can greatly facilitate stem cell-induced differentiation in vitro. In this study, we found that TNF-α in the culture system upregulated the expression of MMP-3, which degraded the extracellular cartilage matrix, resulting in a lack of external support for the induced differentiation of hADSCs.
The NF-κB pathway is the primary signaling pathway that is activated when TNF-α binds to TNFR[11,26]. This pathway is often associated with inflammation by researchers[27] and is widely involved in the homeostatic regulation of the musculoskeletal system[28]. This pathway has been described in many studies to block chondrogenic differentiation[29,30]. In this study, etanercept and infliximab successfully reduced the level of phosphorylated NF-κB p65 and the expression level of MMP-3. This study confirmed that the NF-κB pathway is one of the signaling pathways by which TNF-α blocks the chondrogenic differentiation of hADSCs. It is worth considering whether drugs that inhibit TNF-α can be used to improve the protocol of ADSC chondrogenic induction in vitro. It has been reported that some substances, such as hawthorn[31], saffron extract[13] and resveratrol[14], can not only inhibit the biological effects of TNF-α but also have antioxidant and antifibrosis abilities. Could these substances be more effective components for improving the chondrogenic induction medium? In addition, our study found that the continuous accumulation of inflammation level may be one of the reasons for the decreased cartilage matrix secretion. Are there other substances besides TNF-α involved in the inflammatory response of the culture system? Can therapies targeting other inflammatory targets, such as TIMPs and NSAIDs, also be used to assist the chondrogenic differentiation of ADSCs in vitro? Further research is needed to find out the answers.
Through this study, we confirmed that TNF-α increased and inhibits the secretion of cartilage matrix by activating the NF-κB pathway. The use of TNF-A inhibitors, such as etanercept and infliximab, can maintain the cartilage matrix secretion of ADSCs at the late stage of chondrogenic differentiation. We hope to highlight an approach that combines stem cell therapy and targeted anti-inflammatory drugs to treat diseases that involve cartilage damage. The adverse effects associated with in vitro-induced chondrogenic differentiation can be eliminated and the therapeutic effect of hADSCs can be maximized with specific targeted drugs. Animal and in vivo studies remain to be conducted, which is the future direction of our team.
During the induced differentiation, the inability of adipose-derived mesenchymal stem cells (ADSCs) to secret cartilage matrix durably has been one of the difficulties in cartilage tissue engineering. Therefore, understanding the changes in the culture system before and after ADSCs differentiation and improving the induction program can help improve the chondrogenic differentiation efficiency of ADSCs.
In the previous study, we conducted single-cell sequencing of ADSCs before and after chondrogenic differentiation and found that the expression levels of Matrix metalloproteinase 3 (MMP-3) and Tumor necrosis factor receptor superfamily member 12A (TNFRSF12A) were significantly increased. Therefore, we hypothesized that the accumulation of inflammatory levels in THE culture system resulted in decreased cartilage matrix secretion at the late stage of differentiation and designed this study for this reason.
To investigate the changes of tumor necrosis factor-α (TNF-α) and MMP-3 Levels in the culture system of ADSCs before and after chondrogenic differentiation. To confirm that TNF-α increased and decreased cartilage matrix secretion of ADSCs by activating the NF-KB pathway. To confirm that adding TNF-α inhibitor to chondrogenic medium could improve the chondrogenic differentiation efficiency of ADSCs.
Treat ADSCs with chondrogenic medium containing TNF- α inhibitors, such as etanercept and infliximab. Then observe the changes of cartilage matrix secretion and the level of inflammation in the culture system through western blot, Elisa, immunofluorescence and toluidine blue staining.
During the differentiation of ADSCs, the expression levels of TNF-α and MMP-3 increased gradually, and the activation of NF-κB signaling pathway increased. Adding TNF-α inhibitors, etanercept (1 μg/mL) or inflixib (10 μg/mL), to the chondrogenic medium can reduce the activation of NF-κB pathway, alleviate the inflammation and preserve the secretion of cartilage matrix of ADSCs.
When TNF-α increases and binds to its receptor, activates NF-κB pathway and reduces cartilage matrix secretion of ADSCs. TNF-α inhibitors can block the above process and improve the chondrogenic differentiation efficiency of ADSCs in vitro.
In future studies, the TNF-α inhibitor etanercept or infliximab used in this study could be combined with the scaffold material to optimize the growth environment of ADSCs and make the drug release more durable and gentle, so as to achieve higher chondrogenic differentiation efficiency of ADSCs. And obtain a transplantable cartilage engineering material with similar properties to the natural cartilage tissue for the repair and treatment of cartilage defects, eventually.
Thanks to Peking University Hong Kong University of Science and Technology Medical Center for providing the research instruments and workplace.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Amin A, United Arab Emirates; Ma B, China S-Editor: Gong ZM L-Editor: A P-Editor: Cai YX
| 1. | Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 655] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 2. | Xia X, Chan KF, Wong GTY, Wang P, Liu L, Yeung BPM, Ng EKW, Lau JYW, Chiu PWY. Mesenchymal stem cells promote healing of nonsteroidal anti-inflammatory drug-related peptic ulcer through paracrine actions in pigs. Sci Transl Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Lee J, Abdeen AA, Tang X, Saif TA, Kilian KA. Matrix directed adipogenesis and neurogenesis of mesenchymal stem cells derived from adipose tissue and bone marrow. Acta Biomater. 2016;42:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Zhu P, Liu J, Shi J, Zhou Q, Zhang X, Du Z, Liu Q, Guo Y. Melatonin protects ADSCs from ROS and enhances their therapeutic potency in a rat model of myocardial infarction. J Cell Mol Med. 2015;19:2232-2243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Yu SM, Yeo HJ, Choi SY, Kim SJ. Cytokine-induced apoptosis inhibitor-1 causes dedifferentiation of rabbit articular chondrocytes via the ERK-1/2 and p38 kinase pathways. Int J Biochem Cell Biol. 2016;80:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Cai C, Min S, Yan B, Liu W, Yang X, Li L, Wang T, Jin A. MiR-27a promotes the autophagy and apoptosis of IL-1β treated-articular chondrocytes in osteoarthritis through PI3K/AKT/mTOR signaling. Aging (Albany NY). 2019;11:6371-6384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Yu SM, Choi YJ, Kim SJ. PEP-1-glutaredoxin-1 induces dedifferentiation of rabbit articular chondrocytes by the endoplasmic reticulum stress-dependent ERK-1/2 pathway and the endoplasmic reticulum stress-independent p38 kinase and PI-3 kinase pathways. Int J Biol Macromol. 2018;111:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Duan L, Liang Y, Ma B, Wang D, Liu W, Huang J, Xiong J, Peng L, Chen J, Zhu W. DNA Methylation Profiling in Chondrocyte Dedifferentiation In Vitro. J Cell Physiol. 2017;232:1708-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Costa E, González-García C, Gómez Ribelles JL, Salmerón-Sánchez M. Maintenance of chondrocyte phenotype during expansion on PLLA microtopographies. J Tissue Eng. 2018;9:2041731418789829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Ward-Kavanagh LK, Lin WW, Šedý JR, Ware CF. The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity. 2016;44:1005-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 327] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 11. | Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1399] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 12. | Kerschbaumer A, Sepriano A, Smolen JS, van der Heijde D, Dougados M, van Vollenhoven R, McInnes IB, Bijlsma JWJ, Burmester GR, de Wit M, Falzon L, Landewé R. Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2020;79:744-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 13. | Abdalla Y, Abdalla A, Hamza AA, Amin A. Safranal Prevents Liver Cancer Through Inhibiting Oxidative Stress and Alleviating Inflammation. Front Pharmacol. 2021;12:777500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 14. | Bollmann F, Art J, Henke J, Schrick K, Besche V, Bros M, Li H, Siuda D, Handler N, Bauer F, Erker T, Behnke F, Mönch B, Härdle L, Hoffmann M, Chen CY, Förstermann U, Dirsch VM, Werz O, Kleinert H, Pautz A. Resveratrol post-transcriptionally regulates pro-inflammatory gene expression via regulation of KSRP RNA binding activity. Nucleic Acids Res. 2014;42:12555-12569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Nguyen-Ngo C, Salomon C, Quak S, Lai A, Willcox JC, Lappas M. Nobiletin exerts anti-diabetic and anti-inflammatory effects in an in vitro human model and in vivo murine model of gestational diabetes. Clin Sci (Lond). 2020;134:571-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Mao G, Hu S, Zhang Z, Wu P, Zhao X, Lin R, Liao W, Kang Y. Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J Cell Mol Med. 2018;22:5354-5366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Wang T, Nimkingratana P, Smith CA, Cheng A, Hardingham TE, Kimber SJ. Enhanced chondrogenesis from human embryonic stem cells. Stem Cell Res. 2019;39:101497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Yu C, Kornmuller A, Brown C, Hoare T, Flynn LE. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials. 2017;120:66-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Guo T, Noshin M, Baker HB, Taskoy E, Meredith SJ, Tang Q, Ringel JP, Lerman MJ, Chen Y, Packer JD, Fisher JP. 3D printed biofunctionalized scaffolds for microfracture repair of cartilage defects. Biomaterials. 2018;185:219-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA. 2021;325:568-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 1220] [Article Influence: 305.0] [Reference Citation Analysis (0)] |
| 21. | Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23:335-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 451] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 22. | Wang W, Fujii H, Kim HJ, Hermans K, Usenko T, Xie S, Luo ZJ, Ma J, Celso CL, Dick JE, Schroeder T, Krueger J, Wall D, Egeler RM, Zandstra PW. Enhanced human hematopoietic stem and progenitor cell engraftment by blocking donor T cell-mediated TNFα signaling. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Moens L, Hershfield M, Arts K, Aksentijevich I, Meyts I. Human adenosine deaminase 2 deficiency: A multi-faceted inborn error of immunity. Immunol Rev. 2019;287:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Soteriou D, Fuchs Y. A matter of life and death: stem cell survival in tissue regeneration and tumour formation. Nat Rev Cancer. 2018;18:187-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Ge Y, Fuchs E. Stretching the limits: from homeostasis to stem cell plasticity in wound healing and cancer. Nat Rev Genet. 2018;19:311-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 26. | Dostert C, Grusdat M, Letellier E, Brenner D. The TNF Family of Ligands and Receptors: Communication Modules in the Immune System and Beyond. Physiol Rev. 2019;99:115-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 335] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 27. | Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell. 2017;168:37-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1483] [Article Influence: 185.4] [Reference Citation Analysis (0)] |
| 28. | Novack DV. Role of NF-κB in the skeleton. Cell Res. 2011;21:169-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 29. | Jiang T, Kai D, Liu S, Huang X, Heng S, Zhao J, Chan BQY, Loh XJ, Zhu Y, Mao C, Zheng L. Mechanically cartilage-mimicking poly(PCL-PTHF urethane)/collagen nanofibers induce chondrogenesis by blocking NF-kappa B signaling pathway. Biomaterials. 2018;178:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Kobayashi H, Chang SH, Mori D, Itoh S, Hirata M, Hosaka Y, Taniguchi Y, Okada K, Mori Y, Yano F, Chung UI, Akiyama H, Kawaguchi H, Tanaka S, Saito T. Biphasic regulation of chondrocytes by Rela through induction of anti-apoptotic and catabolic target genes. Nat Commun. 2016;7:13336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 31. | Hamza AA, Lashin FM, Gamel M, Hassanin SO, Abdalla Y, Amin A. Hawthorn Herbal Preparation from Crataegus oxyacantha Attenuates In Vivo Carbon Tetrachloride -Induced Hepatic Fibrosis via Modulating Oxidative Stress and Inflammation. Antioxidants (Basel). 2020;9:1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |