Published online Jan 26, 2022. doi: 10.4252/wjsc.v14.i1.104
Peer-review started: May 5, 2021
First decision: June 23, 2021
Revised: July 20, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: January 26, 2022
Processing time: 260 Days and 1.5 Hours
Type 1 diabetes (T1D), a chronic metabolic and autoimmune disease, seriously endangers human health. In recent years, mesenchymal stem cell (MSC) transplantation has become an effective treatment for diabetes. Menstrual blood-derived endometrial stem cells (MenSC), a novel MSC type derived from the decidual endometrium during menstruation, are expected to become promising seeding cells for diabetes treatment because of their noninvasive collection procedure, high proliferation rate and high immunomodulation capacity.
To comprehensively compare the effects of MenSC and umbilical cord-derived MSC (UcMSC) transplantation on T1D treatment, to further explore the potential mechanism of MSC-based therapies in T1D, and to provide support for the clinical application of MSC in diabetes treatment.
A conventional streptozotocin-induced T1D mouse model was established, and the effects of MenSC and UcMSC transplantation on their blood glucose and serum insulin levels were detected. The morphological and functional changes in the pancreas, liver, kidney, and spleen were analyzed by routine histological and immunohistochemical examinations. Changes in the serum cytokine levels in the model mice were assessed by protein arrays. The expression of target proteins related to pancreatic regeneration and apoptosis was examined by western blot.
MenSC and UcMSC transplantation significantly improved the blood glucose and serum insulin levels in T1D model mice. Immunofluorescence analysis revealed that the numbers of insulin+ and CD31+ cells in the pancreas were significantly increased in MSC-treated mice compared with control mice. Subsequent western blot analysis also showed that vascular endothelial growth factor (VEGF), Bcl2, Bcl-xL and Proliferating cell nuclear antigen in pancreatic tissue was significantly upregulated in MSC-treated mice compared with control mice. Additionally, protein arrays indicated that MenSC and UcMSC transplantation significantly downregulated the serum levels of interferon γ and tumor necrosis factor α and upregulated the serum levels of interleukin-6 and VEGF in the model mice. Additionally, histological and immunohistochemical analyses revealed that MSC transplantation systematically improved the morphologies and functions of the liver, kidney, and spleen in T1D model mice.
MenSC transplantation significantly improves the symptoms in T1D model mice and exerts protective effects on their main organs. Moreover, MSC-mediated angiogenesis, antiapoptotic effects and immunomodulation likely contribute to the above improvements. Thus, MenSC are expected to become promising seeding cells for clinical diabetes treatment due to their advantages mentioned above.
Core Tip: Mesenchymal stem cell (MSC)-based therapies have resulted in promising improvements for patients with type 1 diabetes (T1D). Menstrual blood-derived endometrial stem cells (MenSC) transplantation has therapeutic effects equal to those of umbilical cord-derived MSC, which can significantly improve the symptoms of streptozotocin-induced T1D mice and exert protective effects on their main organs; MSC-induced angiogenesis, antiapoptotic effects and immunomodulation contribute to these protective effects. The results of this study showed that MenSC are expected to become a promising alternative for clinical diabetes treatment due to their advantages, including their regular and noninvasive collection protocol, abundant availability, and superior proliferative capacity.
- Citation: Sun YL, Shang LR, Liu RH, Li XY, Zhang SH, Ren YK, Fu K, Cheng HB, Yahaya BH, Liu YL, Lin JT. Therapeutic effects of menstrual blood-derived endometrial stem cells on mouse models of streptozotocin-induced type 1 diabetes. World J Stem Cells 2022; 14(1): 104-116
- URL: https://www.wjgnet.com/1948-0210/full/v14/i1/104.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i1.104
Diabetes is a common metabolic disease characterized by hyperglycemia, and diabetic patients are prone to chronic damage or dysfunction of their eyes, kidneys, heart, blood vessels, and nerves, as well as other complications, severely affecting their quality of life[1,2]. The loss of functional β-cell mass is the key pathogenesis leading to both type 1 diabetes (T1D) and T2D. T1D is a chronic autoimmune disease resulting from a complete insulin deficiency caused by the attack and destruction of pancreatic islet β cells by the autoimmune system. On the other hand, T2D manifests as the progressive loss of β-cell insulin secretion and frequently occurs in the background of insulin resistance[3,4]. Generally, exogenous insulin supplementation, which serves as the main treatment for diabetes, can control blood glucose and delay the occurrence of diabetic complications. However, the long-term use of exogenous insulin leads to insulin resistance and an increased risk of severe hypoglycemia and does not fundamentally inhibit the pathological development of diabetes or potential diabetic complications. Therefore, to improve the symptoms of diabetes and prevent the occurrence and deterioration of diabetes complications, novel effective treatments for diabetes are required. Mesenchymal stem cell (MSC), which have self-renewal and multilineage differentiation abilities as well as low immunogenicity, have exhibited promising therapeutic potential for various diseases in the clinic, and previous studies have also demonstrated that MSC transplantation is safe and effective in diabetes treatment[5-8].
Umbilical cord-derived MSC (UcMSC), as the main source of MSC for diabetes treatment in the clinic, not only enhance the antiapoptotic capacity of islet β cells and stimulate their regeneration but also differentiate into islet β cells to compensate for insufficient insulin secretion[9-11]. Generally, UcMSC are transplanted into the body through intravenous injection, intrapancreatic artery injection or both, and the improvements after UcMSC transplantation have been extensively confirmed in clinical practice. After 1 year of UcMSC therapy, the mean HbA1c% values were consistently decreased in diabetic patients compared with the baseline levels; the mean C-peptide levels were consistently elevated, and the daily insulin requirement was uniformly decreased compared with their respective baseline[12-14]. In addition to the extensive application of UcMSC in diabetes treatment, menstrual blood-derived endometrial stem cells (MenSC), harvested from the menstrual blood of women of reproductive age, have recently attracted increased attention in the field of regenerative medicine. MenSC can be collected continuously every month, and the noninvasive collection method (menstrual cup) does not impose physiological or psychological burdens on the donor. Furthermore, regarding the richness of stem cells, primary MenSC account for approximately 3%-5% of all karyocytes in menstrual blood samples, quantitatively supporting their extensive application in the clinic[15]. Therefore, guaranteeing the quantity of MenSC satisfies the requirement for not only autologous transplantation but also for the family members of patients. Additionally, nude mice exhibited no tumorigenicity after MenSC transplantation, and no obvious adverse effects were observed after MenSC transplantation in clinical trials on multiple sclerosis[16], congestive heart failure[17], and lower limb ischemia[18]. These results guarantee the safety of MenSC in clinical applications[19]. Additionally, in 2014, Wu et al[20] preliminarily demonstrated the positive effect of MenSC transplantation on streptozotocin (STZ)-induced T1D mice, suggesting that MenSC are promising for clinical diabetes treatment[20].
Therefore, in this study, T1D animal models were generated by the injection of STZ, a strategy that effectively and specifically damages pancreatic β cells and inhibits insulin secretion, resulting in elevated blood glucose levels and the induction of T1D symptoms[21]. This study aimed to evaluate the therapeutic effects of MenSC and UcMSC transplantation on STZ-induced T1D mice, focusing on the improvements in T1D-related symptoms and the underlying mechanism, as well as the influences on the main organs of model mice. We believe that both the therapeutic effect and mechanistic studies of MenSC in T1D will provide support for and accelerate the clinical application of these cells in diabetes treatment strategies.
Both the MenSC and UcMSC used in this study were provided by the Zhongyuan Stem Cell Research Institute of Xinxiang High-tech Zone, and informed consent was provided by all donors who agreed to use their UcMSC and MenSC in scientific research. All the experimental procedures in this study were approved by the Ethics Committee of Xinxiang Medical University and were performed in accordance with the approved guidelines. Male C57BL/6N mice (18-25 g) aged 6 to 8 wk were purchased from Vital River Laboratories [Beijing, China; license no. SCXK (Beijing) 2012-0001]. The mice were randomly grouped in separate cages and housed in a specific pathogen-free environment maintained at a temperature of 25 ℃, a humidity of 50 ± 5% and a 12-h light-dark cycle. The mouse handling and laboratory procedures were performed in accordance with the guidelines of the Animal Health Committee of Xinxiang Medical University.
C57BL/6N mice (n = 50) were adaptively fed for one week and randomly divided into 2 groups: The normal group (n = 10) and the STZ-induced group (n = 40). The mice in the STZ-induced group were intraperitoneally injected with STZ (Sigma, United States; 45 mg/kg/d in citric acid buffer at pH 4.3) each day after fasting overnight for 5 consecutive days. The mice in the control group received equal amounts of citric acid buffer (pH 4.3). The first day of STZ injection was labeled as day-13; after the last STZ injection (day-9), the physiological statuses of the model mice were observed for 1 wk. After that, the model mice with fasting blood glucose (FBG) levels greater than 16 mmol/L were diagnosed with diabetes and randomly divided into 3 groups (n = 10): The PBS, UcMSC group, and MenSC treatment groups. The mice in the MSC treatment group were injected with 1 × 106 cells (suspended in 200 μL of saline) via their tail vein on days 0, day 7, and day 14. The mice in the PBS treatment group received an equal amount of saline. The mice were weighed, and their FBG levels and food intake were monitored every week during the experimental period. At the end of the experiment (day 63), all the mice were sacrificed, and their sera, pancreases, livers, spleens, and kidneys were harvested for further examination.
The isolated serum samples were sent to Xinxiang Assegai Medical Laboratory Center (Xinxiang, China) within 4 h. Routine liver and kidney functional indexes, including alanine transaminase (ALT), aspartic acid transaminase (AST) and alkaline phosphatase (ALP), albumin (ALB), globulin (GLB), total protein (TP), UREA, uric acid (UA), and creatinine (CRE), were assayed using a Chemray240 automated biochemical analyzer (Rayto, Shenzhen, China). The serum insulin levels in the mice were determined using an ELISA Kit (D721159; Sangon Biotech) according to the manufacturer's instructions.
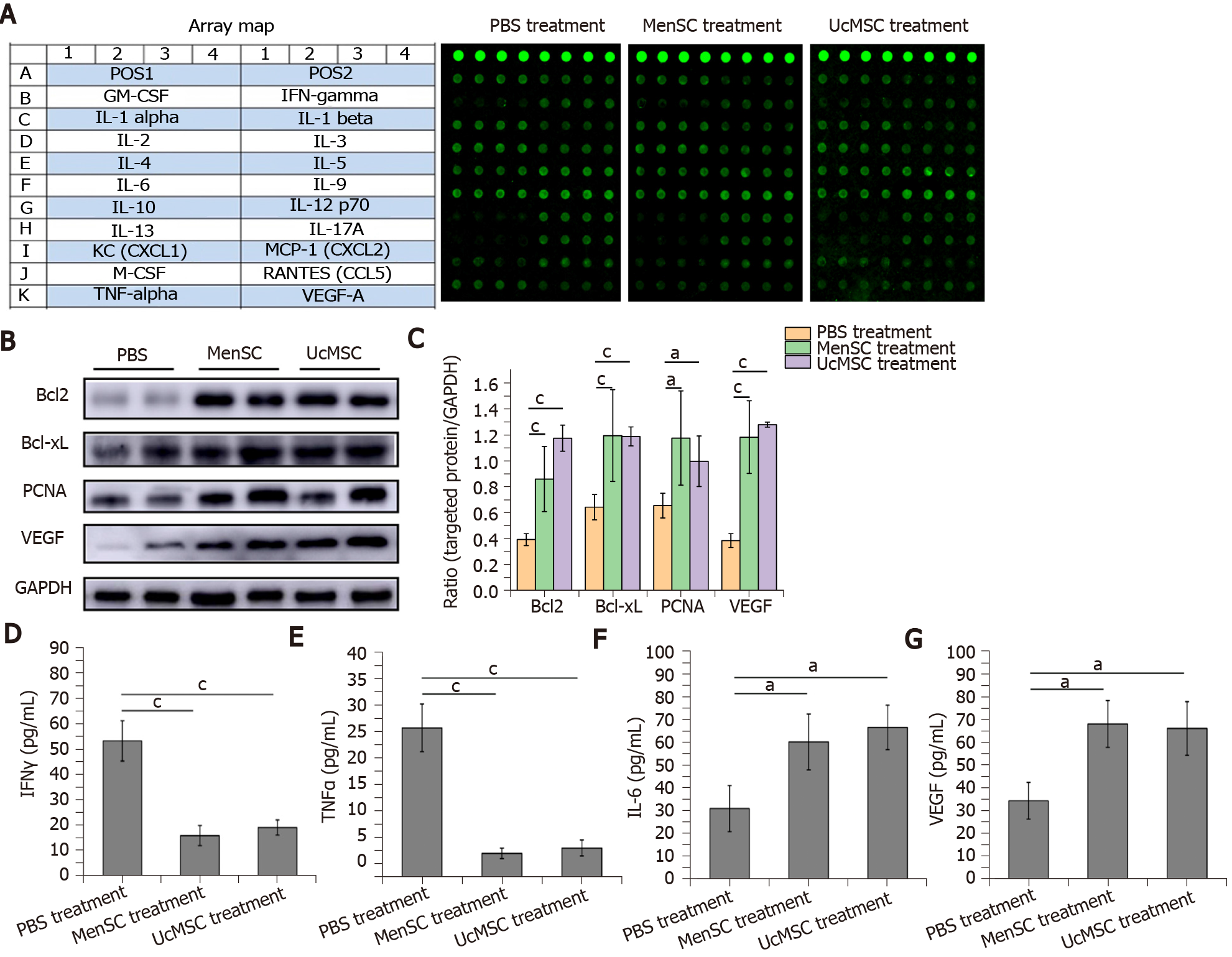
The isolated serum samples were kept on dry ice and sent to RayBiotech (Guangzhou, China). A protein array (AAH-CUST-G1; Norcross, GA, United States) was used according to the manufacturer’s instructions to measure the expression levels of 20 cytokines in the samples (Figure 1A). Positive signals were captured on glass chips using a laser scanner (InnoScan 300 Microarray Scanner; Innopsys, Carbonne, France), and the observed fluorescence intensities were normalized to those of the internal positive controls.
The pancreas, kidney, liver, and spleen samples were routinely fixed with 4% phosphate-buffered paraformaldehyde, embedded in paraffin, and sliced into 5-μm-thick sections for conventional hematoxylin and eosin (HE) staining. Next, the morphologies of the targeted organs were observed and imaged under a microscope (DMi8; Leica, Wetzlar, Germany).
Dewaxed liver sections were successively immersed in 95% and 75% ethanol solutions for 10 min and then rinsed with water for 10 min. After removing the excess water in a fume hood, the samples were stained with a sodium periodate solution for 10 min and rinsed with running water for 10 min. Next, the sections were incubated with Schiff solution at 37 ℃ in the dark for 15-25 min. After removing the excessive Schiff's solution, the sections were washed with sodium sulfite solution for 5 min and then with running water for another 10 min. The sections were soaked in 95% ethanol and xylene for 1-2 min and conventionally sealed after air drying in a fume hood. Finally, the sections were observed and imaged under a microscope.
Dewaxed pancreatic tissue sections were immersed in antigenic repair solution at
TP was extracted from pancreatic tissue using RIPA buffer and a protease inhibitor (Beyotime, China), and the protein concentration was determined by the BCA method. The protein samples were denatured in a metal bath at 95 ℃, separated by SDS-PAGE and transferred onto PVDF membranes. The samples were subsequently blocked with 5% nonfat milk in PBS for 1 h and then incubated with the following primary antibodies overnight at 4 ℃: Rabbit-derived Bcl2 (ab182858; Abcam), Bcl-xL (ab32370; Abcam), proliferating cell nuclear antigen (PCNA) (ab92552; Abcam), and vascular endothelial growth factor (VEGF) (ab32152; Abcam). The membranes were then incubated with goat anti-rabbit HRP-conjugated secondary antibodies at room temperature for 2 h. GAPDH was used as the internal control. Immunoreactions were detected using enhanced an chemiluminescence reagent and an Amersham Imager 600 system (GE Healthcare Life Sciences), and the grayscale values of the bands representing the targeted proteins were quantitated using ImageJ software.
The results are presented as the mean ± SD and were analyzed using Statistical Package for GraphPad Prism 8.0. The nonparametric Mann-Whitney U test was used for comparisons between two independent samples, and one-way ANOVA followed by Dunnett’s test was used for comparisons among ≥ 3 groups. P < 0.05 was considered to be statistically significant.
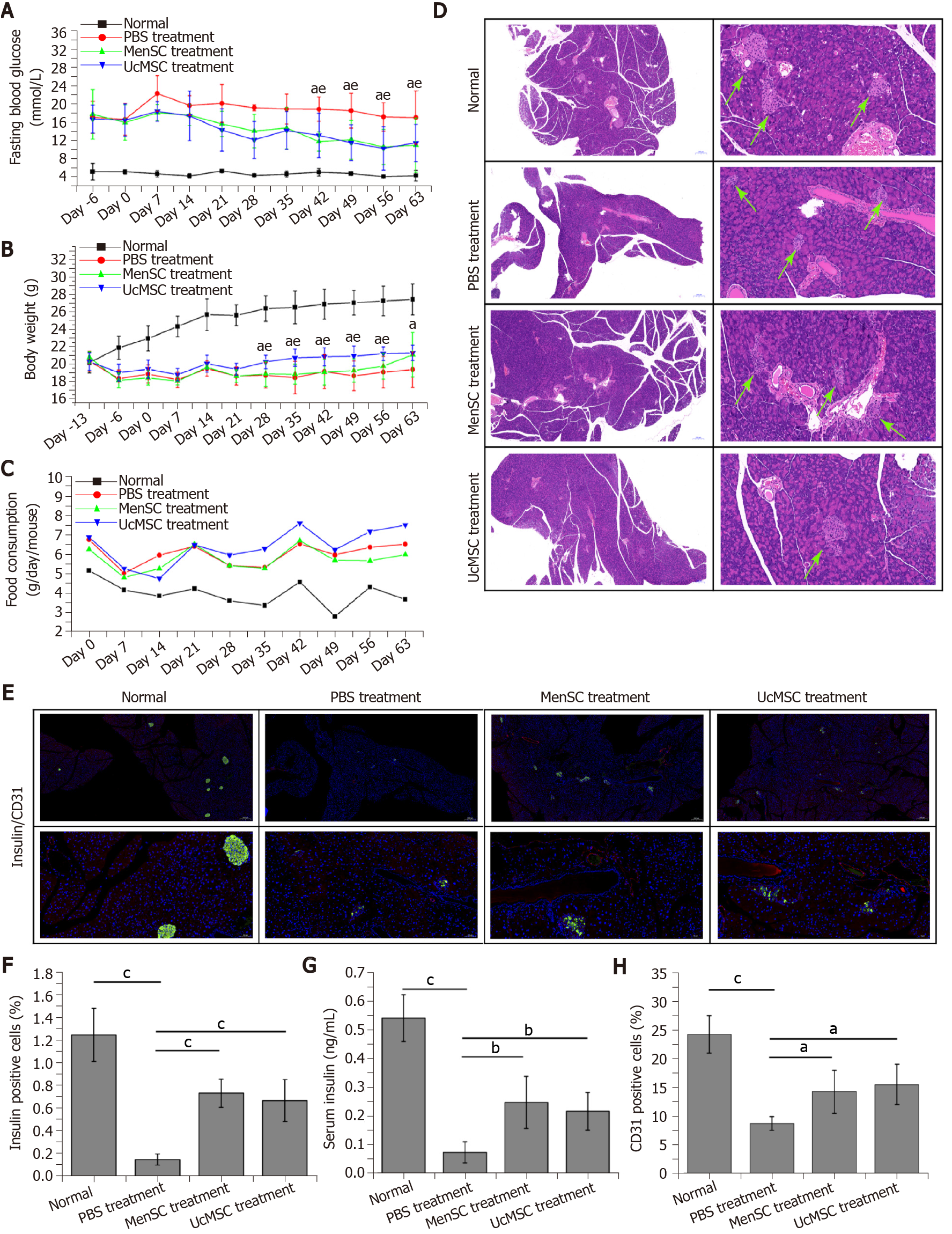
The characteristics of the MenSC and UcMSC used in this study were consistent with the typical characteristics of MSC, which express CD73, CD90, and CD105 but not CD34 and CD29. After MSC transplantation, the FBG levels in MSC-treated mice were significantly decreased starting on day 42 (Figure 2A; P < 0.05), and the body weights of UcMSC-treated mice showed a significantly increasing trend starting on day 28 (Figure 2B; P < 0.05), which was positively related to food consumption (Figure 2C). Subsequently, both the area of islets and the number of insulin+ cells in the pancreas were significantly increased after MSC transplantation (Figure 2D-F). Consistent with the above findings, the serum insulin levels were also upregulated (Figure 2G), but the levels were not significantly different between MenSC- and UcMSC-treated mice. Additionally, the number of CD31+ cells in the pancreas was significantly increased in MSC-treated mice compared with PBS-treated mice (Figure 2E and H), and the expression of VEGF in the pancreas was upregulated (Figure 1B and C). Additionally, the final WB results confirmed that the antiapoptotic markers (Bcl2 and Bcl-xL) and PCNA in the pancreas were significantly upregulated in MSC-treated mice compared with PBS-treated mice (Figure 1B and C).
A protein array was used to detect the expression levels of 20 cytokines in the sera of STZ-treated mice treated with or without MSC. The serum expression levels of interferon (IFN) γ and tumor necrosis factor (TNF) α were significantly downregulated in MSC-treated mice compared with PBS-treated mice (Figure 1A and D-G; P < 0.05), suggesting that the inflammatory response was relieved in vivo. Simultaneously, the serum expression levels of IL-6 and VEGF in the MSC-treated mice were significantly upregulated (P < 0.05).
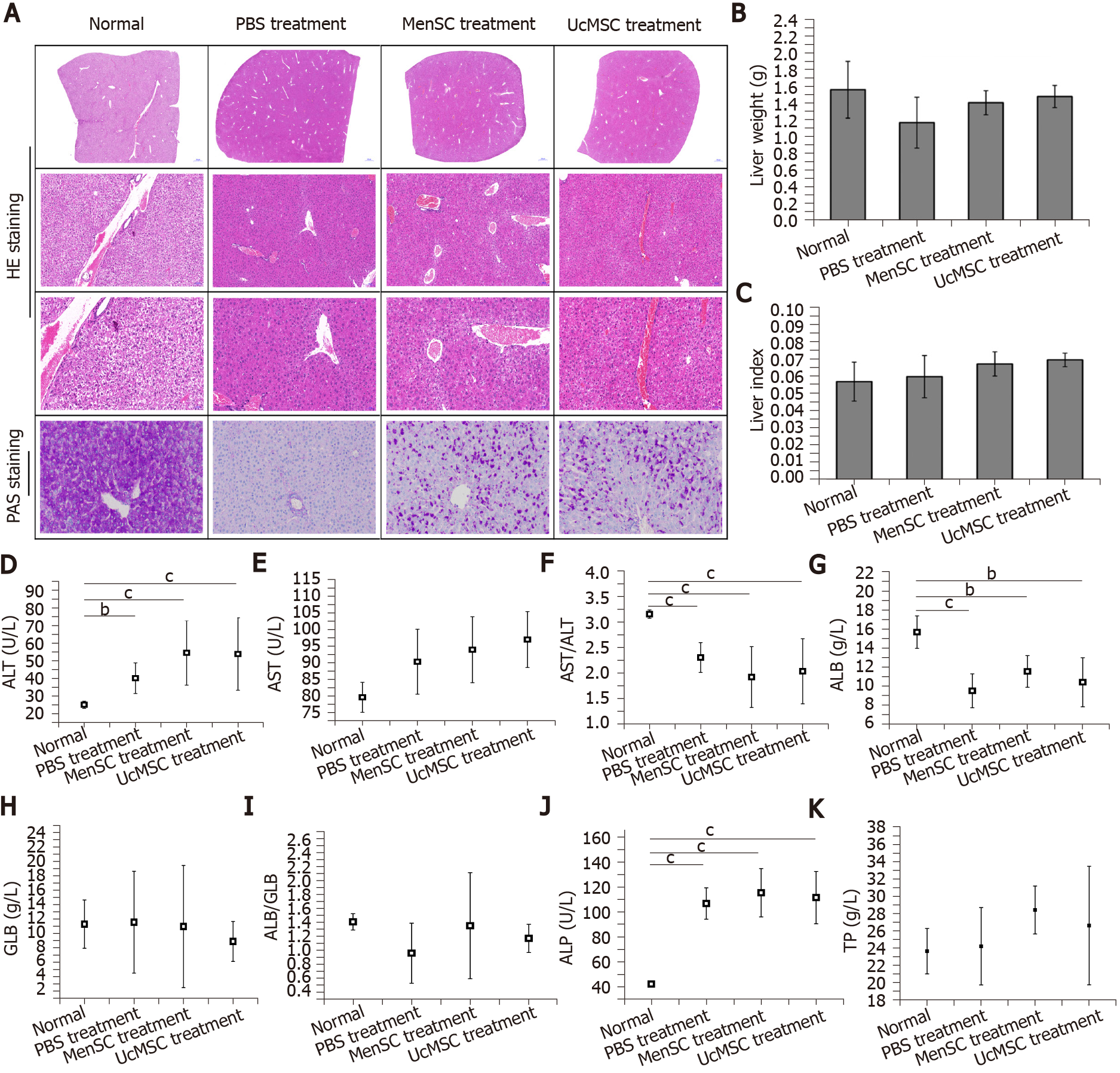
The liver is the main target organ of insulin and is highly likely to be damaged by diabetes-induced chronic complications[22]. After treatment with MenSC and UcMSC, liver lobule destruction and pseudolobule formation were improved, and liver injury was relieved (Figure 3). MenSC and UcMSC transplantation increased the liver weights and liver indexes of T1D mice, but these parameters were not significantly different from those of the PBS-treated mice (Figure 3B and C). Subsequent PAS staining showed that the rose-red plaque areas in the liver tissues of MSC-treated mice were significantly increased (Figure 3A), suggesting that both MenSC and UcMSC transplantation improved the ability of T1D mice to synthesize glycogen in their livers. Additionally, the liver functional indexes of mice treated with MSC were similar to those of the control mice, and no significant differences were observed between the two groups (Figure 3D-K).
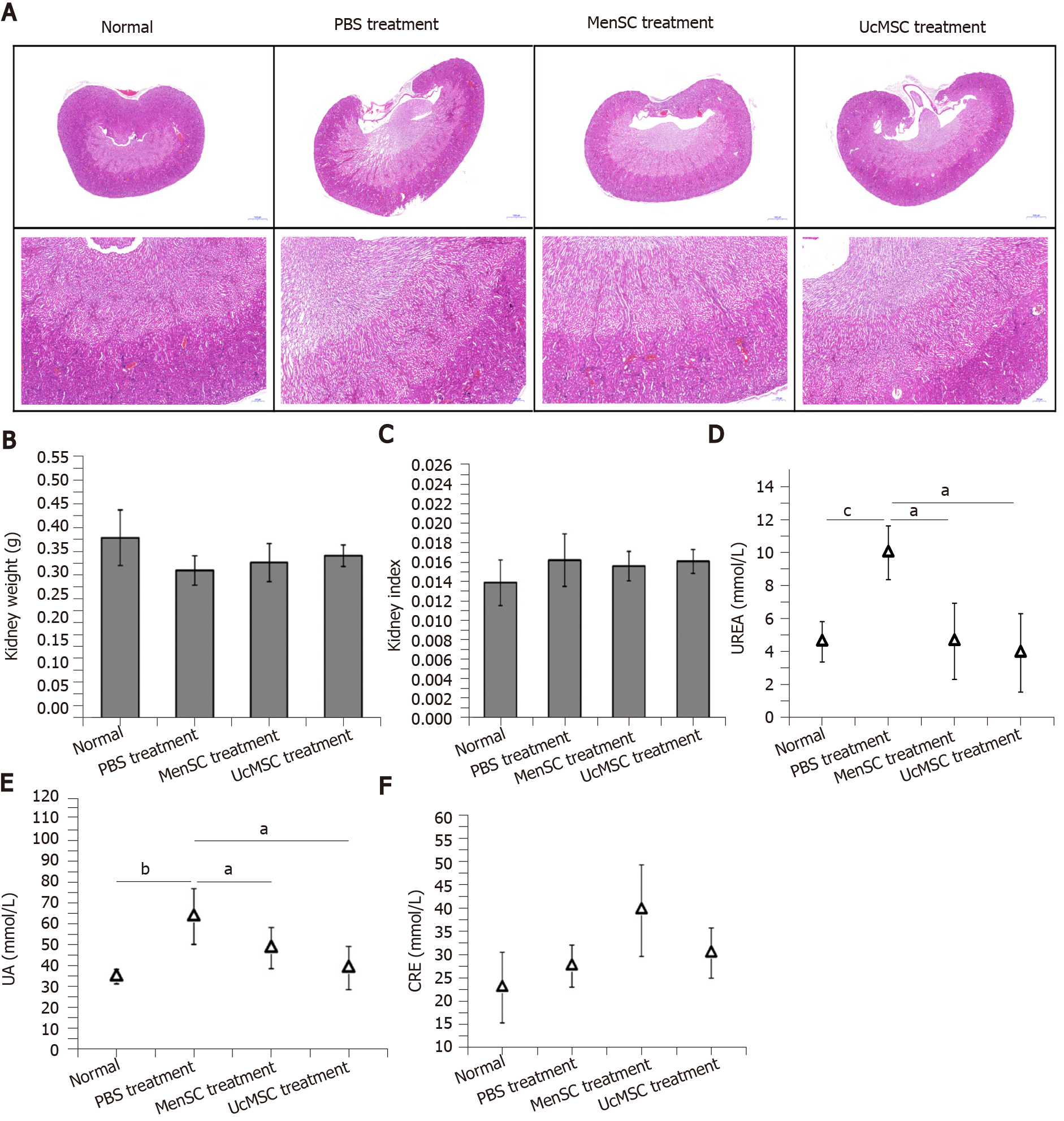
The kidney is another organ commonly affected by diabetes, and early diagnosis and prevention can delay the onset and development of diabetic nephropathy, which is critical for improving the survival rate and quality of life of patients[23]. No marked morphological differences were observed in the kidneys of normal mice and STZ-induced T1D mice treated with or without MSC (Figure 4). Although the kidney weight of MSC-treated mice was slightly increased compared with that of PBS-treated mice, the difference was not significant (Figure 4B and C). Additionally, subsequent biochemical assays showed that the UREA and UA levels in MSC-treated mice were significantly downregulated (Figure 4D and E).
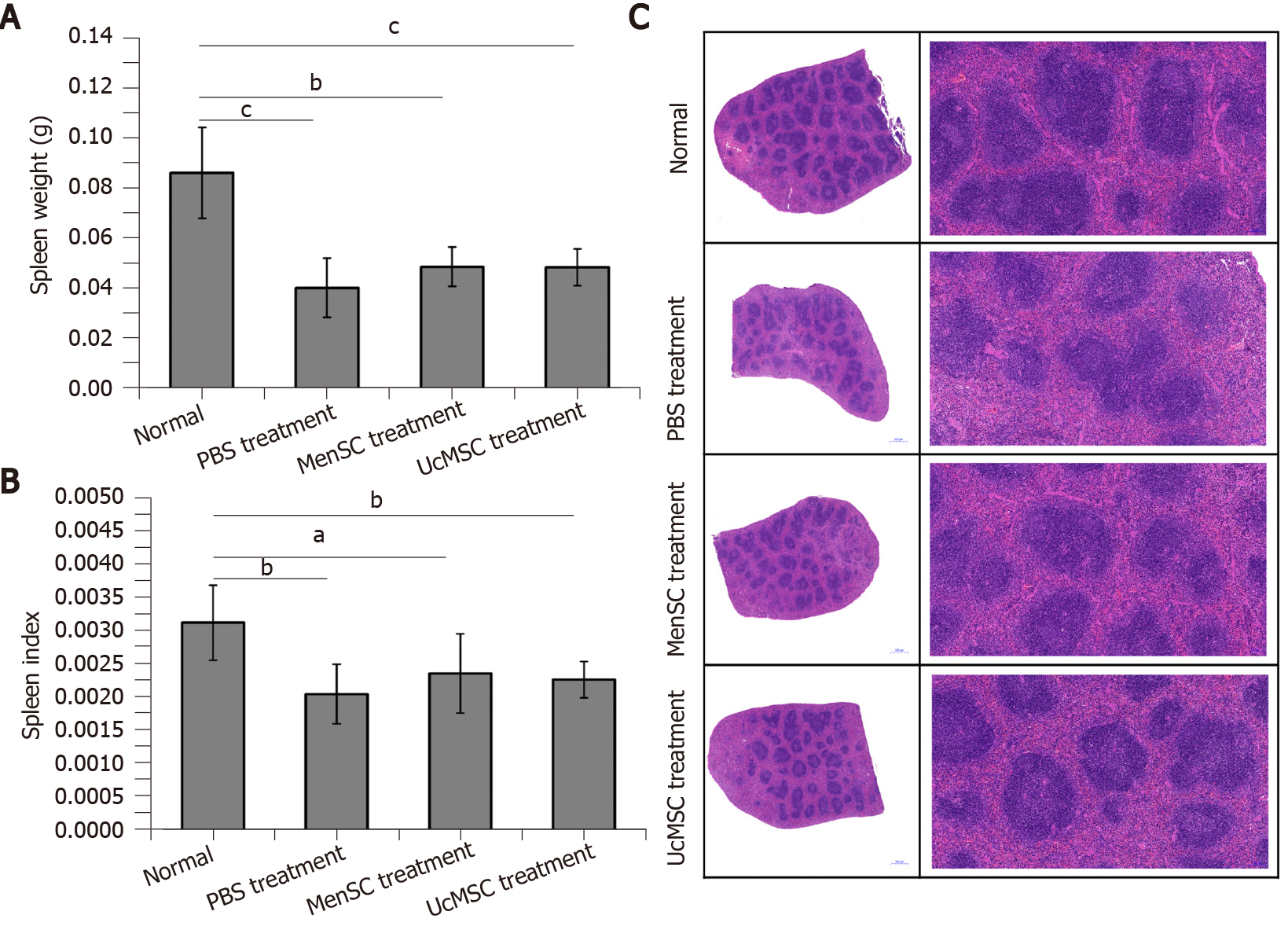
Dysfunction of the spleen, an important immune organ, is closely related to T1D[24]. In the present study, the spleen weights and spleen indexes of STZ-induced T1D mice were significantly reduced compared with those of control mice, and MSC treatment increased the mouse spleen weight; however, the spleen weights were not significantly different between the two groups (P > 0.05; Figure 5A and B). Subsequent HE staining (Figure 5C) indicated that the white pulp of the spleen in normal mice was well developed, the splenic corpuscles were round or oval, the germinal center was obvious, the periarterial lymphatic sheath was located beside the splenic corpuscle, the proportion of white pulp and red pulp was reasonable, and the boundary was clear. However, the area of white pulp in the spleens of STZ-induced T1D mice was significantly reduced and had a disordered structure, and the lymphatic follicles were atrophied. Although the spleen weights and spleen indexes of the STZ-induced T1D mice were not significantly increased after MSC treatment, the degree of lymphatic follicle atrophy in MSC-treated mice was improved, and the white pulp area was increased.
Diabetes, a common metabolic disease, is characterized by long-term high blood sugar levels and hormone disruption, which can cause severe complications in the eyes, liver, kidneys, and immune system. Currently, approximately 451 million patients have diabetes worldwide, and that number is estimated to reach 693 million by 2045[25]; diabetes causes at least five million deaths and costs at least $850 billion in health care costs annually. Additionally, MSC transplantation is considered to be an effective treatment for improving diabetes-derived symptoms and complications when considering the limitations of conventional diabetes treatments, and the efficacy and safety of MSC transplantation to improve diabetic symptoms have been confirmed by many preclinical and clinical studies[15,18,26-28]. In addition to the frequently used UcMSC, MenSC have attracted notable attention as a promising alternative stem cell-based therapy for various diseases based on their wide-ranging advantages, such as their regular and noninvasive collection method, abundant availability, superior proliferative capacity and autologous transplantation potential. Furthermore, published clinical trials on MenSC-based therapies have reported no adverse events for any of the enrolled patients. Medistem Inc. launched a phase II clinical trial on MenSC treatment for patients with congestive heart failure, and the follow-up results revealed no adverse events associated with 17 patients who received MenSC stem cell transplantation[17]. A subsequent clinical report also confirmed the therapeutic effect and safety of autologous MenSC transplantation for patients with Asherman’s syndrome[29]. Additionally, six clinical studies on MenSC-based therapies have been officially approved by the National Health Commission of the People's Republic of China due to the superior biological characteristics of MenSC.
Based on published reports, the therapeutic mechanism of MSC in patients with diabetes is mainly focused on angiogenesis promotion, immunomodulation, islet β cell regeneration and islet β cell apoptosis inhibition[30-32]. Consistent with published results, MenSC treatment exerted similar positive effects on STZ-induced T1D mice and significantly reduced their FBG levels. Subsequent histological examinations revealed that MSC transplantation significantly increased the number of insulin+ islet β cells in the T1D mouse pancreas, and the enhancement of angiogenesis, antiapoptotic effects and regeneration likely contributed to these effects. Furthermore, subsequent protein assays confirmed the presence of systemically reduced inflammation (downregulation of IL-1β and TNFα) and enhanced angiogenesis potential (upregulation of VEGF) in MSC-treated mice. Additionally, MSC treatment was shown to upregulate the expression of IL-6 in the sera of T1D mice, and a low dose of IL-6 counteracted the cytotoxicity of IL-1β on islet β cells and stimulated insulin secretion by islet β cells[33,34].
Furthermore, because of the severe diabetes-derived complications in other organs, attention should be paid to not only the improvement of pancreatic function but also the therapeutic effects of MSC on these other organs. Additionally, the prevention of diabetes-derived complications could effectively increase the survival and improve the quality of life of patients with diabetes. Our previous studies revealed that MenSC resided in the livers and kidneys of mice after injection via the tail vein, providing direct evidence of the MSC-mediated improvement in the functions of these organs. Therefore, the morphologies and functions of the livers and kidneys (highly subject to diabetic complications) of STZ-induced T1D mice were examined after MSC transplantation. As expected, MenSC treatment partially improved the functions of the liver and kidney, and no visible morphological abnormalities were observed in these organs. Additionally, spleen dysfunction plays a role in the onset and development of T1D, and a reasonable ratio of white pulp to red pulp in the spleen plays a critical role in the maintenance of immune homeostasis. Our results indicated that MSC treatment did not significantly increase the spleen weight in STZ-induced T1D mice but did improve the degree of lymphatic follicle atrophy and the ratio of white pulp to red pulp, which contribute to re-establishing immune homeostasis.
In conclusion, the therapeutic effects of MenSC transplantation are equal to those of UcMSC and can significantly improve the symptoms of T1D mice and exert protective effects on their main organs. Moreover, MSC-induced angiogenesis, antiapoptotic effects and immunomodulation contribute to these protective effects. Additionally, MenSC are expected to become a promising alternative for diabetes treatment in the clinic due to their advantages, including their regular and noninvasive collection method, abundant availability, and superior proliferative capacity.
Type 1 diabetes (T1D), a chronic metabolic disease that lacks an effective cure, seriously endangers human health. In recent years, mesenchymal stem cell (MSC) transplantation has become an effective treatment for diabetes. Menstrual blood-derived endometrial stem cells (MenSC), a novel MSC type derived from the decidual endometrium during menstruation, are expected to become promising seeding cells for diabetes treatment due to their therapeutic effects on many diseases.
T1D is a highly prevalent disease and lacks an effective treatment. MenSC are expected to become promising seeding cells for diabetes treatment in the clinic.
The objective of our study was to evaluate the therapeutic effects of MenSC on a T1D mouse model.
Streptozotocin (STZ) was used to induce the T1D mouse model. Then, improvements in the blood glucose levels and biochemical indexes of the mice were detected after the injection of MenSC via their tail vein. Moreover, the morphological and functional improvements in the livers, spleens and kidneys of MenSC-treated T1D model mice were examined.
In the STZ-induced T1D model, MenSC transplantation significantly improved the symptoms of T1D mice. Immunofluorescence and western blot analyses revealed that the numbers of insulin+ cells and CD31+ cells in the pancreas were significantly increased in MenSC-treated mice compared with control mice and inhibited the apoptosis of pancreatic cells. Additionally, protein arrays showed that MenSC transplantation significantly downregulated the serum levels of interferon γ and tumor necrosis factor α and upregulated the serum levels of interleukin-6 and vascular endothelial growth factor in the model mice. Subsequent histological and immunohistochemical analyses demonstrated that MSC transplantation systematically improved the morphologies and functions of the liver, kidneys, and spleen in the T1D model mice and effectively alleviated the complications of T1D.
The therapeutic effects of MenSC transplantation are equal to those of umbilical cord-derived MSC and can significantly improve the symptoms of T1D mice and exert protective effects on their main organs. MenSC are expected to become promising seeding cells for the treatment of T1D.
In the STZ-induced T1D mouse model, MenSC can effectively improve the symptoms and complications of T1D and lay a foundation for the clinical use of MenSC in the treatment of T1D.
The authors would like to thank Ya-Nan He for her assistance with the stem cell cultures.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Hadhrami R, Chrcanovic BR, Hamad ARA, Herold Z S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 946] [Article Influence: 189.2] [Reference Citation Analysis (0)] |
| 2. | Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1776] [Article Influence: 148.0] [Reference Citation Analysis (1)] |
| 3. | Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 519] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 4. | Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 902] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 5. | Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 6. | Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77:2771-2794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 7. | Mishra VK, Shih HH, Parveen F, Lenzen D, Ito E, Chan TF, Ke LY. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 8. | Ghoneim MA, Refaie AF, Elbassiouny BL, Gabr MM, Zakaria MM. From Mesenchymal Stromal/Stem Cells to Insulin-Producing Cells: Progress and Challenges. Stem Cell Rev Rep. 2020;16:1156-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Stiner R, Alexander M, Liu G, Liao W, Liu Y, Yu J, Pone EJ, Zhao W, Lakey JRT. Transplantation of stem cells from umbilical cord blood as therapy for type I diabetes. Cell Tissue Res. 2019;378:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Chandravanshi B, Bhonde RR. Human Umbilical Cord-Derived Stem Cells: Isolation, Characterization, Differentiation, and Application in Treating Diabetes. Crit Rev Biomed Eng. 2018;46:399-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Choi KS, Shin JS, Lee JJ, Kim YS, Kim SB, Kim CW. In vitro trans-differentiation of rat mesenchymal cells into insulin-producing cells by rat pancreatic extract. Biochem Biophys Res Commun. 2005;330:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Chen J, Zheng CX, Jin Y, Hu CH. Mesenchymal stromal cell-mediated immune regulation: A promising remedy in the therapy of type 2 diabetes mellitus. Stem Cells. 2021;39:838-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Li Y, Wang F, Liang H, Tang D, Huang M, Zhao J, Yang X, Liu Y, Shu L, Wang J, He Z. Efficacy of mesenchymal stem cell transplantation therapy for type 1 and type 2 diabetes mellitus: a meta-analysis. Stem Cell Res Ther. 2021;12:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 14. | Hsiao CY, Chen TH, Huang BS, Chen PH, Su CH, Shyu JF, Tsai PJ. Comparison between the therapeutic effects of differentiated and undifferentiated Wharton's jelly mesenchymal stem cells in rats with streptozotocin-induced diabetes. World J Stem Cells. 2020;12:139-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 15. | Chen JY, Mou XZ, Du XC, Xiang C. Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins. Asian Pac J Trop Med. 2015;8:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Riordan NH, Morales I, Fernández G, Allen N, Fearnot NE, Leckrone ME, Markovich DJ, Mansfield D, Avila D, Patel AN, Kesari S, Paz Rodriguez J. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J Transl Med. 2018;16:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 17. | Bockeria L, Bogin V, Bockeria O, Le T, Alekyan B, Woods EJ, Brown AA, Ichim TE, Patel AN. Endometrial regenerative cells for treatment of heart failure: a new stem cell enters the clinic. J Transl Med. 2013;11:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Murphy MP, Wang H, Patel AN, Kambhampati S, Angle N, Chan K, Marleau AM, Pyszniak A, Carrier E, Ichim TE, Riordan NH. Allogeneic endometrial regenerative cells: an "Off the shelf solution" for critical limb ischemia? J Transl Med. 2008;6:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Liu Y, Niu R, Yang F, Yan Y, Liang S, Sun Y, Shen P, Lin J. Biological characteristics of human menstrual blood-derived endometrial stem cells. J Cell Mol Med. 2018;22:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Wu X, Luo Y, Chen J, Pan R, Xiang B, Du X, Xiang L, Shao J, Xiang C. Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev. 2014;23:1245-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Goyal SN, Reddy NM, Patil KR, Nakhate KT, Ojha S, Patil CR, Agrawal YO. Challenges and issues with streptozotocin-induced diabetes - A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem Biol Interact. 2016;244:49-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 266] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 22. | Dewidar B, Kahl S, Pafili K, Roden M. Metabolic liver disease in diabetes - From mechanisms to clinical trials. Metabolism. 2020;111S:154299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 23. | Nistala R, Savin V. Diabetes, hypertension, and chronic kidney disease progression: role of DPP4. Am J Physiol Renal Physiol. 2017;312:F661-F670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Bellinger DL, Lorton D. Sympathetic Nerve Hyperactivity in the Spleen: Causal for Nonpathogenic-Driven Chronic Immune-Mediated Inflammatory Diseases (IMIDs)? Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Haddad D, Al Madhoun A, Nizam R, Al-Mulla F. Role of Caveolin-1 in Diabetes and Its Complications. Oxid Med Cell Longev. 2020;2020:9761539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | Bozorgmehr M, Gurung S, Darzi S, Nikoo S, Kazemnejad S, Zarnani AH, Gargett CE. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front Cell Dev Biol. 2020;8:497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 27. | Rodrigues MC, Lippert T, Nguyen H, Kaelber S, Sanberg PR, Borlongan CV. Menstrual Blood-Derived Stem Cells: In Vitro and In Vivo Characterization of Functional Effects. Adv Exp Med Biol. 2016;951:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 28. | Garrido R, Castillo L, Hernández G, Abarca J, Montes JM, Monsalve V, Espinoza R, Valenzuela S, Pérez G. [Systemic leptospirosis as a cause of multiple organ failure. Report of a case]. Rev Med Chil. 1996;124:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 29. | Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, Xu X, Kong L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman's syndrome. Hum Reprod. 2016;31:2723-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 30. | Ohnishi S, Yasuda T, Kitamura S, Nagaya N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells. 2007;25:1166-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic β Cell Regeneration as a Possible Therapy for Diabetes. Cell Metab. 2018;27:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 32. | Chen J, Chen J, Cheng Y, Fu Y, Zhao H, Tang M, Lin N, Shi X, Lei Y, Wang S, Huang L, Wu W, Tan J. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res Ther. 2020;11:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 33. | Buschard K, Aaen K, Horn T, Van Damme J, Bendtzen K. Interleukin 6: a functional and structural in vitro modulator of beta-cells from islets of Langerhans. Autoimmunity. 1990;5:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Shimizu H, Sato N, Tanaka Y, Ohtani K, Fukatsu A, Mori M. Interleukin-6 stimulates insulin secretion in HIT-T 15 cells. Horm Metab Res. 1995;27:37-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |