Copyright
©The Author(s) 2023.
World J Stem Cells. May 26, 2023; 15(5): 490-501
Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.490
Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.490
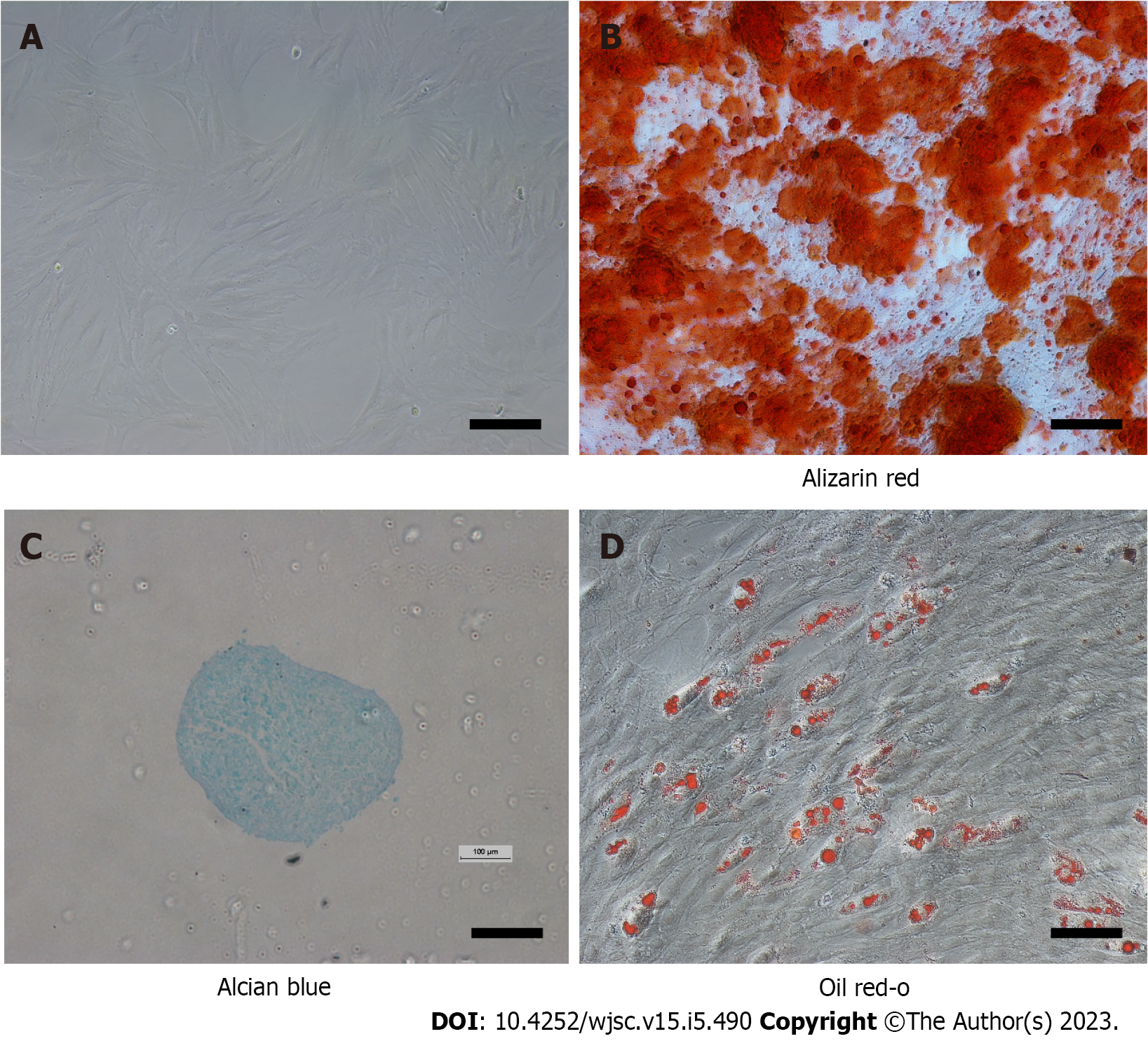
Figure 1 Characterization of mesenchymal stem cells.
A: At passage 3, the cells resembled fibroblasts. Scale bar = 100 μm; B: Differentiation into bone cells was demonstrated by alizarin red S staining. Scale bar = 100 μm; C: Alcian blue staining indicated that the cells had successfully transformed into chondrocytes. Scale bar = 500 μm; D: Oil red O staining confirmed differentiation of the cells into adipose cells. Scale bar = 100 μm.
Figure 2 Expression of C-X-C chemokine receptor type 4 on rat mesenchymal stem cells.
Representative image of the expression of C-X-C chemokine receptor type 4 (green fluorescence) on mesenchymal stem cell membranes. CXCR4: C-X-C chemokine receptor type 4; DAPI: 4',6-diamidino-2-phenylindole.
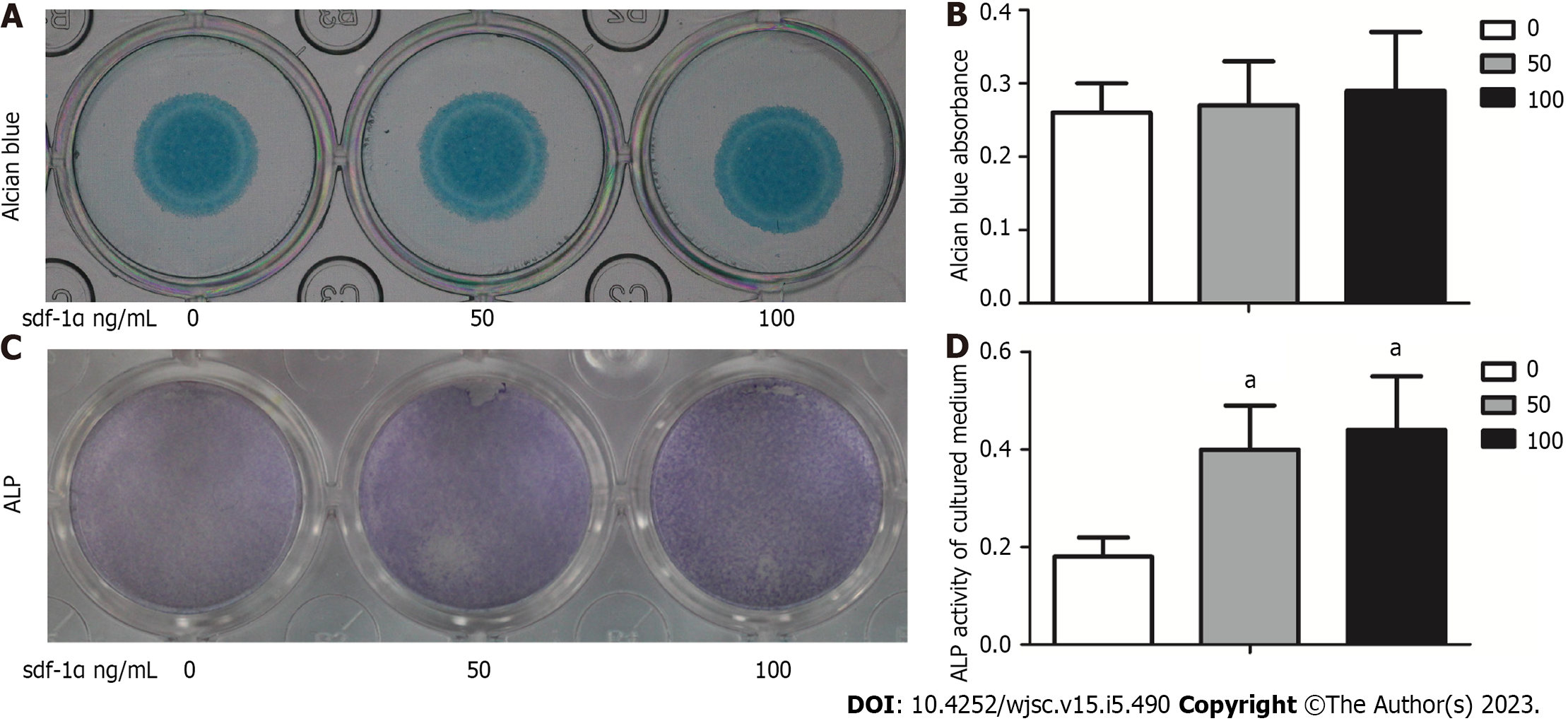
Figure 3 Alkaline phosphatase activity levels in mesenchymal stem cells treated with stromal cell-derived factor-1α were noted in the absence of an effect on cartilage formation.
A: Mesenchymal stem cells (MSCs) were cultured in vitro and stained with Alcian blue following 7 d of culture with or without stromal cell-derived factor-1α treatment; B: Alcian blue staining was measured after chemical extraction by measuring the absorbance of the supernatant at 600 nm; C: MSCs were positive for alkaline phosphatase (ALP; light purple staining); D: ALP expression was quantitatively analyzed. The values were representative of the mean ± standard deviation (n = 3). aP < 0.05 vs control. ALP: Alkaline phosphatase; sdf-1α: Stromal cell-derived factor-1α.
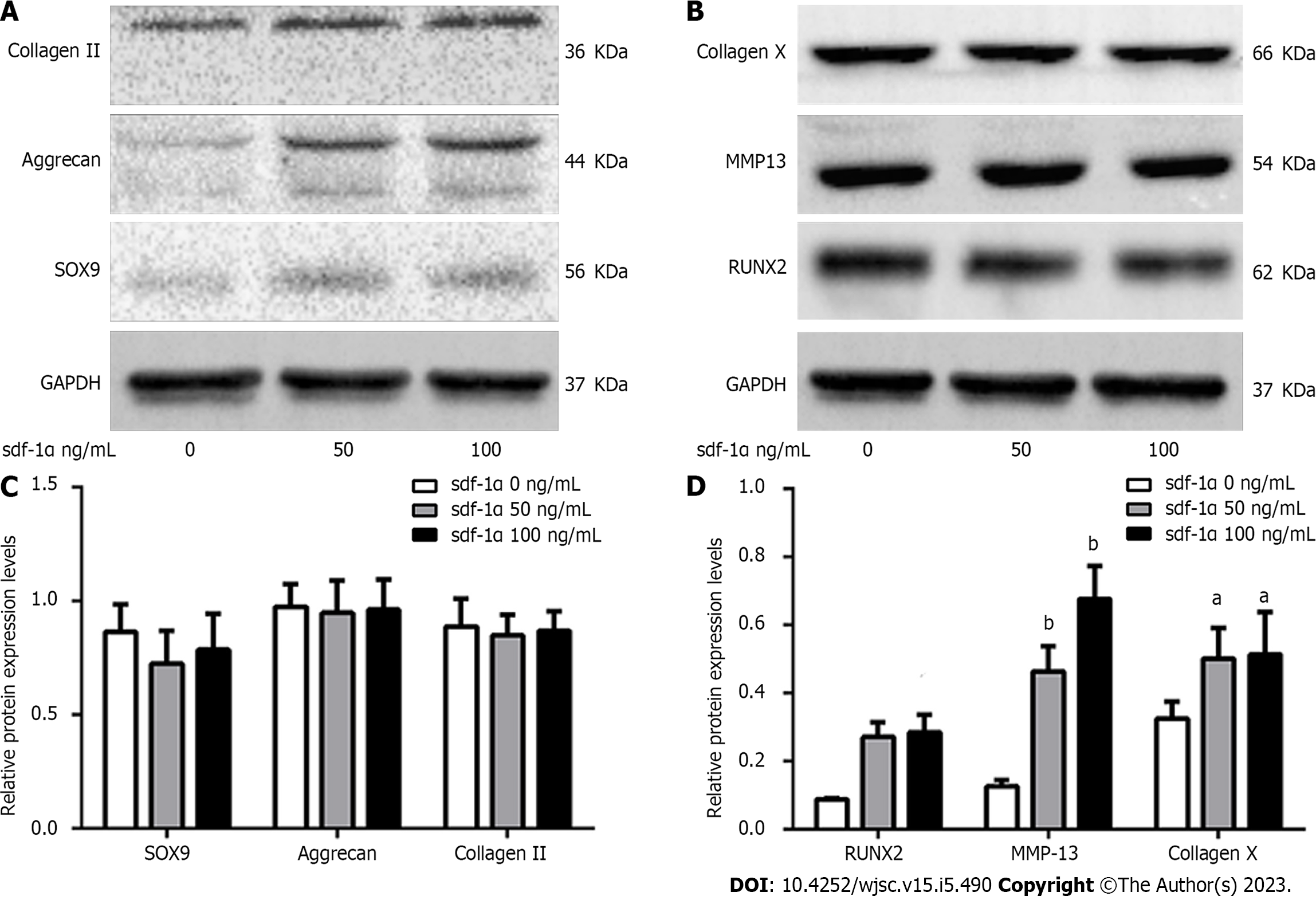
Figure 4 Effects of stromal cell-derived factor-1α on cartilage differentiation of mesenchymal stem cells.
Representative images of western blot analysis of rat mesenchymal stem cells treated with stromal cell-derived factor-1α. A: No changes in the expression levels of SRY-box transcription factor 9 (Sox9), aggrecan, and collagen II were observed; B: Increased expression levels of Runt-related transcription factor 2 (RUNX2), collagen X, and matrix metalloproteinase 13 (MMP13) were observed; C: Relative Sox9, aggrecan, and collagen II protein expression; D: Relative RUNX2, collagen X, and MMP13 protein expression. aP < 0.05 vs control (Student’s t-test). bP < 0.01 vs control (Student’s t-test). sdf-1α: Stromal cell-derived factor-1α; Sox9: SRY-box transcription factor 9; RUNX2: Runx family transcription factor 2; MMP13: Matrix metalloproteinase 13.
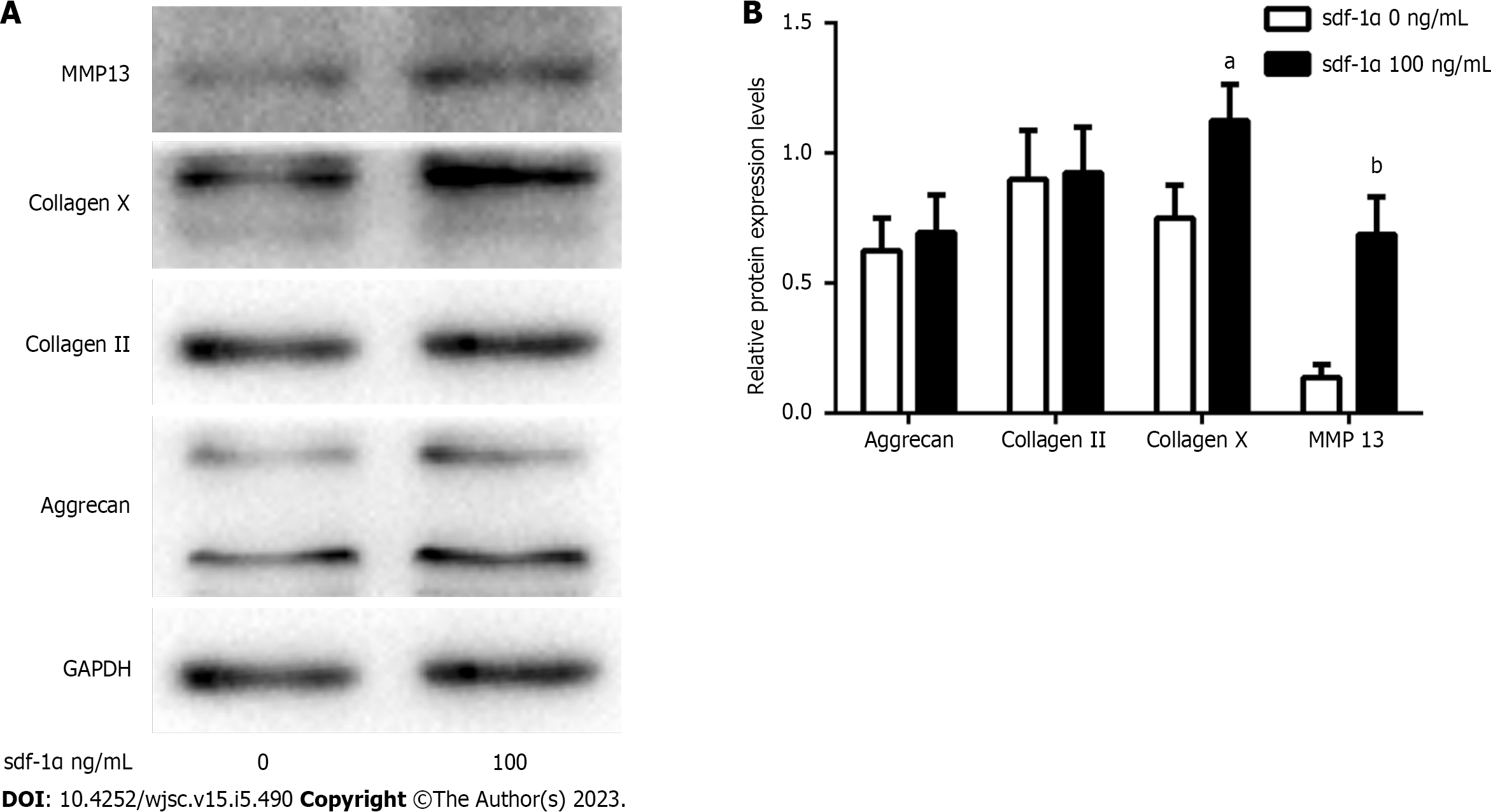
Figure 5 Effects of stromal cell-derived factor-1α on the cartilage phenotype of primary rat chondrocytes.
A: Expression levels of collagen II, aggrecan, collagen X, and matrix metalloproteinase 13 (MMP13) were determined by western blotting in primary chondrocytes treated with stromal cell-derived factor-1α (100 ng/mL); B: Relative collagen II, aggrecan, collagen X, and MMP13 protein expression. aP < 0.05 vs control (Student’s t-test), bP < 0.01 vs control (Student’s t-test). MMP13: Matrix metalloproteinase 13; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; sdf-1α: Stromal cell-derived factor-1α.
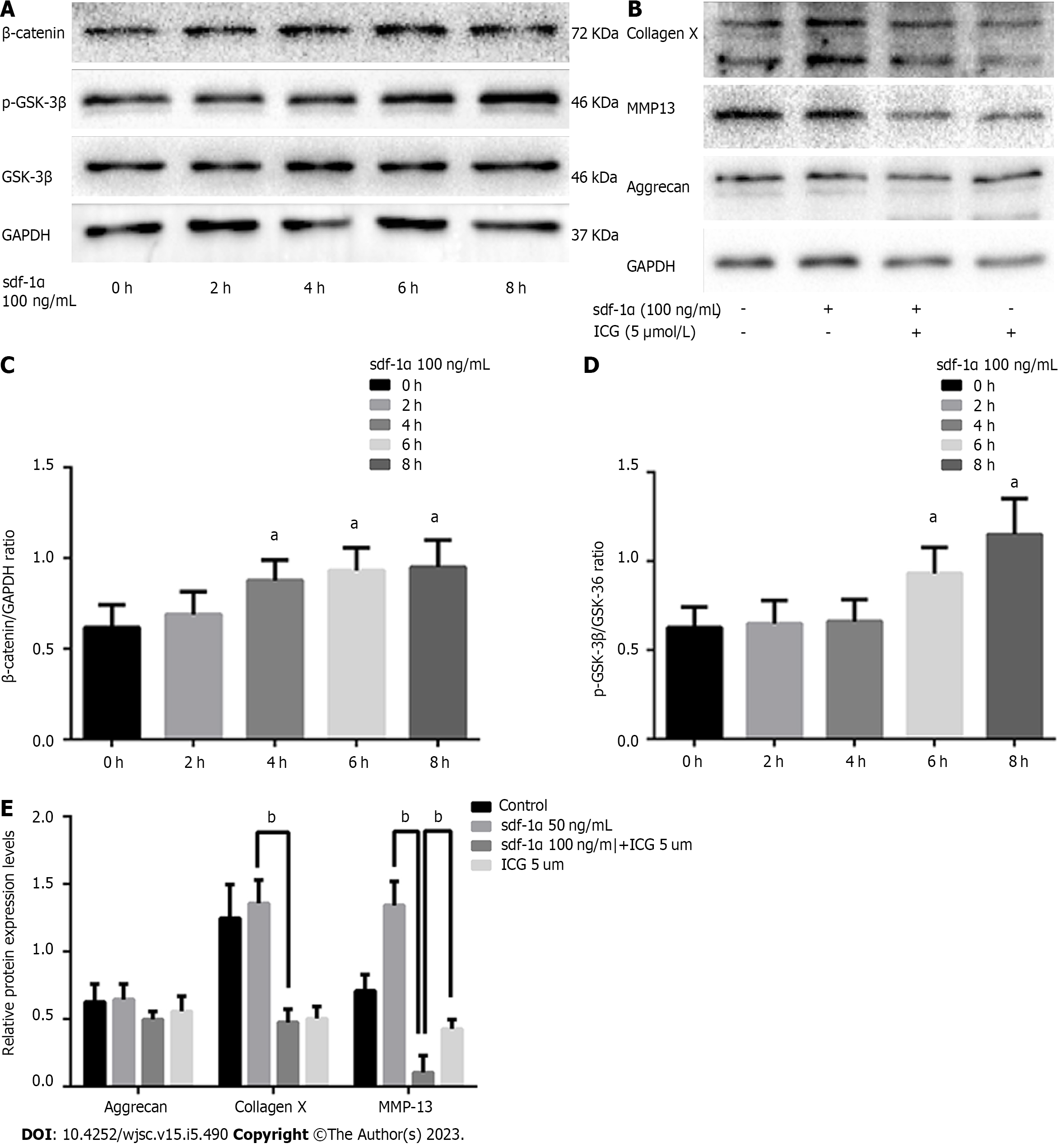
Figure 6 Wnt/β-catenin pathway involvement in the effects of stromal cell-derived factor-1α on cartilage differentiation.
A: Expression levels of β-catenin, p-glycogen synthase kinase 3β (p-GSK-3β), and GSK-3β by western blotting; B: Blockage of the Wnt/β-catenin pathway with ICG-001 inhibited the expression levels of collagen X and matrix metalloproteinase 13; C: Relative β-catenin protein expression; D: Ratio of relative protein expression of p-GSK-3β to relative protein expression of GSK-3β (p-GSK-3β/GSK-3β); E: Relative aggrecan, collagen X, and MMP13 protein expression. 1P < 0.05, bP < 0.01, Student’s t-test. p-GSK-3β: p-glycogen synthase kinase 3β; GSK-3β: Glycogen synthase kinase 3β; MMP13: Matrix metalloproteinase 13; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; sdf-1α: Stromal cell-derived factor-1α.
- Citation: Chen X, Liang XM, Zheng J, Dong YH. Stromal cell-derived factor-1α regulates chondrogenic differentiation via activation of the Wnt/β-catenin pathway in mesenchymal stem cells. World J Stem Cells 2023; 15(5): 490-501
- URL: https://www.wjgnet.com/1948-0210/full/v15/i5/490.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i5.490