Published online Sep 14, 2022. doi: 10.3748/wjg.v28.i34.5036
Peer-review started: February 7, 2022
First decision: April 10, 2022
Revised: May 1, 2022
Accepted: July 25, 2022
Article in press: July 25, 2022
Published online: September 14, 2022
Processing time: 212 Days and 5.7 Hours
Severe alcoholic hepatitis (AH) is one of the most lethal manifestations of alcohol-associated liver disease. In light of the increase in alcohol consumption worldwide, the incidence of AH is on the rise, and data examining the trends of AH admission is needed.
To examine inpatient admission trends secondary to AH, along with their clinical outcomes and epidemiological characteristics.
The National Inpatient Sample (NIS) database was utilized, and data from 2011 to 2017 were reviewed. We included individuals aged ≥ 21 years who were admitted with a primary or secondary diagnosis of AH using the International Classification of Diseases (ICD)-9 and its correspondent ICD-10 codes. Hepatitis not related to alcohol was excluded. The national estimates of inpatient admissions were obtained using sample weights provided by the NIS.
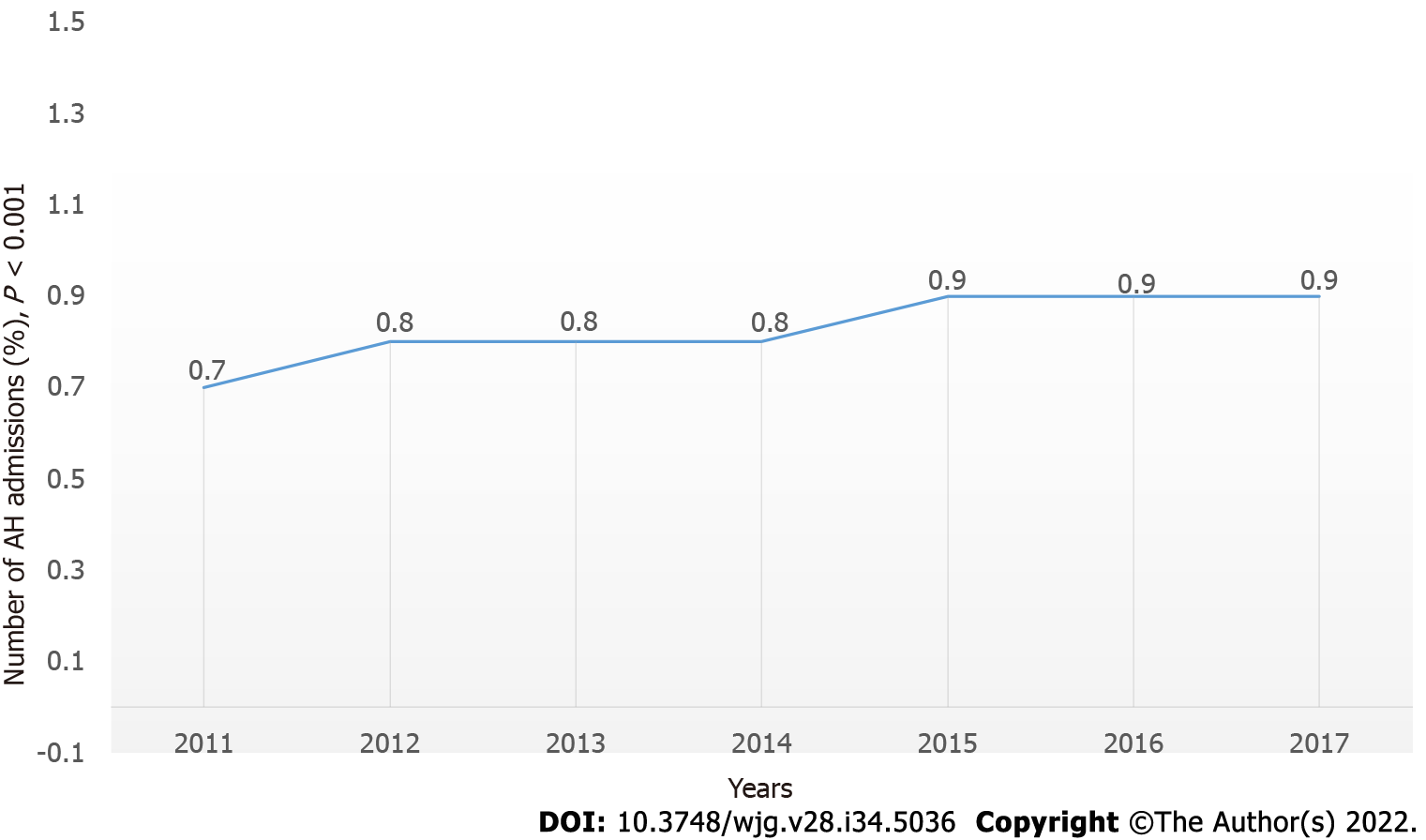
AH-related hospitalization demonstrated a significant increase in the USA from 281506 (0.7% of the total admission in 2011) to 324050 (0.9% of the total admi
The number of AH inpatient hospitalizations significantly increased from 2011 to 2017. This could have a substantial financial impact with increasing healthcare costs and utilization. AH-mortality remained the same.
Core tip: This study demonstrated a significant increase in the number of hospitalizations due to alcoholic-associated hepatitis (AH) throughout the USA, with an overall increase in the cost and financial burden of the disease. These trends were in line with the increase in the incidence of alcohol misuse across the years. This study provides potential data for future prospective research to help trigger more aggressive screening and prevention methods for alcohol abuse to prevent AH. Additionally, there is a need for the development of novel therapeutic agents targeting the disease since AH treatment is limited.
- Citation: Wakil A, Mohamed M, Tafesh Z, Niazi M, Olivo R, Xia W, Greenberg P, Pyrsopoulos N. Trends in hospitalization for alcoholic hepatitis from 2011 to 2017: A USA nationwide study. World J Gastroenterol 2022; 28(34): 5036-5046
- URL: https://www.wjgnet.com/1007-9327/full/v28/i34/5036.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i34.5036
Alcohol-associated liver disease (ALD) comprises a spectrum of liver diseases ranging from reversible fatty liver to severe alcohol-associated hepatitis (AH) and cirrhosis, to acute-on-chronic liver failure[1]. All manifestations of ALD may overlap and can develop after heavy alcohol consumption for at least 6 mo[2]. AH is a clinical syndrome with acute onset jaundice in the presence of heavy alcohol misuse[2]. Mild AH patients with alcohol abstinence can have a good outcome. However, those with severe disease defined by Maddrey’s discriminant function ≥ 32, or model for end-stage liver disease score ≥ 20, have a high 30-d mortality rate[1]. ALD is one of the leading causes of cirrhosis and it is the second leading indication for liver transplantation in the USA according to the Scientific Registry of Transplant Recipients data[3]. Alcohol consumption continues to be on the rise, with global data extrapolated from the World Health Organization showing the average annual per capita alcohol consumption has risen from 5.5 L in 2005 to 6.4 L in 2018[4]. Consequently, global alcohol-attributable mortality in 2016 has increased to 38.8 per 100000 people and 1759 disability-adjusted life-years per 100000 people[4]. According to a study from Denmark, during the study period (1999–2008), the annual incidence rate of AH was reported to increase for both men and women from 37 to 46 per 100000 and from 24 to 34 per 100 000, respectively[5]. The 5-year overall mortality in this study was found to be 56% and was significantly higher in patients with cirrhosis compared to patients without cirrhosis (69% vs 47%, respectively)[5]. In the USA, a study evaluating the United States National Inpatient Sample (NIS) database showed that out of 325 000 hospital admissions in 2010, 0.8% were AH-related[6]. With the availability of highly effective direct-acting antivirals therapy for chronic hepatitis C, the burden of chronic liver disease (CLD) is shifting towards ALD and non-ALD[7,8]. Studies have demonstrated that there is an increasing trend in consuming alcohol in the USA[9-12], with the initiation of alcohol at younger ages[13-15], and binging representing the most frequent pattern of alcohol consumption[16]. As a consequence of this upward trend in alcohol use disorder, it is anticipated that the correlating rise in ALD will have significant health, social and economic burdens accredited to the increase in hospitalization rate, as well as the elevated support required for these patients in an outpatient setting[8]. According to the 2007 NIS database, a significant healthcare cost and use of resources were reported[17]. Another study using the NIS database from 2012 to 2016, reported a higher admissions rate for CLD with a 26.2% increase in hospitalization costs and an $18.8 billion economic burden in 2016[18]. Despite the advances in medicine, the currently available therapeutics for severe AH are scarce and only limited to corticosteroids, which contributes to the high mortality of this disease[1].
Given the increased prevalence of alcohol misuse in the USA, this study aimed to provide a relatively recent descriptive analysis of trends in AH hospitalization within the USA. Data were obtained using the NIS database from 2011 to 2017.
The Healthcare Cost and Utilization Project (HCUP) is a collection of databases and contains the NIS database[19]. The NIS is the largest publicly available inpatient database that encompasses a range of different data encoded by International Classification of Diseases (ICD) codes from more than 1000 hospitals, constituting a 20% sample data of all US hospitals. The database enables data extraction on a broad range of health conditions and specific populations, including cost and quality of health services, medical practice patterns, and outcomes of treatments on a national level.
This was a retrospective analysis. All subjects in the database ≥ 21 years old who were hospitalized with a discharge diagnosis of AH from 2011 to 2017 were included. To minimize ascertainment bias, we classified hospitalization as AH-related if it was associated with a primary admission diagnosis of AH or a primary discharge diagnosis. A secondary AH-related hospitalization was classified as having the AH diagnosis anywhere in the admission diagnosis (25 diagnoses) or anywhere in the discharge diagnoses. We excluded all non-alcohol-related hepatitis diagnoses using ICD-9 and ICD-10. This included autoimmune hepatitis, acute and chronic viral hepatitis A–E, and nonalcoholic steatohepatitis. All data were weighted using discharge level values, to produce an accurate estimate of the patient population nationwide. AH-related hospitalization was identified by ICD-9 and ICD-10 discharge diagnosis codes. The ICD codes included were alcohol-associated hepatitis, alcoholic fatty liver disease, and alcohol-associated cirrhosis (Table 1). Previous validation studies have shown that the discharge diagnosis captures the cause of hospitalization accurately, such as in primary biliary cholangitis[20], coronary artery disease[21], and hepatitis B and C[11]. Other outcomes examined were inpatient mortality. We identified known risk factors related to mortality in patients with the following: acute renal failure (ARF), gastrointestinal bleeding, and sepsis (Table 2). Patient age, sex, household income, race, and geographic region (Northeast, Midwest, South and West) were obtained. The primary payer for the hospitalization was categorized as Medicare, Medicaid, private insurance, self-pay, or others. The types of hospitals were categorized into teaching, non-teaching community, and rural hospitals. Hospitalization characteristics were presented separately where the primary reason for hospitalization was AH versus AH presenting as a secondary condition. These characteristics included in-hospital mortality, length of stay, and discharge disposition.
| ICD-9 | Correspondent ICD-10 |
| 571.0 alcoholic fatty liver | K.70 alcoholic fatty liver |
| 571.1 acute alcoholic hepatitis | K.70.11 alcoholic hepatitis with ascites |
| K.70.10 alcoholic hepatitis without ascites | |
| 571.2 alcoholic cirrhosis of liver | Correspondent ICD-10 found |
| 571.3 alcoholic liver damage, unspecified | K70.40 alcoholic hepatic failure without coma |
| K70.41 alcoholic hepatic failure with coma | |
| K70.9 alcoholic liver disease, unspecified |
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | P | |
| Total admission, n | 38590733 | 36484846 | 35597792 | 35358818 | 35769942 | 35675421 | 35798453 | |
| AH-related admission, n (%) | 281506 (0.7) | 274365 (0.8) | 278580 (0.8) | 291435 (0.8) | 308765 (0.9) | 313235 (0.9) | 324050 (0.9) | < 0.001 |
| Admission when primary admission diagnosis is AH, n (%) | 47140 (0.1) | 45710 (0.1) | 46715 (0.1) | 48395 (0.1) | 54955 (0.2) | 66170 (0.2) | 71290 (0.2) | < 0.001 |
Patients were first screened and selected based on the presence of an AH diagnosis. The temporal trend of the AH-related hospitalization was tested by Cochran–Armitage test. The patient demographics, additional clinical characteristics, and clinical outcome measures were then summarized using the median with interquartile range for continuous variables, or frequencies with percentages for categorical variables. To compare these variables among different years, Kruskal–Wallis and χ2 tests were used. These demographics and variables were tested against the year. Overall and between-categorical group P values were reported for categorical variables. Prevalence of AH-related diagnosis among HCUP dataset and mortality-related risk factors among AH patients who died during hospitalization in 2011–2017 were tested using the χ2 test. A multivariable logistic model was fitted and resulting odds ratios (OR) with 95% confidence intervals were used to test for association between the risk factors and the primary outcome of in-hospital mortality, further adjusting for demographics and additional clinical characteristics. Due to the desire to have population-level interpretations, survey weights were applied to all patient-level observations as provided in the NIS database. All reported P values were two-sided, and the significance cut-off was set at 0.05. The Bonferroni adjustment was used to adjust for multiple testing in between-categorical χ2 test and logistic regression. Data analyses were completed in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R studio (R version 3.6.0, R Foundation for Statistical Computing, Vienna, Austria).
AH-related hospitalization showed an increase from 281506 (0.7% of the total in 2011) to 324050 (0.9% of the total in 2017; P < 0.01) (Figure 1). This included all AH-related admissions when AH was not the primary or secondary diagnosis. There was also an increase in the number of admissions when the primary admission diagnosis was AH (47140 in 2011 to 71290 in 2017) (P < 0.01; Table 2).
Results from demographic observations showed that the median age of patients hospitalized with AH was 54 years. The most common age group was 45–65 years (58.4%–60.7%; P < 0.01); the most common race was white (63.2%–66.4%; P < 0.01); patients were predominantly male (69.7%–71.2%; P < 0.01); and the primary healthcare payers were Medicare (29.4%–30.7%; P < 0.01) and Medicaid (21.5%–32.5%; P < 0.01). The most common geographical location was the southern region of the USA (33.6%–34.4%; P = 0.017). Most patients were admitted to tertiary hospitals (50.2%–62.3%; P < 0.01) in urban areas. The most common presenting diagnosis was AH (63.5%–69%; P < 0.01). The most common outcome was routine discharge (60%–63.3%; P < 0.01). The median length of stay was 5.98 d (SD = 7.11) in 2011 which increased to 6.14 d (SD = 7.43) in 2017 (P < 0.01). Total charge for hospitalized patients with AH ranged between $46507.47 (SD = 87193.29) and $63574.52 (SD = 108850.63; P < 0.01) (Table 3).
| Characteristic | AH-related admission | P1 | |||||||
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Overall | Between-group | |
| n | 281506 | 274365 | 278580 | 291435 | 308765 | 313235 | 324050 | ||
| Age in years at admission [median (Q1, Q3)] | 54.0 (46.0, 61.0) | 54.0 (46.0, 61.0) | 54.0 (46.0, 62.0) | 54.0 (46.0, 62.0) | 54.0 (46.0, 62.0) | 54.0 (46.0, 62.0) | 55.0 (46.0, 62.0) | < 0.001 | |
| Age group (yr) | < 0.001 | ||||||||
| < 25, n (%) | 1545 (0.5) | 1215 (0.4) | 1135 (0.4) | 1295 (0.4) | 1320 (0.4) | 1635 (0.5) | 1535 (0.5) | 0.011 | |
| 25-44, n (%) | 57738 (20.5) | 57040 (20.8) | 58155 (20.9) | 61335 (21.0) | 66670 (21.6) | 69165 (22.1) | 70935 (21.9) | < 0.001 | |
| 45-64, n (%) | 170876 (60.7) | 166030 (60.5) | 167810 (60.2) | 174765 (60.0) | 181845 (58.9) | 182905 (58.4) | 187350 (57.8) | < 0.001 | |
| ≥65, n (%) | 51348 (18.2) | 50080 (18.3) | 51480 (18.5) | 54040 (18.5) | 58930 (19.1) | 59530 (19.0) | 64230 (19.8) | < 0.001 | |
| Gender | < 0.001 | ||||||||
| Male, n (%) | 198491 (70.5) | 195350 (71.2) | 196820 (70.7) | 203735 (69.9) | 216650 (70.2) | 218295 (69.7) | 227080 (70.1) | < 0.001 | |
| Female, n (%) | 83016 (29.5) | 79000 (28.8) | 81725 (29.3) | 87615 (30.1) | 92075 (29.8) | 94810 (30.3) | 96965 (29.9) | < 0.001 | |
| Missing, n (%) | 0 (0.0) | 15 (0.0) | 35 (0.0) | 85 (0.0) | 40 (0.0) | 130 (0.0) | 5 (0.0) | ||
| Race | < 0.001 | ||||||||
| White, n (%) | 178031 (63.2) | 180550 (65.8) | 183125 (65.7) | 193560 (66.4) | 202205 (65.5) | 207155 (66.1) | 214760 (66.3) | < 0.001 | |
| Black, n (%) | 27402.8 (9.7) | 27045 (9.9) | 26625 (9.6) | 27630 (9.5) | 29145 (9.4) | 28025 (8.9) | 29290 (9.0) | < 0.001 | |
| Hispanic, n (%) | 37280 (13.2) | 36670 (13.4) | 38575 (13.8) | 38905 (13.3) | 43390 (14.1) | 45870 (14.6) | 49135 (15.2) | < 0.001 | |
| Asian or Pacific Islander, n (%) | 2235 (0.8) | 2370 (0.9) | 2680 (1.0) | 2855 (1.0) | 3565 (1.2) | 3520 (1.1) | 3840 (1.2) | < 0.001 | |
| Native American, n (%) | 5056 (1.8) | 5985 (2.2) | 5720 (2.1) | 5910 (2.0) | 6725 (2.2) | 7140 (2.3) | 7565 (2.3) | < 0.001 | |
| Other, n (%) | 7134 (2.5) | 7760 (2.8) | 6940 (2.5) | 7785 (2.7) | 7780 (2.5) | 8305 (2.7) | 9085 (2.8) | 0.030 | |
| Missing, n (%) | 24368.5 (8.7) | 13985 (5.1) | 14915 (5.4) | 14790 (5.1) | 15955 (5.2) | 13220 (4.2) | 10375 (3.2) | ||
| Median household income national quartile for patient ZIP code | < 0.001 | ||||||||
| $1-$38999, n (%) | 79993 (28.4) | 83510 (30.4) | 80530 (28.9) | 85525 (29.3) | 95660 (31.0) | 94940 (30.3) | 95785 (29.6) | < 0.001 | |
| $39000-$47999, n (%) | 69137 (24.6) | 66095 (24.1) | 71125 (25.5) | 76895 (26.4) | 72860 (23.6) | 77800 (24.8) | 83130 (25.7) | < 0.001 | |
| $48000-$62999, n (%) | 68313 (24.3) | 62360 (22.7) | 65010 (23.3) | 65695 (22.5) | 72025 (23.3) | 73690 (23.5) | 75805 (23.4) | < 0.001 | |
| $63000 or more, n (%) | 56576 (20.1) | 53795 (19.6) | 53035 (19.0) | 54360 (18.7) | 59515 (19.3) | 58905 (18.8) | 60560 (18.7) | < 0.001 | |
| Missing, n (%) | 7487.7 (2.7) | 8605 (3.1) | 8880 (3.2) | 8960 (3.1) | 8705 (2.8) | 7900 (2.5) | 8770 (2.7) | ||
| Primary expected payer | < 0.001 | ||||||||
| Medicare, n (%) | 82774 (29.4) | 81905 (29.9) | 85335 (30.6) | 88860 (30.5) | 93460 (30.3) | 94160 (30.1) | 99510 (30.7) | < 0.001 | |
| Medicaid, n (%) | 60601 (21.5) | 61975 (22.6) | 62490 (22.4) | 86040 (29.5) | 96230 (31.2) | 100255 (32.0) | 105305 (32.5) | < 0.001 | |
| Private including HMO, n (%) | 71261 (25.3) | 65885 (24.0) | 65405 (23.5) | 70445 (24.2) | 76095 (24.6) | 75795 (24.2) | 76200 (23.5) | < 0.001 | |
| Self-pay, n (%) | 45272 (16.1) | 43865 (16.0) | 42785 (15.4) | 31705 (10.9) | 28095 (9.1) | 27930 (8.9) | 28890 (8.9) | < 0.001 | |
| No charge, n (%) | 4980.6 (1.8) | 3455 (1.3) | 5330 (1.9) | 3235 (1.1) | 3195 (1.0) | 2995 (1.0) | 2610 (0.8) | < 0.001 | |
| Other, n (%) | 15296.9 (5.4) | 16515 (6.0) | 16670 (6.0) | 10555 (3.6) | 11150 (3.6) | 11625 (3.7) | 10805 (3.3) | < 0.001 | |
| Missing, n (%) | 1320 (0.5) | 765 (0.3) | 565 (0.2) | 595 (0.2) | 540 (0.2) | 475 (0.2) | 730 (0.2) | ||
| Bed size of hospital | < 0.001 | ||||||||
| Small, n (%) | 32567 (11.6) | 36820 (13.4) | 37640 (13.5) | 54105 (18.6) | 54870 (17.8) | 57875 (18.5) | 64035 (19.8) | < 0.001 | |
| Medium, n (%) | 73628 (26.2) | 77055 (28.1) | 76680 (27.5) | 86355 (29.6) | 94260 (30.5) | 91795 (29.3) | 97445 (30.1) | < 0.001 | |
| Large, n (%) | 175312 (62.3) | 160490 (58.5) | 164260 (59.0) | 150975 (51.8) | 159635 (51.7) | 163565 (52.2) | 162570 (50.2) | < 0.001 | |
| Region of hospital | 0.001 | ||||||||
| Northeast, n (%) | 53252 (18.9) | 51120 (18.6) | 51855 (18.6) | 53795 (18.5) | 56100 (18.2) | 57590 (18.4) | 59600 (18.4) | 0.16 | |
| Midwest, n (%) | 61095 (21.7) | 59575 (21.7) | 60330 (21.7) | 64160 (22.0) | 67170 (21.8) | 68655 (21.9) | 72150 (22.3) | 0.42 | |
| South, n (%) | 95441 (33.9) | 94365 (34.4) | 95705 (34.4) | 98035 (33.6) | 105260 (34.1) | 105730 (33.8) | 108555 (33.5) | 0.017 | |
| West, n (%) | 71718 (25.5) | 69305 (25.3) | 70690 (25.4) | 75445 (25.9) | 80235 (26.0) | 81260 (25.9) | 83745 (25.8) | 0.046 | |
| Location teaching status of hospital | < 0.001 | ||||||||
| Rural, n (%) | 28721 (10.2) | 27135 (9.9) | 26909.9 (9.7) | 23195 (8.0) | 23990 (7.8) | 24250 (7.7) | 25035 (7.7) | < 0.001 | |
| Urban non-teaching, n (%) | 127976 (45.5) | 113625 (41.4) | 112610 (40.4) | 84150 (28.9) | 88965 (28.8) | 87650 (28.0) | 77930 (24.0) | < 0.001 | |
| Urban teaching, n (%) | 124809 (44.3) | 133605 (48.7) | 139060 (49.9) | 184090 (63.2) | 195810 (63.4) | 201335 (64.3) | 221085 (68.2) | < 0.001 | |
The mortality of AH-related hospitalizations was 5.3% in 2011 and 5.5% in 2017 (P < 0.01). Risk factors that could be associated with AH mortality were sepsis (increased from 7.4% in 2011 to 48.3% in 2017; P < 0.01), renal failure (59.6% in 2011 to 72.1% in 2018; P < 0.01), and gastrointestinal (GI) hemorrhage (17.2% in 2011 to 20.3% in 2017; P = 0.048) (Table 4). Multivariable regression analysis on death during hospitalization was performed and demonstrated higher odds of mortality in the following group: age > 65 years (OR 3.55; P < 0.0001), female (OR 1.13; P < 0.0001), large academic hospital (OR 1.3; P < 0.0001), sepsis (OR 3.33; P < 0.0001), GI hemorrhage (OR 2.31; P < 0.0001), and ARF (OR 7.19; P < 0.0001) (Table 5).
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | P | |
| n | 15002 | 14845 | 15310 | 15885 | 16385 | 17435 | 17785 | |
| Sepsis, n (%) | 1111 (7.4) | 950 (6.4) | 980 (6.4) | 1170 (7.4) | 2850 (17.4) | 8145 (46.7) | 8595 (48.3) | < 0.001 |
| GI hemorrhage, n (%) | 2582 (17.2) | 2755 (18.6) | 2715 (17.7) | 3230 (20.3) | 3025 (18.5) | 3355 (19.2) | 3395 (19.1) | 0.042 |
| Acute renal failure, n (%) | 8941 (59.6) | 9450 (63.7) | 9780 (63.9) | 10485 (66.0) | 11265 (68.8) | 12325 (70.7) | 12825 (72.1) | < 0.001 |
| Variables | Levels | Odds ratio | ||
| Estimate | 95% confidence interval | P after Bonferroni correction2 | ||
| Age groups (yr) | < 25 | 1.0 | Reference | |
| 25-44 | 1.89 | 1.28-2.79 | 0.039 | |
| 45-64 | 2.85 | 1.93-4.21 | < 0.0001 | |
| 65/65+ | 3.55 | 2.4-5.25 | < 0.0001 | |
| Sex | Male | Reference | ||
| Female | 1.13 | 1.09-1.17 | < 0.0001 | |
| Race | White | 1.0 | Reference | |
| Black | 0.82 | 0.77-0.86 | < 0.0001 | |
| Hispanic | 0.94 | 0.9-0.98 | 0.20 | |
| Asian or Pacific Islander | 0.92 | 0.8-1.07 | 1.00 | |
| Native American | 1.04 | 0.94-1.15 | 1.00 | |
| Other | 1.12 | 1.02-1.22 | 0.36 | |
| Primary expected payer | Medicare | 1.0 | Reference | |
| Medicaid | 1.12 | 1.07-1.18 | 0.00020 | |
| Private including HMO | 1.11 | 1.06-1.17 | 0.00025 | |
| Self-pay | 1.37 | 1.29-1.45 | < 0.0001 | |
| No charge | 0.92 | 0.78-1.09 | 1.00 | |
| Other | 1.45 | 1.34-1.57 | < 0.0001 | |
| Median household income national quartile for patient ZIP code | $1-$38999 | 1.0 | Reference | |
| $39000-$47999 | 0.98 | 0.94-1.02 | 1.00 | |
| $48000-$62999 | 0.95 | 0.92-1 | 0.84 | |
| $63000 or more | 0.9 | 0.86-0.94 | 0.00021 | |
| Bed size of hospital | Small | 1.0 | Reference | |
| Medium | 1.2 | 1.14-1.26 | < 0.0001 | |
| Large | 1.3 | 1.25-1.37 | < 0.0001 | |
| Region of hospital | Northeast | 1.0 | Reference | |
| Midwest | 0.91 | 0.87-0.96 | 0.0062 | |
| South | 1 | 0.96-1.05 | 1.00 | |
| West | 1.08 | 1.03-1.14 | 0.023 | |
| Location/teaching status of hospital | Rural | 1.0 | Reference | |
| Urban non-teaching | 1.03 | 0.97-1.1 | 1.00 | |
| Urban teaching | 1.08 | 1.02-1.15 | 0.28 | |
| Sepsis | Yes | 3.33 | 3.2-3.47 | < 0.0001 |
| GI hemorrhage | Yes | 2.31 | 2.22-2.4 | < 0.0001 |
| Acute renal failure | Yes | 7.19 | 6.96-7.42 | < 0.0001 |
ALD is a spectrum of diseases ranging from hepatic steatosis to fibrosis and eventually cirrhosis, with continued alcohol use[22]. Alcohol consumption has been on the rise in the USA. Previously, a large survey conducted by the National Epidemiologic Survey showed a prevalence of alcohol use over any 12-mo period to rise from 65% to 72%, with an overall increase in alcohol consumption between 2001 and 2012[23].
Our study included all AH-related admissions from 2011 to 2017 in the NIS database. Out of the 38.5 million admissions in 2011, about 281 506 (0.7%) were due to AH. This number increased to 324050 (0.9%) out of 35.7 million admissions in 2017 (P < 0.001; Figure 1). Moreover, the number of admissions due to a primary diagnosis of AH increased by almost 1.5 times between 2011 and 2017 from 47140 (0.1%) to 71290 (0.2%) (P < 0.001). These are alarming figures, and they match the results of increased alcohol consumption and binge drinking in the USA[8,12]. This has major consequences as AH continues to cause significant morbidity and mortality. Additionally, we found that each AH-related admission costs on average $46000 to $63000 with an average in-hospital length of stay of 4 d per admission. About 60% of those patients had Medicare or Medicaid insurance as the primary expected payer. These increasing admissions, as reported previously, escalates the burden on the healthcare system and the public payer funded by tax dollars[24].
The increase in AH burden has a huge impact on trends of liver transplantation and consequently, organ allocation. Recent data showed that in select patients with severe AH, early liver transplantation resulted in high survival rates in the early transplant of AH at 6 mo vs no transplant (77% vs 23%)[25,26]. Based on these results, many liver transplant centers around the USA are performing liver transplants for select severe AH patients. Consequently, this has resulted in ALD surpassing chronic hepatitis C virus infection as the leading indication for liver transplantation[27].
Our study showed that the majority of AH hospitalized individuals were middle-aged white men. This is not surprising, as more men consume alcohol above the recommended safety levels compared to women[24]; although women are more susceptible to developing AH within a shorter period and less exposure to alcohol compared to men[28].
We observed that the mortality of hospitalized patients with AH was about 5%, which has remained similar from 2011 to 2017. We also examined some of the major mortality-related risk factors among AH patients who expired during hospitalization. ARF was the most common finding in these patients and increased from 59.6% in 2011 to 72% in 2017. A possible explanation of these results may include the lack of therapy for severe AH with ARF since the benefit of steroids is unknown in this patient population as the steroids or pentoxifylline for AH trial excluded patients with ARF defined as creatinine > 5.7 mg/dL[29]. In addition, hepatorenal syndrome is associated with high mortality, which could be as high as 80% at 2 wk[30]. Sepsis and GI bleeding were also noted as mortality-risk factors in almost 48% and 19%, respectively, in patients who died from AH in 2017. Sepsis had a significant increase from 7.4% in 2014 to 46.7% in 2016 and 48.3% in 2017. This likely resulted from implementing the conversion of ICD-9 to ICD-10 on October 1, 2015. Sepsis is coded for by one code in ICD-9 (995.91), in contrast, there are 26 codes for different types of sepsis in ICD-10, which could potentially explain the sharp rise in sepsis rate. Additionally, AH patients usually present with fever and leukocytosis meeting Systemic Inflammatory Response Syndrome, criteria leading clinicians to code for sepsis even without an active infection present.
A regression analysis was performed that showed that advanced age, female gender, ARF, sepsis and GI bleeding were the most prominent risk factors. These mortality risk factors do not establish causality, since there is no etiology of death in the NIS database. Moreover, there is no information as to whether these risk factors were resolved or not during hospitalization since it was captured by the ICD codes used.
One limitation of this study was the accuracy of the NIS database in capturing ICD-9 and ICD-10 codes. For instance, some AH-related admissions could have been reported as jaundice or hepatic failure, which would exclude those patients from our analysis. There was also no information regarding the outcomes of AH patients once they were discharged. This has resulted in a lower in-hospital mortality rate (about 5%) compared to the established high 30-d mortality of AH patients (30%–50%)[31]. The mortality rate in our study is the percentage of patients who died while hospitalized only.
We observed that AH-related hospitalization continued to increase during the study period. This could have a substantial impact on increasing healthcare costs and utilization among patients hospitalized for AH. Mortality remained the same throughout the study period. These findings are alarming and should trigger more aggressive screening and prevention of alcohol abuse to prevent the increasing cases of AH and its consequences.
Alcoholic hepatitis (AH) is a significant healthcare issue with rising alcohol use in the USA. Alcohol-associated liver disease is the second leading indication for liver transplantation after surpassing chronic hepatitis C infection.
With increasing alcohol consumption there is a need to measure the magnitude of AH effects.
The study aimed to examine the trends in hospitalization of AH patients across the USA. The second aim was to look at the mortality of hospitalized patients, along with the risk factors associated with death while hospitalized.
Data were extracted from National Inpatient Sample database using discharge diagnosis codes of International Classification of Diseases (ICD)-9 and their corresponding ICD-10. We included hospitalization for the years from 2011 to 2017.
AH inpatient hospitalization increased from 0.7% of total admissions to 0.9% of total admissions. Mortality for admitted patients remained the same.
There has been an increase in AH hospitalization that could affect the healthcare system. Acute renal failure, sepsis and gastrointestinal hemorrhage are highly associated with increased mortality in AH patients.
New studies should focus on finding new therapeutic targets of AH. New studies should look for improved strategies to limit alcohol misuse.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases, No. 218688; American College of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lu XL, China; Radford-Smith DE, United Kingdom; Xu CF, China S-Editor: Yan JP L-Editor: Kerr C P-Editor: Yan JP
| 1. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1495] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 2. | Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12:555-64; quiz e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Kwong AJ, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Foutz J, Booker SE, Cafarella M, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2019 Annual Data Report: Liver. Am J Transplant. 2021;21 Suppl 2:208-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 259] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Global Status Report on Alcohol and Health 2018. Poznyak V, Rekve D, editor. Geneva: World Health Organization, 2018. |
| 5. | Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999-2008: a nationwide population based cohort study. J Hepatol. 2011;54:760-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Singal AK, Kuo YF, Anand BS. Hepatitis C virus infection in alcoholic hepatitis: prevalence patterns and impact on in-hospital mortality. Eur J Gastroenterol Hepatol. 2012;24:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Williams R. The pervading influence of alcoholic liver disease in hepatology. Alcohol Alcohol. 2008;43:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Neff GW, Duncan CW, Schiff ER. The current economic burden of cirrhosis. Gastroenterol Hepatol (N Y). 2011;7:661-671. [PubMed] |
| 9. | Yörük BK. Legalization of Sunday alcohol sales and alcohol consumption in the United States. Addiction. 2014;109:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Xuan Z, Nelson TF, Heeren T, Blanchette J, Nelson DE, Gruenewald P, Naimi TS. Tax policy, adult binge drinking, and youth alcohol consumption in the United States. Alcohol Clin Exp Res. 2013;37:1713-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Niu B, Forde KA, Goldberg DS. Coding algorithms for identifying patients with cirrhosis and hepatitis B or C virus using administrative data. Pharmacoepidemiol Drug Saf. 2015;24:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Shirazi F, Singal AK, Wong RJ. Alcohol-associated Cirrhosis and Alcoholic Hepatitis Hospitalization Trends in the United States. J Clin Gastroenterol. 2021;55:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Siegel M, DeJong W, Naimi TS, Heeren T, Rosenbloom DL, Ross C, Ostroff J, Jernigan DH. Alcohol brand preferences of underage youth: results from a pilot survey among a national sample. Subst Abus. 2011;32:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Miller TR, Levy DT, Spicer RS, Taylor DM. Societal costs of underage drinking. J Stud Alcohol. 2006;67:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Siegel MB, Naimi TS, Cremeens JL, Nelson DE. Alcoholic beverage preferences and associated drinking patterns and risk behaviors among high school youth. Am J Prev Med. 2011;40:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Bor J, Basu S, Coutts A, McKee M, Stuckler D. Alcohol use during the great recession of 2008-2009. Alcohol Alcohol. 2013;48:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Jinjuvadia R, Liangpunsakul S; Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in Alcoholic Hepatitis-related Hospitalizations, Financial Burden, and Mortality in the United States. J Clin Gastroenterol. 2015;49:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 18. | Hirode G, Saab S, Wong RJ. Trends in the Burden of Chronic Liver Disease Among Hospitalized US Adults. JAMA Netw Open. 2020;3:e201997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 19. | Myers RP, Shaheen AA, Fong A, Wan AF, Swain MG, Hilsden RJ, Sutherland L, Quan H. Validation of coding algorithms for the identification of patients with primary biliary cirrhosis using administrative data. Can J Gastroenterol. 2010;24:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Rawson NS, Malcolm E. Validity of the recording of ischaemic heart disease and chronic obstructive pulmonary disease in the Saskatchewan health care datafiles. Stat Med. 1995;14:2627-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Agency for Healthcare Research and Quality. Overview of the National (Nationwide) Inpatient Sample (NIS). Sep 13, 2021. [cited Feb 6, 2022]. Available from: http://www.hcup-us.ahrq.gov/nisoverview.jsp. |
| 22. | O'Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 853] [Article Influence: 56.9] [Reference Citation Analysis (2)] |
| 23. | Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001-2002 to 2012-2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 1019] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 24. | Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, Duhamel A, Pageaux GP, Leroy V, Dharancy S, Louvet A, Boleslawski E, Lucidi V, Gustot T, Francoz C, Letoublon C, Castaing D, Belghiti J, Donckier V, Pruvot FR, Duclos-Vallée JC. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 658] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 25. | Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 569] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 26. | Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, Charlton M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152:1090-1099.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 469] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 27. | Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol. 2011;45:714-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Roerecke M, Vafaei A, Hasan OSM, Chrystoja BR, Cruz M, Lee R, Neuman MG, Rehm J. Alcohol Consumption and Risk of Liver Cirrhosis: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2019;114:1574-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 29. | Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A, Hood S, Masson S, McCune A, Mellor J, O'Grady J, Patch D, Ratcliffe I, Roderick P, Stanton L, Vergis N, Wright M, Ryder S, Forrest EH; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 556] [Article Influence: 55.6] [Reference Citation Analysis (1)] |
| 30. | Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 550] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 31. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 693] [Article Influence: 43.3] [Reference Citation Analysis (0)] |