Published online Apr 28, 2022. doi: 10.3748/wjg.v28.i16.1656
Peer-review started: October 19, 2021
First decision: December 12, 2021
Revised: December 26, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: April 28, 2022
Processing time: 186 Days and 15.9 Hours
Gastric cancer (GC) is considered as one of the most widespread malignancies. Emerging evidence has shown that lncRNAs can function as important oncogenes or tumor suppressors during GC progression.
To investigate the effect and mechanism of lncRNA cancer susceptibility 20 (CASC20) in the proliferation and metastasis of GC cells.
Data mining and clinical samples were used to evaluate the expression of CASC20 in GC and adjacent tissues. CASC20 was down-regulated in GC cells by short-interfering RNA. Cell proliferation was evaluated by CCK-8 assay, and cell migration and invasion were detected by wound healing and Transwell assays. The expressions of proteins related to epithelial-mesenchymal transition were detected by western blot assay.
The expression of CASC20 was increased in GC tumor tissues and various GC cell lines. High CASC20 expression was correlated with a high risk of lymphatic metastasis and poor prognosis in GC patients. In vitro assays showed that silencing CASC20 reduced cell proliferation, migration, and invasion in GC cells. Mechanistic studies revealed that CASC20 exhibits oncogenic functions by regulating MEMO1 expression through competitive endogenous binding to miR-143-5p, leading to induction of epithelial-mesenchymal transition.
Our findings indicate that CASC20 serves as a tumor promoter by regulating metastasis in GC via the miR-143-5p/MEMO1 axis. CASC20 may be a potential therapeutic target for GC.
Core Tip: LncRNA cancer susceptibility 20 (CASC20) is upregulated in gastric cancer (GC) and associated with a higher risk of lymphatic metastasis and poor prognosis of patients. CASC20 can promote the proliferation, invasion and metastasis of GC cells by absorbing miR-143-5p to upregulate MEMO1, thereby induced epithelial-mesenchymal transition in vitro.
- Citation: Shan KS, Li WW, Ren W, Kong S, Peng LP, Zhuo HQ, Tian SB. LncRNA cancer susceptibility 20 regulates the metastasis of human gastric cancer cells via the miR-143-5p/MEMO1 molecular axis. World J Gastroenterol 2022; 28(16): 1656-1670
- URL: https://www.wjgnet.com/1007-9327/full/v28/i16/1656.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i16.1656
In 2020, gastric cancer (GC) was the fifth most common cancer and the fourth for mortality of cancer-related in the world[1]. Many patients are already at an advanced stage when diagnosed with GC. Surgical resection is the main treatment for GC. However, postoperative metastasis is the main cause of death from GC[2]. In 2011, Robert A. Weinberg published an article classifying metastasis as one of the top ten characteristics of cancer[3]. Therefore, finding effective molecular markers and therapeutic targets for metastasis is important for developing treatments for GC. The mechanisms underlying metastasis in GC have not yet been fully clarified and require further elucidation.
LncRNAs are RNA molecules more than 200 nucleotides in length that are located in the nucleus or cytoplasm and lack any protein-coding ability. Increasing studies have shown that lncRNAs play important roles in chromosome silencing, chromatin epigenetic modification, gene transcription, protein translation and protein localization[4,5]. In addition, lncRNAs have been shown to function in tumor progression[6,7]. Some lncRNAs function as competing endogenous RNA (ceRNA) and regulate the expression of target genes by binding miRNA elements in target miRNAs and “sponging” miRNAs[8]. MiRNAs can specifically target the 3’UTR of target mRNAs, thereby inhibiting mRNA translation. LncRNA 00473 is highly expressed in GC tissues and is closely related to lymph node metastasis and late TNM staging. LncRNA 00473 regulates the expression of CCND2 by adsorbing miR-16-5p to promote the proliferation and metastasis of GC cells[9]. LncRNAs also play emerging roles in endothelial-mesenchymal transition (EMT) process of GC[10]. The process of EMT involves downregulation of the epithelial cell specific proteins and upregulation of the mesenchymal cell specific proteins and it is an important cause of tumor metastasis[11]. LncRNA CASC19 is highly expressed in colorectal cancer, and CASC19 overexpression increased the invasion and migration ability of colorectal cancer cells. CASC19 regulates cell migration inducing hyaluronidase 1 (CEMIP) by targeting miR-140-5p and affecting the EMT process[12].
Several studies have revealed key roles for lncRNAs in GC progression, and multiple lncRNAs have been identified as diagnostic and prognostic markers or treatment targets for GC[4]. A recent study identified the HOXC-AS3 lncRNA as highly expressed in GC tissues. HOXC-AS3 binds YBX1 protein to regulate downstream gene transcription and participates in GC progression[13]. The lncRNA CASC9 is highly expressed in GC tissues and cells. CASC9 negatively regulates miR-370 and promotes the progression of GC through the EGFR/AKT pathway[14]. However, the precise mechanisms and roles of lncRNAs in GC progression are still unclear.
Cancer susceptibility 20 (CASC20) is a 1484 bp lncRNA mapped to chromosome 20p12.3. In this study, we explored the role and possible mechanism of the lncRNA CASC20 in GC metastasis to gain more insights into the mechanism of GC metastasis. We found that the CASC20 lncRNA is highly expressed in GC and closely associated with lymph node metastasis and poor prognosis of GC patients. Through in vitro and in vivo experiments, we found that CASC20 promotes the proliferation, migration and invasion of GC cells. Mechanistic studies revealed that CASC20 “sponges” miR-143-5p and promotes EMT by regulating MEMO1. This function of CASC20 plays an important role in the metastasis of GC.
All clinical samples and information in this study were from the Department of Gastrointestinal Surgery, Provincial Hospital Affiliated to Shandong First Medical University. Fresh pathological specimens (GC and adjacent normal tissues) were from patients undergoing radical GC surgery at our hospital. All patients provided informed consent and were approved by the Medical Ethics Committee.
Human GC cell lines (MKN45, MKN28, HGC27, MGC803, N87, AGS and SGC7901) and the immortalized gastric mucosa cell line (GES-1) were provided by the Cell Center of the Chinese Academy of Medical Sciences. All cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS, Gibco, United States). Cells were cultured in incubator at 37 °C and 5% CO2. Cells were passaged when they were 70% confluent.
The miR-143-5p mimics and inhibitor were purchased from RiboBio (Guangzhou, China). siRNAs targeting CASC20 (si-CASC20) and MEMO1 (si-MEMO1) were designed and synthesized by RiboBio (Guangzhou, China). Lipofectamine2000 (Invitrogen, United States) was used to transfect siRNA or miRNA mimics/inhibitor into cells following the manufacturer’s instructions. GC cells in logarithmic phase were collected and inoculated in a 6-well plate. Cells were transfected with siRNAs when cell confluence reached approximately 50%.
Trizol reagent (Invitrogen, United States) was used to extract total RNA from GC tissues and cells. RNA integrity was determined by evaluating the absorbance at OD260/280 using a NanoDrop analyzer and confirmed at 1.8–2.0. Total RNA was reverse transcribed into cDNA using a reverse transcription kit (Takara, Dalian, China) in accordance with the manufacturer’s instructions. The quantitative real-time polymerase chain reaction (qRT-PCR) three-step method was used, and the 2-ΔΔCt method was used to calculate the relative expression levels of CASC20 and miR-143-5p. The primer sequences are as follows: CASC20, Forward 5′-ATAACCACCCTCCCCTCCTC-3′, Reverse 5′-GCTCCTCCCACTTTAACCCC-3′; miR-143-5p, Forward 5′-ATGGTTCGTGGGGTCCAGTTTTCCCAG-3′, Reverse 5′-GTGTCGTGGAGTCGGCAATTC-3′; MEMO1, Forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′, Reverse 5'-CGCTTCACGAATTTGCGTGTCAT-3′; and β-actin, Forward 5′-TGGCACCCAGCACAATGAA-3′, Reverse 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. U6, Forward 5′-CAGCACATATACTAAAATTGGAACG-3′ Reverse 5′-ACGAATTTGCGTGTCATCC-3′. β-actin (cytoplasm control) and U6 (nucleus control) were used for normalization.
GC cells were inoculated into 96-well plate at 5 × 103 cells per well. At 24 h, 48 h, 72 h, 96 h and 120 h after transfection, 90 μL of fresh culture medium containing 10% FBS and 10 μL of CCK-8 reagent (Dojindo, Mashikimachi, Japan) were simultaneously added to each well. Cells were cultured for another 4 h. The OD value of each well at 450 nm was measured and proliferation curves were drawn.
GC cells were collected after trypsin digestion and centrifuged at 1000 rpm/min for 5 min. The cells were washed twice with phosphate buffer solution, and 500 μL binding buffer was added to adjust the cell concentration to 1 × 106 cells/mL. Annexin V-FITC (5 μL) and propidium iodide solution (10 μL) (NeoBioscience, China) were added to the cell suspension, and cells were analyzed on a flow cytometer (BD Accuri C6).
After 48 h of transfection, cells were collected and the cell density was adjusted to 1 × 105 cells/mL. The cell suspension was inoculated into a 6-well plate at 100 μL/well and the plates were incubated for 14 d. Cells were washed twice with phosphate buffered saline and fixed with 4% paraformaldehyde for 15 min; the cells were then stained with 0.1% crystal violet staining solution for 10 min. We counted the number of cell clones in five fields under the microscope and determined the clone formation rate as follows: Clone formation rate (%) = (number of clones/number of inoculated cells) × 100%. The experiment was repeated three times.
GC cells were cultured in a 6-well plate at 5 × 105 cells per well. Linear scratch wounds were created in the cell monolayer with a sterile 100 μL pipette tip. Cells were cultured for 24 h, and the wound widths were photographed and measured.
Assays using a Transwell chamber (8 μm, Corning, United States) were used to evaluate cell migration and invasion abilities. GC cells were collected and resuspended in serum-free RPMI 1640 medium at 1.5 × 104 cells/mL, and 200 μL of the cell suspension was inoculated into the upper chambers. Complete medium containing 10% FBS was added to the lower chamber. After culturing for 24 h, the cells were fixed with methanol. Cells in the upper chamber were removed with cotton swabs, and cells in the lower chambers were stained with 0.1% crystal violet solution for 30 min. The cells were observed under a microscope and photographed; five random fields were obtained to count the number of migrated cells. Each experimental group was performed in triplicate, and the average value was taken. For the invasion experiment, the upper chamber was coated with Matrigel; the assay was then conducted following the methods for the migration assay.
The online tool lncRNASNP2 was used to predict miRNAs that potentially bind to CASC20. We used miRDB, Targetscan, and miRTarbase to predict putative target genes of miR-143-5p.
Luciferase reporter pGL3 vectors with wild-type CASC20 (termed WT-CASC20), wild-type MEMO1 (MEMO1-WT), mutant CASC20 (MUT-CASC20) and mutant MEMO1 (MEMO1-MUT) were constructed (Promega, Madison, United States). The reporter plasmids were transfected into MGC803 cells along with miR-143-5p mimics. At 48 h after transfection, the luciferase activity was measured using the Dual Luciferase Reporter Assay system (Promega, United States) in accordance with the manufacturer’s instructions.
Cells were collected and lysed with RIPA lysis buffer (Beyotime, Shanghai, China). After lysates were centrifuged, the supernatant was collected and protein concentration was evaluated by the BCA method. The samples (60 μg/well) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electro-transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, United States). After blocking in 5% skim milk at room temperature for 2 h, the membrane was incubated with primary antibody overnight at 4 °C. The membrane was then washed three times with Tris buffered saline tween and incubated with the secondary antibody at room temperature for 2 h. ECL luminescent solution was used for chemiluminescence and Image J software was used to analyze the protein expression level. MEMO1 and β-actin monoclonal antibodies were obtained from Sigma-Aldrich (St Louis, MO, United States). The other antibodies were purchased from Abcam (Cambridge, England).
Statistical analyses were performed using GraphPad Prism (version 7.0; GraphPad Prism software, San Diego, CA, United States). Data are shown as mean ± SD. For measurement data, the difference between two or more groups was calculated using the Student’s t-test or one-way ANOVA analysis. Chi-square test was used to analyze the correlation between CASC20 and clinicopathological parameters of GC patients. The survival curve was determined by the Kaplan–Meier method and log-rank test. P < 0.05 was considered statistically significant.
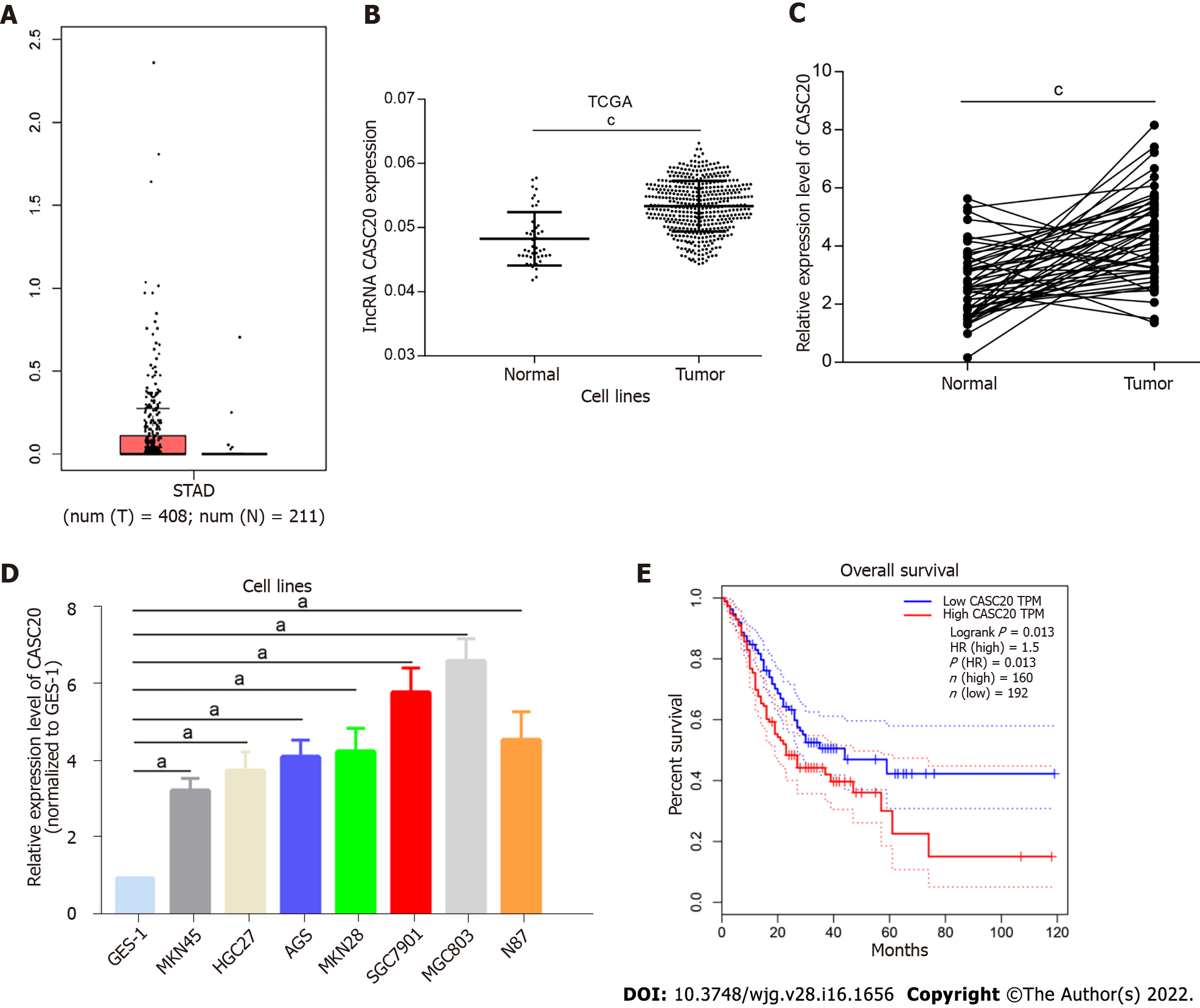
Analysis of TCGA database revealed that CASC20 expression was significantly higher in 408 GC tumor tissues than that in 211 normal gastric tissues (P < 0.01, Figure 1A and B). We performed qRT-PCR to assess the expression of CASC20 in 50 cases of GC tissues and paired normal gastric mucosa tissues adjacent to tumors. The results showed that the expression of CASC20 in GC tissues was significantly higher than that in normal adjacent gastric mucosa tissues (P < 0.01, Figure 1C). We further found that the expression of CASC20 in seven GC cell lines was significantly higher than that of GES-1 normal gastric mucosal epithelial cells (Figure 1D). We next analyzed the relationship between CASC20 expression and the survival rate of GC patients in TCGA database. The results indicated that the survival rate of GC patients with high expression of CASC20 was significantly lower than that of patients with low expression (Figure 1E). Together these data indicate that CASC20 is highly expressed in GC and may play an important role in the development of GC.
Next, we examined the correlation between CASC20 expression and the pathological characteristics of 50 GC patients. GC patients were divided into high and low CASC20 expression groups based on the median ratio of relative CASC20 expression. High CASC20 expression in GC patients was significantly correlated with a higher risk of lymphatic metastasis (P = 0.011), but did not show associations with age, gender, TNM stage and differentiation degree (Table 1). These results suggest that CASC20 is closely related to the malignant biological behavior of GC.
| Characteristics | Number of cases | CASC20 expression | P value | |
| High (n = 26) | Low (n = 24) | |||
| Gender | 0.382 | |||
| Male | 23 | 14 | 9 | |
| Female | 27 | 12 | 15 | |
| Age | 0.546 | |||
| ≤ 55 | 22 | 13 | 9 | |
| > 55 | 28 | 13 | 15 | |
| TNM stage | 0.262 | |||
| I + II | 24 | 10 | 14 | |
| III | 26 | 16 | 10 | |
| Lymphatic metastasis | 0.011a | |||
| Positive | 25 | 18 | 7 | |
| Negative | 25 | 8 | 17 | |
| Differentiation | 0.073 | |||
| Moderate/well | 30 | 12 | 18 | |
| Poor | 20 | 14 | 6 | |
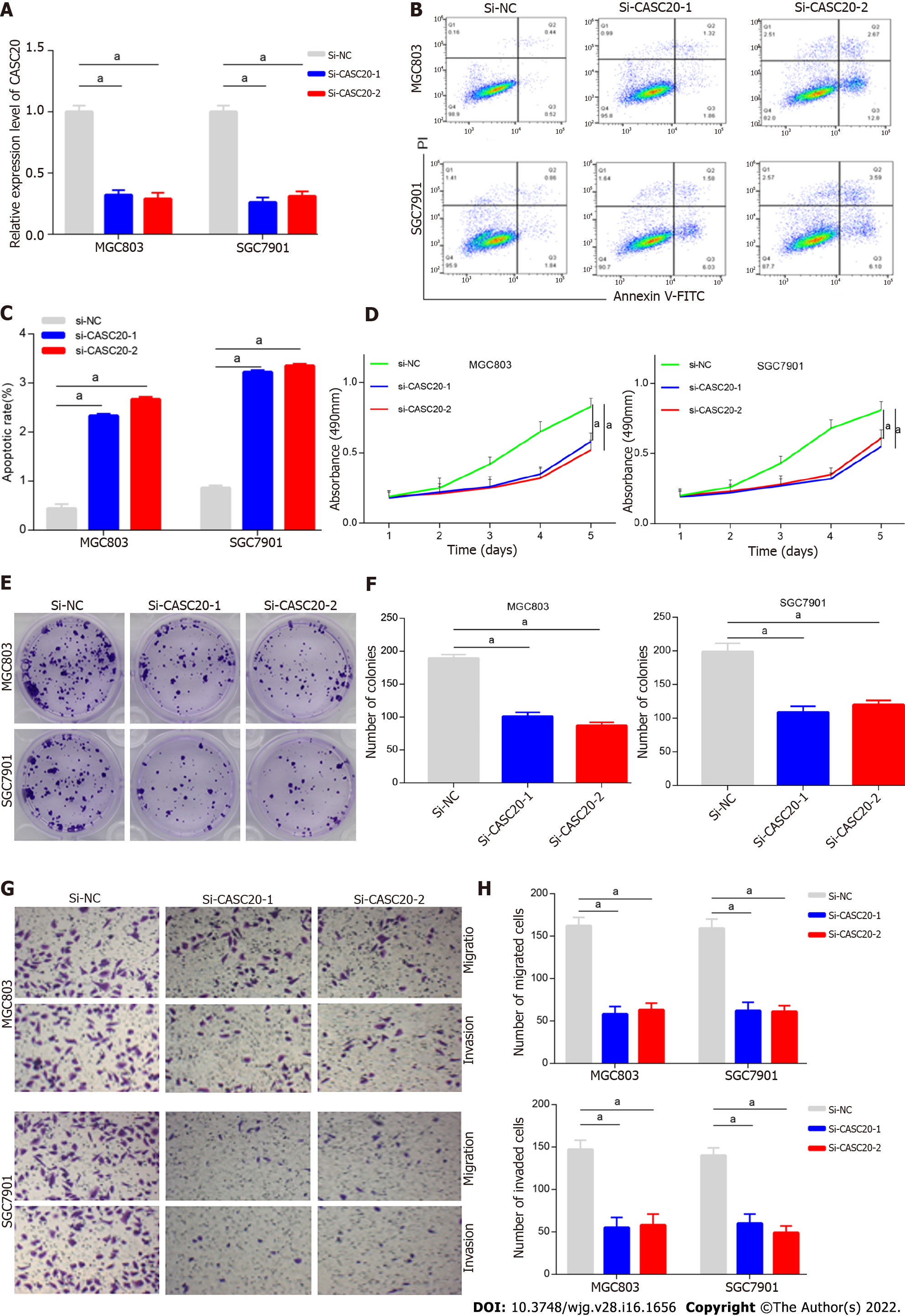
We next evaluated the role and effects of CASC20 in GC cells. We transfected siRNA targeting CASC20 into GC cells and qRT-PCR confirmed that the expression of CASC20 was significantly down-regulated after siRNA transfection (Figure 2A). Flow cytometry results showed that knockdown of CASC20 significantly increased the apoptosis rate of SGC7901 and MGC803 GC cells compared with the control cells (Figure 2B and C). CCK8 assays demonstrated that knockdown of CASC20 significantly inhibited the proliferation of SGC7901 and MGC803 cells (Figure 2D). Colony formation experiments revealed that inhibiting CASC20 expression resulted in decreased clonogenic survival (Figure 2E and F). Transwell experiments showed that knocking down CASC20 significantly reduced the numbers of invading and migrating GC cells (Figure 2G and H). Together, these findings show that knockdown of CASC20 inhibits the malignant phenotype of GC cells, suggesting that CASC20 exhibits oncogenic functions in GC.
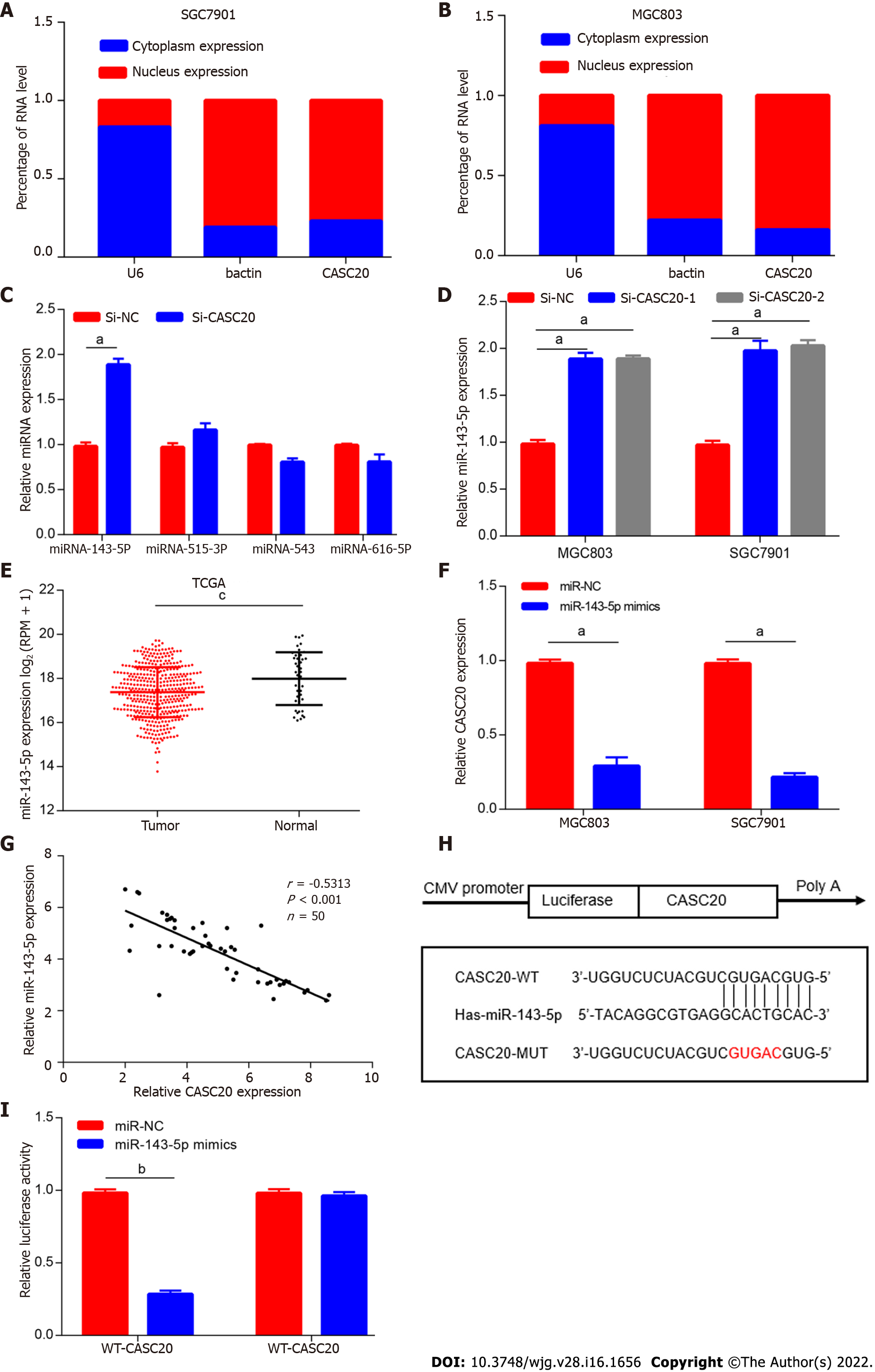
To gain better insights into the function of CASC20, we first determined the subcellular location of CASC20 by GeneCards software (https://www.genecards.org). The results showed that CASC20 is mainly located in the cytoplasm. The results of nuclear cytoplasmic experiments further confirmed that CASC20 mostly localizes in the cytoplasm (Figure 3A and B), suggesting that CASC20 may function as a ceRNA to “sponge” miRNA.
We next used bioinformatics tools (lncRNASNP2) to predict miRNAs with potential binding sites for CASC20 and the results identified miR-143-5p, miR-515-3p, miR-543 and miR-616-5p. We evaluated the expressions of these miRNAs in cells transfected with si-CASC20 and found that the expression of miR-143-5p was up-regulated when CASC20 was knocked down, while miR-515-3p, miR-543 and miR-616-5p showed no significant changes (Figure 3C and D). We thus selected miR-143-5p for further analyses.
TCGA database analysis showed that miR-143-5p was expressed at low levels in GC tissues (Figure 3E), which was consistent with previous studies on miR-143-5p[15]. We transfected miR-143-5p mimics into GC cells and observed significantly decreased expression of CASC20 (Figure 3F). In addition, we identified a negative correlation between CASC20 and miR-143-5p in 50 GC tissues (Figure 3G).
To further determine the regulatory mechanism between CASC20 and miR-143-5p, we performed dual luciferase reporter assays. The results showed that transfection of miR-143-5p mimics decreased the luciferase activity of the WT-CASC20 reporter in MGC803 cells, while miR-143-5p mimics had no impact on the MUT-CASC20 reporter activity (Figure 3H and I). This result suggests that the regulatory relationship of CASC20 and miR-143-5p in GC cells involves interactions between CASC20 and miR-143-5p.
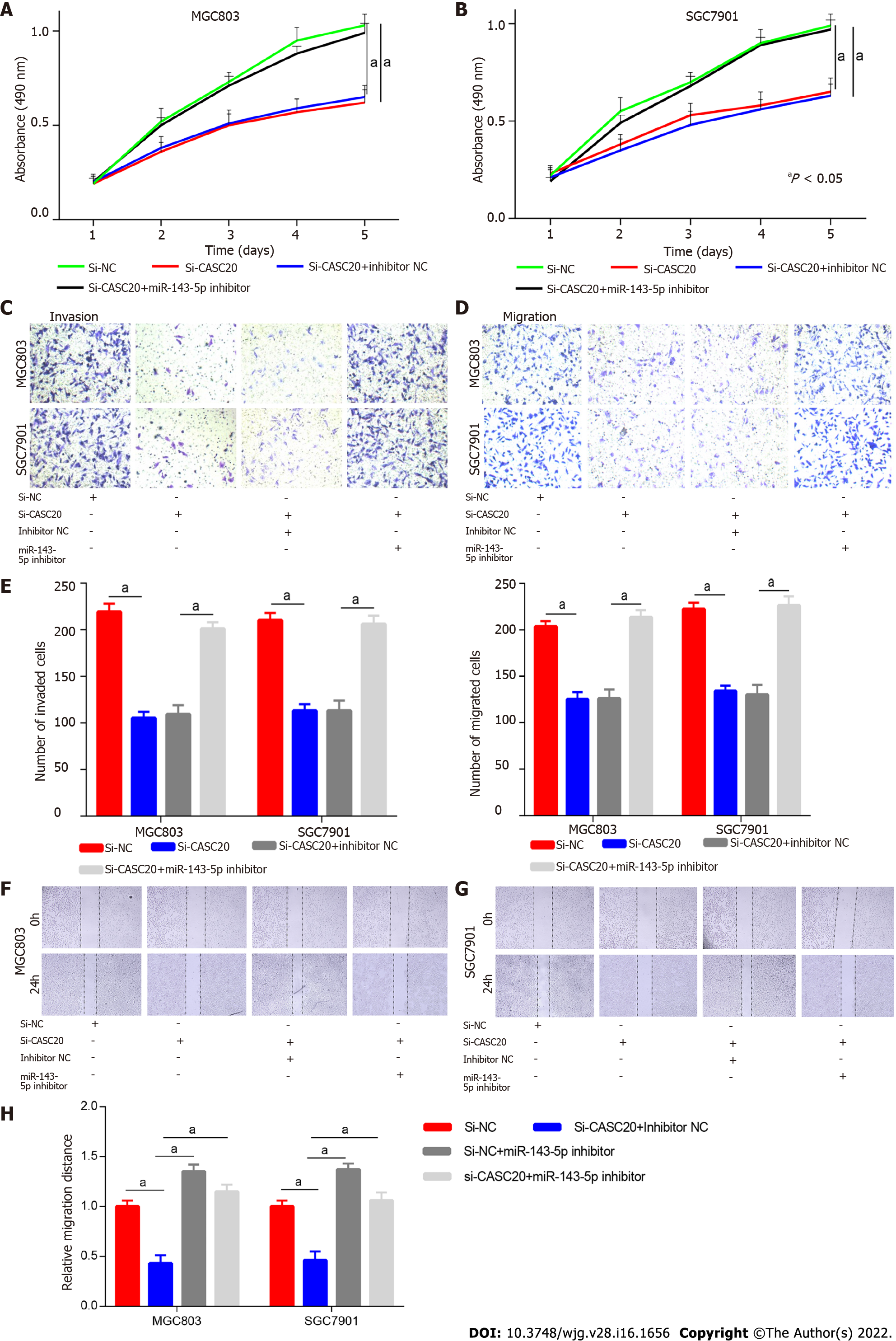
To determine whether miR-143-5p played a role in the effects of CASC20 in GC cells, we co-transfected si-CASC20 and miR-143-5p inhibitor into SGC7901 and MGC803 cells. CCK8 assays showed that si-CASC20 transfection inhibited GC cell proliferation, and co-transfection of miR-143-5p inhibitor restored proliferation (Figure 4A and B). Transwell experiments showed while transfection of si-CASC20 inhibited the invasion and migration of GC cells, co-transfection of miR-143-5p inhibitor restored the invasion and migration abilities (Figure 4C–E). Wound healing assays also confirmed that co-transfection of miR-143-5p inhibitor partly rescued the inhibitory effect of knockdown of CASC20 on GC cells (Figure 4F–H). These results indicate that CASC20 regulates GC cell activities through miR-143-5p.
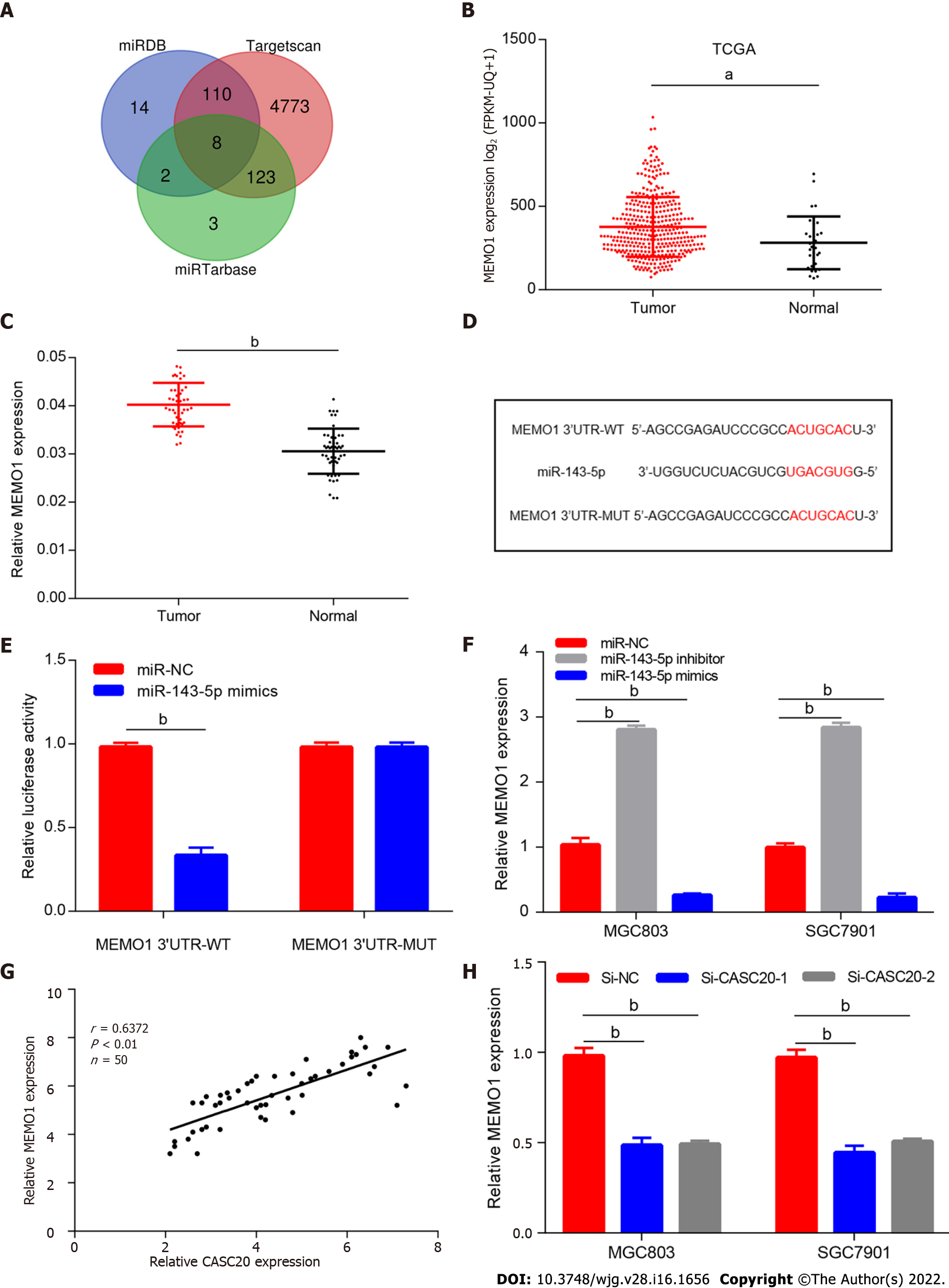
To further explore the role of miR-143-5p in GC, we screened and identified the potential target mRNAs of miR-143-5p. Bioinformatics tools miRDB, Targetscan and miRTarbase predicted several mRNAs (HDAC7, ZNF85, ZNF347, MEMO1, ESCO1, MLXIP, MAP3K2 and DYRK1A mRNAs) that potentially interact with miR-143-5p (Figure 5A). Among them, MEMO1 was selected as the target gene of miR-143-5p because it had the highest score in the prediction analysis.
TCGA database analysis showed that MEMO1 was highly expressed in GC tissues (Figure 5B). In addition, the mRNA expression of MEMO1 was significantly increased in 50 GC tissues compared with its expression in adjacent tissues (Figure 5C).
We next designed luciferase reporter vectors containing the wild-type 3′UTR of MEMO1 mRNA or the 3′UTR with mutated binding sites (Figure 5D). Dual luciferase reporter assay showed that miR-143-5p significantly reduced the luciferase activity of the reporter vector with the wild-type 3′UTR of MEMO1 mRNA but had no effects on the mutated reporter (Figure 5E), suggesting that MEMO1 mRNA may be a target gene of miR-143-5p. We then transfected miR-143-5p mimics into MGC803 cells and found that the expression of MEMO1 was significantly reduced at both gene and protein levels (Figure 5F). These data indicate that miR-143-5p regulates MEMO1 mRNA expression.
We also found that the expression of CASC20 was positively correlated to MEMO1 expression in GC tissues (Figure 5G). In cells with CASC20 knockdown, MEMO1 expression was downregulated, suggesting a positive regulatory relationship between CASC20 and MEMO1 (Figure 5H).
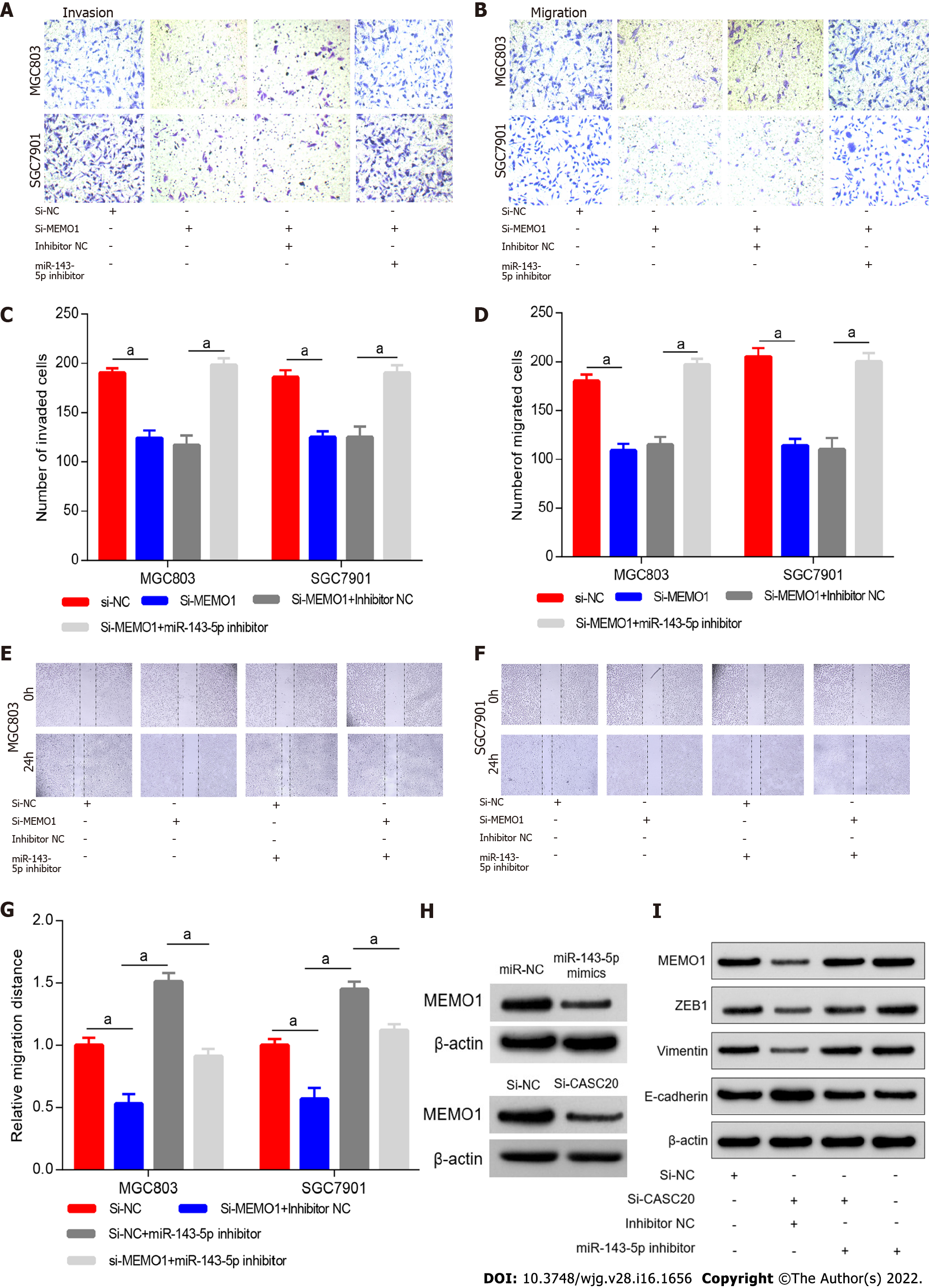
To explore the role of MEMO1 in GC, we transfected siRNA targeting MEMO1 into GC cells. Scratch experiments and Transwell experiments revealed that knockdown of MEMO1 inhibited the invasion and migration of GC cells. The migration and invasion abilities were partially rescued by co-transfection with miR-143-5p inhibitor (Figure 6A–G), indicating that miR-143-5p inhibition reversed the effects of MEMO1 knockdown.
To investigate whether CASC20 regulates MEMO1 through miR-143-5p, we transfected si-CASC20 and miR-143-5p inhibitor into MGC803 cells. Western blot results indicated that MEMO1 expression decreased in GC cells after transfection with si-CASC20, while MEMO1 expression was restored after co-transfection with si-CASC20 and miR-143-5p inhibitor (Figure 6H).
We next evaluated the effects of CASC20 on EMT. In MGC803 cells with CASC20 knockdown, the expression level of the epithelial protein marker E-cadherin was up-regulated, while the expression levels of the mesenchymal protein markers Vimentin and ZEB1 were down-regulated. After transfection with miR-143-5p inhibitor, the expressions of MEMO1, Vimentin and ZEB1 were up-regulated, and the expression of E-cadherin was down-regulated (Figure 6H and I).
Together these results indicated that CASC20 may sequester miR-143-5p to increase the MEMO1 expression, leading to the induction of EMT and metastasis of GC.
Mounting evidence confirmed that lncRNAs play extensive regulatory roles in the initiation and progression of human cancers. A complex ceRNA crosstalk mechanism has been identified in GC[16]. However, only a small fraction of lncRNAs has been thoroughly studied, with no research on CASC20 in tumors. We analyzed the TCGA GC database and found that CASC20 is highly expressed in GC and associated with poor prognosis of GC patients. Functional experiments revealed that knock down of CASC20 significantly inhibited the proliferation, invasion, and migration of GC cells. These findings indicate a potential oncogenic function of CASC20 in GC.
miRNAs are 18–24 nt in length and negatively regulate gene expression by interacting with mRNA, thereby playing important physiological functions in several cellular processes. In tumors, the combination of miRNA and the 3'UTR of the target gene can function as an oncogene or tumor suppressor gene by regulating its expression[17,18]. miRNAs may thus become potential therapeutic targets for cancer metastasis, and the application of miRNA mimics and their antagonists for therapeutic purposes is expanding[19].
Our study showed that CASC20 was mainly distributed in the cytoplasm in GC cells, which suggested that CASC20 may function as a ceRNA for sponging miRNA. LncRNASNP2 was used to predict miRNAs that potentially bind CASC20, and our in vitro experiments indicated that CASC20 acts as a molecular sponge for miR-143-5p.
miR-143-5p is located in the human 5q32 chromosome region. Wu et al[15] found that miR-143-5p is expressed at low levels in GC and functions as tumor suppressor gene by targeting COX-2[15]. The lncRNA ZEB2-AS is highly expressed in GC tissues and cell lines, and ZEB2-AS was shown to promote the proliferation and metastasis of GC cells through the miR-143-5p/HIF-1α pathway[20]. These studies have shown that miR-143-5p is expressed at low levels in GC and exhibits tumor suppressor functions. Our qRT-PCR results showed that miR-143-5p was markedly down-regulated in GC tissues, and its expression level was negatively correlated with CASC20. Downregulation of miR-143-5p partially reversed the effects of CASC20 knockdown on the proliferation, invasion and migration of GC cells.
Bioinformatics analysis predicted that miR-143-5p interacts with the 3′UTR of MEMO1 (mediator of ErbB2-driven cell motility 1), a key mediator of receptor tyrosine kinase activation. MEMO1 plays an important role in breast cancer cell invasion and migration by activating the PI3K/Akt signaling pathway, leading to up-regulated Snail1 and induction of the EMT program[21]. MEMO1 is highly expressed in colon cancer tissues and cells, and miR-219a-1 regulates the proliferation, invasion and metastasis of colon cancer cells by targeting MEMO1, thereby functioning as a tumor suppressor gene[22]. The role of MEMO1 in the progression of GC has not yet been reported. In this study, we found that MEMO1 is highly expressed in GC in TCGA GC database and clinical samples. Overexpression of miR-143-5p reduced the expression of MEMO1 at the gene and protein level. Dual luciferase reporter assay was performed to reveal the regulatory relationship between miR-143-5p and MEMO1. Reduced migration and migration abilities of GC cells caused by MEMO1 knockdown were reversed by miR-143-5p inhibitor. EMT is a prerequisite for distant metastasis of malignant tumor cells, and many studies reported roles for lncRNAs in the tumor EMT process[23]. Our western blot results showed that CASC20 expression resulted in decreased expression of the epithelial cell marker E-cadherin and increased expression of the mesenchymal cell marker Vimentin. Together, our results indicate that CASC20 functions as ceRNA to sponge miR-143-5p, leading to up-regulated MEMO1 expression and initiation of the EMT process in GC.
This study has several limitations. Only 50 GC tissues were collected and analyzed in this study. The small clinical sample size may be one of the reasons why CASC20 expression was only related to lymph node metastasis, but showed no significant association with tumor size and clinical stage. In addition, in vivo experiments are required to further establish the biological function of CASC20 in GC.
In summary, this study identified the lncRNA CASC20/miR-143-5p/MEMO1 axis in GC and provided insights into the mechanistic relationships among CASC20, miR-143-5p and MEMO. These findings enrich the molecular regulatory network driving the occurrence and development of GC and deepen our understanding of the function and mechanism of CASC20 in cancer. Our results suggest that CASC20 may be a potential therapeutic target and clinical prognostic marker for GC.
LncRNAs have been indicated to play critical roles in gastric cancer (GC) tumorigenesis and progression. However, their roles in GC remain to be further elucidated.
To discover promising diagnostic and therapeutic targets for GC.
To investigate the underlying mechanisms of the lncRNA cancer susceptibility 20 (CASC20) in GC.
The expression of CASC20, miR-143-5p and MEMO1 genes were detected by quantitative real-time polymerase chain reaction. The oncogenic functions of CASC20 was investigated by experiments including cell counting kit-8, colony formation, wound-healing and Transwell assays in GC cells in vitro. Bioinformatics and dual-luciferase report assay were performed for determining the regulatory relationship of lncRNA-miRNA-mRNA.
We found that the expression of lncRNA CASC20 was significantly upregulated in GC tissues and cell lines, and increased expression of CASC20 was positively correlated with lymph node metastasis in patients with GC. Additionally, knockdown of CASC20 could inhibit GC cells growth, migration and invasion, whereas its overexpression could reverse these effects. Mechanistically, CASC20 specifically inhibited miR-143-5p expression, thus up-regulating MEMO1 expression.
CASC20 promotes GC cell metastasis via adsorbing miR-143-5p and up-regulating MEMO1, it plays an important oncogenic function in GC.
This study identified the lncRNA CASC20/miR-143-5p/MEMO1 axis in GC and provided insights into the mechanistic relationships among CASC20, miR-143-5p and MEMO. The results of our research suggest that CASC20 may act as a promising diagnostic marker for GC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Senchukova M, Russia; Tanabe S, Japan S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64611] [Article Influence: 16152.8] [Reference Citation Analysis (176)] |
| 2. | Xie Y, Shi L, He X, Luo Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol Rep (Oxf). 2021;9:91-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 3. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47126] [Article Influence: 3366.1] [Reference Citation Analysis (5)] |
| 4. | Yu H, Rong L. Emerging role of long non-coding RNA in the development of gastric cancer. World J Gastrointest Oncol. 2018;10:260-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | McDonel P, Guttman M. Approaches for Understanding the Mechanisms of Long Noncoding RNA Regulation of Gene Expression. Cold Spring Harb Perspect Biol. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Chi Y, Wang D, Wang J, Yu W, Yang J. Long Non-Coding RNA in the Pathogenesis of Cancers. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 589] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 7. | Huang Y, Guo Q, Ding XP, Wang X. Mechanism of long noncoding RNAs as transcriptional regulators in cancer. RNA Biol. 2020;17:1680-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4127] [Cited by in RCA: 5558] [Article Influence: 397.0] [Reference Citation Analysis (0)] |
| 9. | Zhuo S, Sun M, Bai R, Lu D, Di S, Ma T, Zou Z, Li H, Zhang Z. Long intergenic non-coding RNA 00473 promotes proliferation and migration of gastric cancer via the miR-16-5p/CCND2 axis and by regulating AQP3. Cell Death Dis. 2021;12:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Landeros N, Santoro PM, Carrasco-Avino G, Corvalan AH. Competing Endogenous RNA Networks in the Epithelial to Mesenchymal Transition in Diffuse-Type of Gastric Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Hanly DJ, Esteller M, Berdasco M. Interplay between long non-coding RNAs and epigenetic machinery: emerging targets in cancer? Philos Trans R Soc Lond B Biol Sci. 2018;373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 12. | Wang XD, Lu J, Lin YS, Gao C, Qi F. Functional role of long non-coding RNA CASC19/miR-140-5p/CEMIP axis in colorectal cancer progression in vitro. World J Gastroenterol. 2019;25:1697-1714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Zhang E, He X, Zhang C, Su J, Lu X, Si X, Chen J, Yin D, Han L, De W. A novel long noncoding RNA HOXC-AS3 mediates tumorigenesis of gastric cancer by binding to YBX1. Genome Biol. 2018;19:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 14. | Li C, Zhang J, Zhou Y, Li B. Long non-coding RNA CASC9 promotes the progression and development of gastric cancer via regulating miR-370/EGFR axis. Dig Liver Dis. 2021;53:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Wu XL, Cheng B, Li PY, Huang HJ, Zhao Q, Dan ZL, Tian DA, Zhang P. MicroRNA-143 suppresses gastric cancer cell growth and induces apoptosis by targeting COX-2. World J Gastroenterol. 2013;19:7758-7765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Ye J, Li J, Zhao P. Roles of ncRNAs as ceRNAs in Gastric Cancer. Genes (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Bartel DP. Metazoan MicroRNAs. Cell. 2018;173:20-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2329] [Cited by in RCA: 2657] [Article Influence: 379.6] [Reference Citation Analysis (0)] |
| 18. | Mori MA, Ludwig RG, Garcia-Martin R, Brandão BB, Kahn CR. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019;30:656-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 643] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 19. | Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2492] [Cited by in RCA: 3509] [Article Influence: 438.6] [Reference Citation Analysis (0)] |
| 20. | Wu F, Gao H, Liu K, Gao B, Ren H, Li Z, Liu F. The lncRNA ZEB2-AS1 is upregulated in gastric cancer and affects cell proliferation and invasion via miR-143-5p/HIF-1α axis. Onco Targets Ther. 2019;12:657-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Sorokin AV, Chen J. MEMO1, a new IRS1-interacting protein, induces epithelial-mesenchymal transition in mammary epithelial cells. Oncogene. 2013;32:3130-3138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Xu K, Shi J, Mo D, Yang Y, Fu Q, Luo Y. miR-219a-1 inhibits colon cancer cells proliferation and invasion by targeting MEMO1. Cancer Biol Ther. 2020;21:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1763] [Cited by in RCA: 1969] [Article Influence: 164.1] [Reference Citation Analysis (0)] |