Published online Feb 28, 2021. doi: 10.13105/wjma.v9.i1.88
Peer-review started: January 22, 2021
First decision: February 10, 2021
Revised: February 10, 2021
Accepted: February 25, 2021
Article in press: February 25, 2021
Published online: February 28, 2021
Processing time: 39 Days and 10.2 Hours
Mortality after hepatectomy has decreased, and the quality of various surgical approaches to hepatectomy have been evaluated. Various assessments of quality of life (QOL) after hepatectomy have been developed and investigated in different clinical settings.
To conduct a systematic review and meta-analysis to examine two clinical topics: Laparoscopic hepatectomy vs open hepatectomy, and preoperative QOL status vs postoperative QOL status.
A systematic literature search was performed using PubMed and MEDLINE, including the Cochrane Library Central. The following inclusion criteria were set for inclusion in this meta-analysis: (1) Studies comparing preoperative QOL and postoperative QOL; and (2) Studies comparing QOL between laparoscopic hepatectomy and open hepatectomy.
A total of 8 articles were included in this meta-analysis. QOL was better after laparoscopic hepatectomy than after open hepatectomy.
The outcomes of evaluations of QOL after hepatectomy can depend on the type of questionnaire used, the timing of the assessment, and the etiology of the hepatic disease.
Core Tip: A systematic review and meta-analysis of post-hepatectomy quality of life (QOL) assessments were conducted. A total of ten studies were included in the meta-analysis. QOL was better after hepatectomy than after transarterial chemoembolization. QOL was also better after laparoscopic hepatectomy than after open hepatectomy. The outcomes of post-hepatectomy QOL evaluations could depend on the type of questionnaire used, the timing of the assessment, and the etiology of the hepatic disease.
- Citation: Ishinuki T, Ota S, Harada K, Tatsumi H, Harada K, Miyanishi K, Nagayama M, Takemasa I, Ohyanagi T, Hui TT, Mizuguchi T. Health-related quality of life in patients that have undergone liver resection: A systematic review and meta-analysis. World J Meta-Anal 2021; 9(1): 88-100
- URL: https://www.wjgnet.com/2308-3840/full/v9/i1/88.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i1.88
Recently, hepatectomy has become safe, and the mortality rate of the procedure is now less than 1%[1,2]. Besides surgery, various other approaches have been developed for managing liver tumors, such as ablation, chemotherapy, molecular targeted therapy, and immunotherapy[3,4]. Furthermore, various surgical approaches have been developed, such as laparoscopic hepatectomy, robot-assisted hepatectomy, hybrid methods, hand-assisted methods, and classic open hepatectomy[5-9]. Therefore, selecting the optimal approach is essential for ensuring patients receive high-quality treatment.
Patient-reported outcomes (PRO) are considered to be gold-standard methods for evaluating quality of life (QOL) and comparing different management strategies[10]. Various PRO, such as the Functional Assessment of Cancer Therapy–Hepatobiliary (FACT-Hep)[11], the 36-Item Short-Form Health Survey (SF-36)[12], the European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire (QLQ-C30)[13], the EuroQol 5-dimension, 5-level questionnaire[14], and others, have been investigated in patients who underwent hepatectomy. The FACT-Hep consists of 5 subscales, physical well-being, social well-being, emotional well-being, functional well-being, and the hepatobiliary cancer subscale[15]. The sum of the scores for the five subscales gives a total score ranging from 0 to 180. A higher score indicates better QOL. The SF-36 consists of eight subscales, which are used to produce a physical component score and a mental component score[16]. The EORTC developed the QLQ-C30. The QLQ-C30 consists of three subscales: global health status, functional scales, and symptom scales[17]. Each subscale gives a score ranging from 0 to 100. Higher scores in the global health status and functional scales represent better QOL.
Although many studies have investigated PRO-QOL after hepatectomy, even the best QOL questionnaires are imprecise. Also, the timing of the evaluation is usually unknown. We attempted to examine QOL in patients who had undergone hepatectomy. The first clinical question we investigated was whether postoperative QOL was better among patients who underwent hepatectomy or transarterial chemoembolization (TACE). The second question was whether QOL was better among patients who underwent laparoscopic hepatectomy or classic open hepatectomy. Finally, we compared the changes in QOL scores seen after hepatectomy. This systematic review and meta-analysis examined the current status of QOL studies of patients who underwent hepatectomy. In addition, it revealed a future clinical question and provided an idea for a future clinical study.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines were followed when obtaining and reporting the meta-analysis data[18]. The PICOS scheme was followed when reporting the inclusion criteria. A systematic literature search was performed independently by two authors (Ishinuki T and Ota S) using PubMed and MEDLINE, including the Cochrane Library. The search was limited to human studies whose findings were reported in English. No restriction was set for the type of publication, the publication date, or publication status. Patients of any age or sex who underwent liver resection for any type of hepatic lesion were considered as outlined in the PICOS scheme. The search strategy was based on different combinations of words for each database. For the PubMed database the following combination was used: ("qol"[All Fields] AND ("liver"[MeSH Terms] OR "liver"[All Fields] OR "livers"[All Fields] OR "liver s"[All Fields]) AND ("surgery"[MeSH Subheading] OR "surgery"[All Fields] OR "surgical procedures, operative"[MeSH Terms] OR ("surgical"[All Fields] AND "procedures"[All Fields] AND "operative"[All Fields]) OR "operative surgical procedures"[All Fields] OR "general surgery"[MeSH Terms] OR ("general"[All Fields] AND "surgery"[All Fields]) OR "general surgery"[All Fields] OR "surgery s"[All Fields] OR "surgerys"[All Fields] OR "surgeries"[All Fields]). For the Medline database, the following combination was used: (QOL and Liver and Surgery).
The two independent authors screened the titles and abstracts of the primary studies identified in the database search. Duplicate studies were excluded. The following inclusion criteria were set for inclusion in the meta-analysis: (1) Studies comparing preoperative QOL and postoperative QOL in patients who underwent liver resection for any type of hepatic lesion; (2) Studies comparing QOL between laparoscopic hepatectomy and open hepatectomy in patients who underwent liver resection for any type of hepatic lesion; (3) Studies reporting at least one QOL outcome; and (4) If the same institute reported more than one study, only the most recent or the highest level study was included.
The following exclusion criteria were set: (1) Original studies assessing the outcomes of liver transplantation; (2) Review articles, letters, comments, and case reports; and (3) Studies for which it was impossible to retrieve or calculate the data of interest. The Cohen kappa statistic was used to quantify the agreement between the investigators.
The protocol was registered with PROSPERO (#CRD42021225970).
The same two authors extracted the following primary data: (1) The questionnaires used for each QOL evaluation; (2) The first author, year of publication, and type of study; (3) The etiology of the disease and the number of times each intervention was performed; and (4) The timing of the evaluations. The reasons why studies were excluded from the full-text evaluation are shown in Supplementary Tables 1-3. All excluded references are listed in the supplemental references.
The Newcastle–Ottawa Scale (NOS) was used to assess the quality of the included studies, as they included observational studies (http://www.ohri.ca/). The NOS consists of three domains, patient selection, comparability of study groups, and outcome assessment. The minimum risk of bias gain 9 points. We considered studies that scored ≥ 7, 4-6, and < 4 to be high quality, moderate quality, and low quality, respectively[19].
All analyses were performed using the RevMan software (version 5.3.; The Cochrane Collaboration). The mean differences (MD) between groups were calculated for continuous variables. The interquartile ranges of the data were transformed by dividing them by 1.35 to produce alternative standard deviation values[20]. Multiple means and standard deviations were combined using the StatsToDo online web program (https://www.statstodo.com/index.php).
The χ2 test was used to evaluate heterogeneity, and the Cochran Q and I2 statistics were reported. The I2 value describes the percentage variation between studies in degrees of freedom. Low, moderate, and high heterogeneity were defined based on cut-off values of 25%, 50%, and 75%, respectively, using the obtained I2 test values[21].
All results were considered significant at P values of < 0.05.
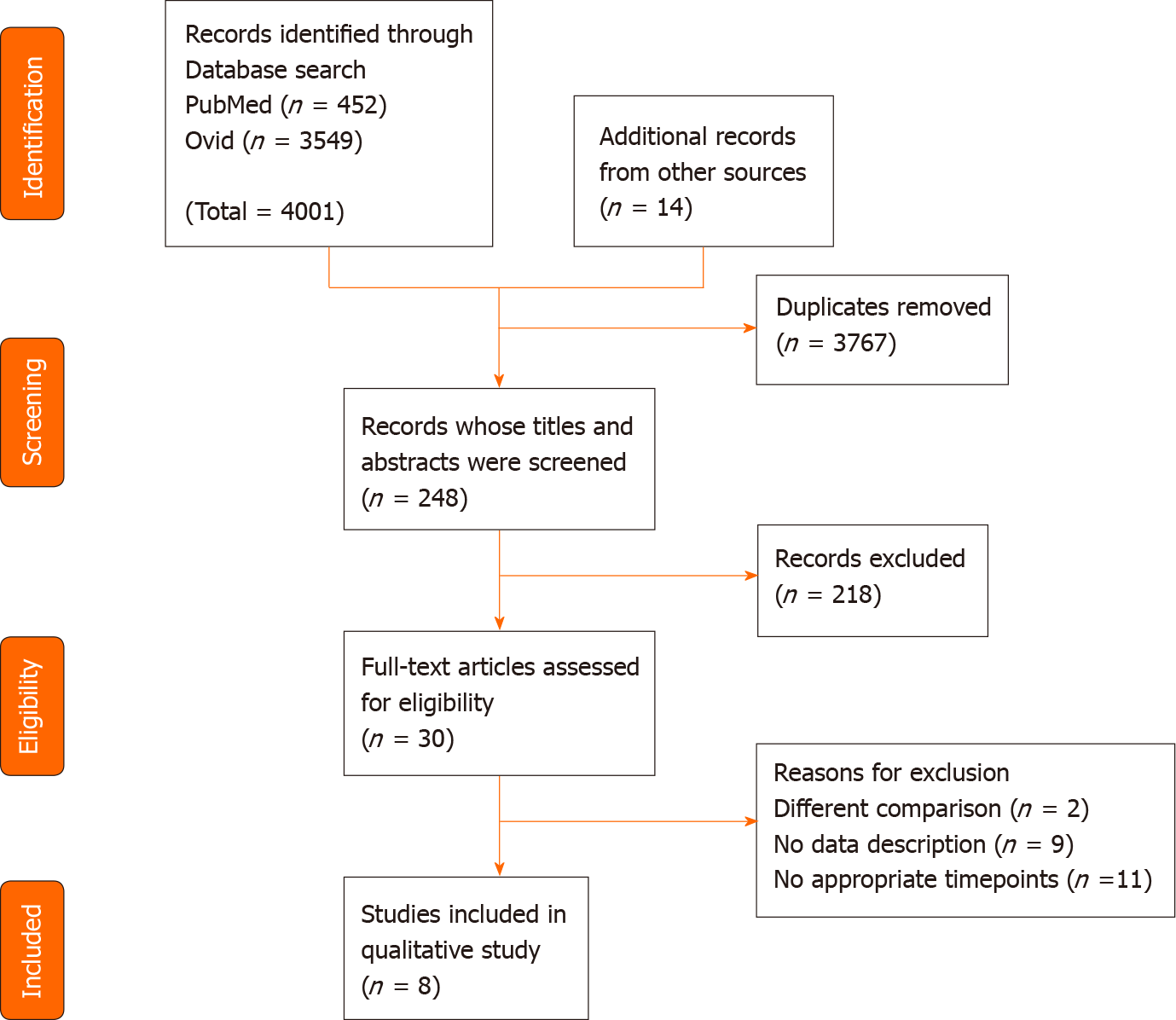
The literature search yielded 248 articles, and the abstracts were reviewed by two independent researchers (Figure 1). Of these, 30 articles were selected for full-text review. Two articles were excluded due to different comparison. Nine articles were excluded due to no data description being provided. Eleven articles were excluded as they did not involve appropriate timepoints. Detailed information about the excluded articles is shown in Supplementary Tables 1-3. Finally, a total of 8 articles were included in this meta-analysis (Table 1). Two studies used the FACT-Hep[22,23], four studies used the SF-36[24-27], and two studies used the QLQ-C30[28,29]. None of them were randomized controlled studies.
| Questionnaire | Ref. | Type of study | Etiology | Number of each intervention | Timing of the evaluations |
| FACT-Hep | Martin et al[22], 2007 | Prospective | HCC; CCC; CRLM | 24 hepatectomies | Pre, 6 wk, 3 mo |
| Liu et al[23], 2012 | Retrospective | HCC | 65 hepatectomies; 50 chemotherapies | Pre, 3 mo, 6 mo, 9 mo, 12 mo | |
| SF-36 | Giuliani et al[24], 2014 | Retrospective | Miscellaneous | 38 open hepatectomies; 28 laparoscopic hepatectomies | 6 mo, 12 mo |
| Qiu et al[25], 2015 | Prospective | Hemangioma | 344 enucleations; 386 hepatectomies | Pre, 1 mo, 3 mo, 6 mo | |
| Chiu et al[26], 2018 | Prospective | HCC | 332–324 hepatectomies | Pre, 3 mo, 6 M mo | |
| Liu et al[27], 2019 | Prospective | Hemangioma | 73 open hepatectomies; 73 laparoscopic hepatectomies | Pre, 1 mo, 3 mo | |
| QLQ-C30 | Dasgupta et al[28], 2008 | Prospective | CRLM; CCC; HCC | 101–33 hepatectomies | Pre, 6 mo, 12 mo, 36–48 mo |
| Rees et al[29], 2012 | Prospective | CRLM | 232–193 hepatectomies | Pre, 3 mo, 6 mo, 12 mo |
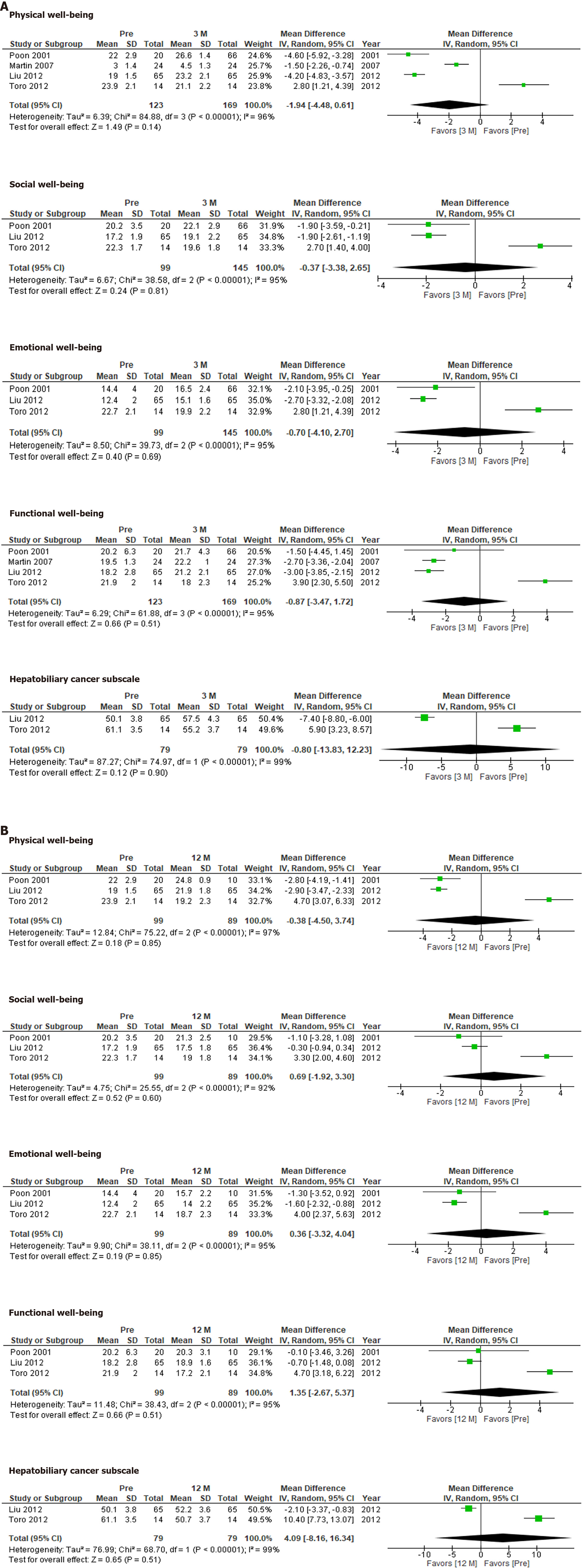
The FACT-Hep was used to compare QOL before and after hepatectomy. None of the FACT-Hep domains differed significantly from their preoperative levels at 3 mo (Figure 2A) or 12 mo (Figure 2B), although several domains at 3 mo after hepatectomy tends to be better than those at 12 mo.
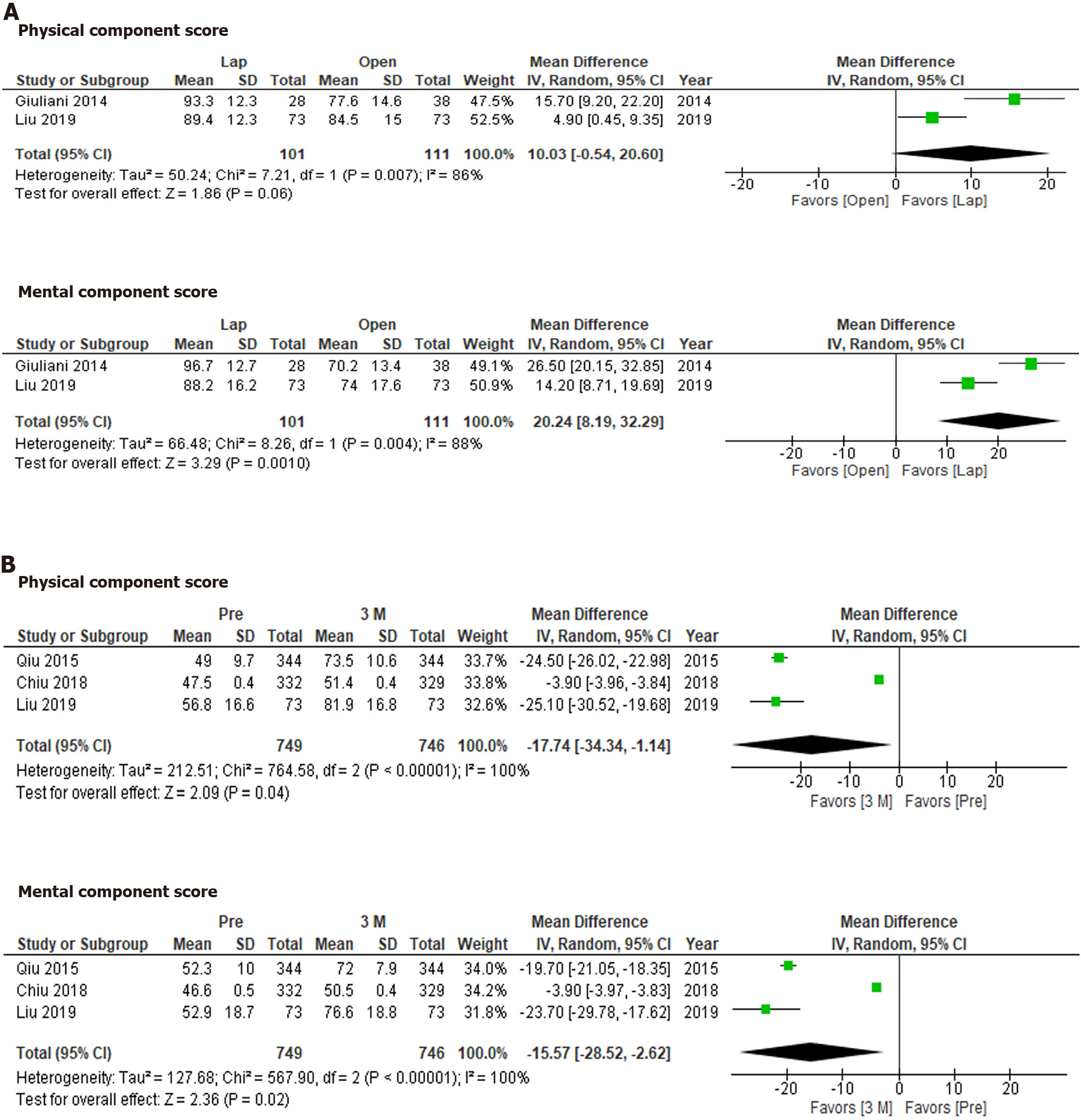
The SF-36 was used to compare QOL between laparoscopic hepatectomy and open hepatectomy at 3-6 mo after treatment (Figure 3A). Although the physical component score did not differ significantly between the groups (P = 0.08), the mental component score for the laparoscopic hepatectomy group was significantly more favorable than that for the open hepatectomy group (P = 0.001). On the other hand, the physical component scores and mental component scores seen at 3 mo after hepatectomy were significantly more favorable than those observed before hepatectomy (Figure 3B; P = 0.04 and P = 0.02, respectively).
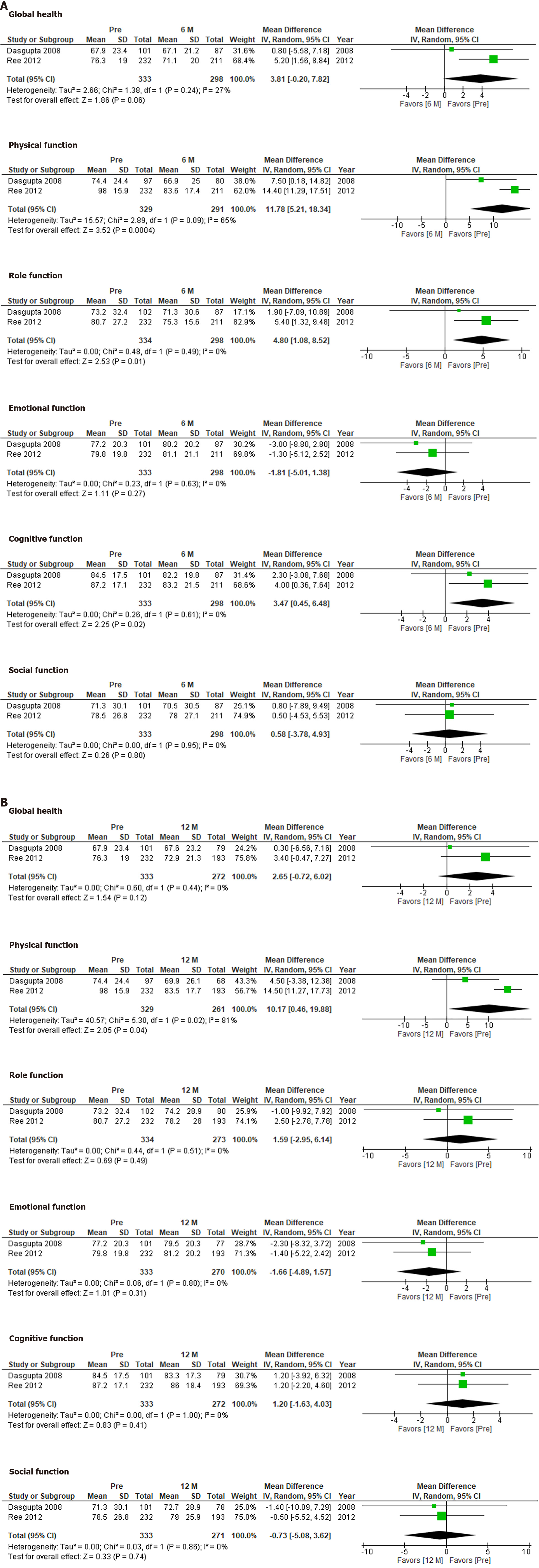
The QLQ-C30 was used to evaluate QOL at 6 mo and 12 mo after hepatectomy (Figure 4). No significant differences in global health, emotional function, or social function were observed between the preoperative assessment and 6 mo or 12 mo after hepatectomy. However, the patients’ preoperative physical function scores were better than those seen at 6 mo or 12 mo after hepatectomy (P = 0.0004 and P = 0.04, respectively). Although role function and cognitive function differed significantly between the preoperative assessment and 6 mo after hepatectomy (P = 0.01 and P = 0.02, respectively), they did not differ significantly between the preoperative assessment and 12 mo after hepatectomy.
The quality assessment was conducted using the NOS score (Supplementary Table 4). Three studies were of moderate quality, and seven studies were of high quality.
Liver resection has become a safe surgical procedure for liver tumors and is now used for living transplantation[2,3,5,13]. The clinical outcomes of hepatectomy have been reported based on quality assessments since 2000, and evidence has accumulated rapidly within the last decade[30]. We evaluated two crucial clinical questions in this study. The first was whether hepatectomy or TACE resulted in better QOL. The second was whether laparoscopic or open hepatectomy resulted in better QOL. Furthermore, we examined the changes in QOL seen at 3 mo, 6 mo, and 12 mo after hepatectomy.
The findings of postoperative QOL assessments can vary according to the type of questionnaire used, the surgical approach, the etiology of the disease, and the timing of the evaluations[30].
Most of the studies examined in the present review were conducted using a paper-based approach or face-to-face interviews. A more mobile approach would allow the comprehensive collection of a greater variety of data[31]. However, some questionnaires are not suitable for extensive prospective surveys due to cost issues. In addition, language translation is also an obstacle to international comparisons among questionnaires. Therefore, the statistical power of the studies was limited.
The FACT-Hep did not identify any significant changes in QOL after hepatectomy. Our results indicate that HCC patients’ QOL recovered within 12 mo after hepatectomy. Although the QOL scores for each subdomain at 3 mo did not differ significantly from those observed before hepatectomy, the integrated mean tended to be more favorable at 3 mo after hepatectomy than before hepatectomy. This would depend on the condition of the patients who were eligible for the studies. Therefore, the QOL scores for these patients would have improved after hepatectomy.
According to the SF-36, QOL was significantly better at 3 mo after hepatectomy than before hepatectomy. On the other hand, different results might have been obtained if the studies had involved asymptomatic patients[26]. Another concern is the sensitivity of each questionnaire. It is possible that the SF-36 is more sensitive than the FACT-Hep in these circumstances.
Laparoscopic hepatectomy has become the standard approach for liver resection[5,7,9]. It is considered that the reduced invasiveness associated with the minimal wound length of the laparoscopic approach allows patients to recover faster than is possible with the open approach[5]. As we demonstrated in this study, QOL could be better after laparoscopic hepatectomy than after open hepatectomy. In addition, the mental component scores of the patients that underwent laparoscopic hepatectomy were significantly better than their physical component scores. The reduced invasiveness of laparoscopic hepatectomy is considered to improve physical outcomes. However, this was not proven, presumably due to the long period of time between the surgery and the assessments. The degree to which mental QOL was preserved is a unique feature of laparoscopic hepatectomy.
The QLQ-C30 produced different results from the other QOL questionnaires. Physical function and role function had deteriorated significantly at 6 mo after hepatectomy, but had recovered at 12 mo after hepatectomy. The changes in the QLQ-C30 seen after hepatectomy seem to be more reasonable. They indicate that surgery itself temporarily reduces patients’ QOL. In addition, the examined studies included asymptomatic patients who had metastatic liver tumors from colorectal cancer[28,29], which could also explain why QOL deteriorated after surgery.
Laparoscopic hepatectomy resulted in better QOL than open hepatectomy. The results of QOL evaluations performed after hepatectomy could depend on the type of questionnaire used, the timing of the assessment, and the etiology of the hepatic disease.
The quality of life (QOL) assessment after hepatectomy has never been summarized. Therefore, comprehensive systematic review and meta-analysis would have great scientific value.
Lack of randomized controlled trial motivate us to plan prospective study. However, sample size calculation is difficult due to lack of the QOL value. This analysis would be helpful to conduct future trials.
Research objectives were to elucidate QOL after hepatectomy.
Systematic review and meta-analysis was conducted according to PROSPERO guidelines with Preferred Reporting Items for Systematic Reviews and Meta-Analyses check lists.
A total of 8 articles were included in this meta-analysis. QOL was better after laparoscopic hepatectomy than after open hepatectomy. Physical and mental component score of the 36-Item Short-Form Health Survey at 3 mo was significantly better than before hepatectomy.
The outcomes of evaluations of QOL after hepatectomy can depend on the type of questionnaire used, the timing of the assessment, and the etiology of the hepatic disease.
The values from this study could be useful to plan future randomized control trial.
We thank Tan S and Nara M for their help in preparing this manuscript and valuable discussions.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: The Japanese Society of Gastroenterology, No. 30537; The Japan Surgical Society, No. O411084; The Japanese Society of Gastrointestinal Surgery, No. G0220360; The Japanese Society of Clinical Surgery, No. 18343; and The Japanese Society of Surgical Infection, No. 5154651376.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Donadon M, Kordzaia D, Lo Tesoriere R S-Editor: Wang JL L-Editor: A P-Editor: Li X
| 1. | Ishii M, Mizuguchi T, Harada K, Ota S, Meguro M, Ueki T, Nishidate T, Okita K, Hirata K. Comprehensive review of post-liver resection surgical complications and a new universal classification and grading system. World J Hepatol. 2014;6:745-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, Arakawa S, Yoshida R, Isetani M. Recent advances in the surgical treatment of hepatocellular carcinoma. World J Gastroenterol. 2014;20:14381-14392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Kaneko J, Kokudo T, Inagaki Y, Hasegawa K. Innovative treatment for hepatocellular carcinoma (HCC). Transl Gastroenterol Hepatol. 2018;3:78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Akateh C, Black SM, Conteh L, Miller ED, Noonan A, Elliott E, Pawlik TM, Tsung A, Cloyd JM. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J Gastroenterol. 2019;25:3704-3721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Mizuguchi T, Kawamoto M, Meguro M, Shibata T, Nakamura Y, Kimura Y, Furuhata T, Sonoda T, Hirata K. Laparoscopic hepatectomy: a systematic review, meta-analysis, and power analysis. Surg Today. 2011;41:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Meguro M, Mizuguchi T, Kawamoto M, Ota S, Ishii M, Nishidate T, Okita K, Kimura Y, Hirata K. Clinical comparison of laparoscopic and open liver resection after propensity matching selection. Surgery. 2015;158:573-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Mizuguchi T, Kawamoto M, Nakamura Y, Meguro M, Hui TT, Hirata K. New technique of extracorporeal hepatic inflow control for pure laparoscopic liver resection. Surg Laparosc Endosc Percutan Tech. 2015;25:e16-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ye SP, Qiu H, Liao SJ, Ai JH, Shi J. Mini-invasive vs open resection of colorectal cancer and liver metastases: A meta-analysis. World J Gastroenterol. 2019;25:2819-2832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Liu R, Wakabayashi G, Kim HJ, Choi GH, Yiengpruksawan A, Fong Y, He J, Boggi U, Troisi RI, Efanov M, Azoulay D, Panaro F, Pessaux P, Wang XY, Zhu JY, Zhang SG, Sun CD, Wu Z, Tao KS, Yang KH, Fan J, Chen XP. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol. 2019;25:1432-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (2)] |
| 10. | Patel BY, White L, Gavriilidis P, Satyadas T, Frampton AE, Pai M. A systematic review into patient reported outcomes following pancreaticoduodenectomy for malignancy. Eur J Surg Oncol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Li L, Yeo W. Value of quality of life analysis in liver cancer: A clinician's perspective. World J Hepatol. 2017;9:867-883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Sajid MS, Iftikhar M, Rimple J, Baig MK. Use of health-related quality of life tools in hepatobiliary surgery. Hepatobiliary Pancreat Dis Int. 2008;7:135-137. [PubMed] |
| 13. | Gandhi S, Khubchandani S, Iyer R. Quality of life and hepatocellular carcinoma. J Gastrointest Oncol. 2014;5:296-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 14. | McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making. 2008;28:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Heffernan N, Cella D, Webster K, Odom L, Martone M, Passik S, Bookbinder M, Fong Y, Jarnagin W, Blumgart L. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol. 2002;20:2229-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-13; discussion 415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 429] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 17. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9802] [Cited by in RCA: 11469] [Article Influence: 358.4] [Reference Citation Analysis (0)] |
| 18. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 11040] [Article Influence: 690.0] [Reference Citation Analysis (0)] |
| 19. | Islam MM, Iqbal U, Walther B, Atique S, Dubey NK, Nguyen PA, Poly TN, Masud JH, Li YJ, Shabbir SA. Benzodiazepine Use and Risk of Dementia in the Elderly Population: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2016;47:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 20. | Schumm WR, Higgins M, Lockett L, Huang S, Abdullah N, Asiri A, Clark K, McClish K. Does Dividing the Range by Four Provide an Accurate Estimate of a Standard Deviation in Family Science Research? Marri Fami Rev. 2017;53:1-23. [DOI] [Full Text] |
| 21. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46547] [Article Influence: 2115.8] [Reference Citation Analysis (3)] |
| 22. | Martin RC, Eid S, Scoggins CR, McMasters KM. Health-related quality of life: return to baseline after major and minor liver resection. Surgery. 2007;142:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Liu J, Wang Y, Zhang D, Liu B, Ou Q. Comparison of survival and quality of life of hepatectomy and thrombectomy using total hepatic vascular exclusion and chemotherapy alone in patients with hepatocellular carcinoma and tumor thrombi in the inferior vena cava and hepatic vein. Eur J Gastroenterol Hepatol. 2012;24:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Giuliani A, Migliaccio C, Ceriello A, Aragiusto G, La Manna G, Calise F. Laparoscopic vs. open surgery for treating benign liver lesions: assessing quality of life in the first year after surgery. Updates Surg. 2014;66:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Qiu J, Chen S, Wu H. Quality of life can be improved by surgical management of giant hepatic haemangioma with enucleation as the preferred option. HPB (Oxford). 2015;17:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Chiu CC, Lee KT, Lee HH, Wang JJ, Sun DP, Huang CC, Shi HY. Comparison of Models for Predicting Quality of Life After Surgical Resection of Hepatocellular Carcinoma: a Prospective Study. J Gastrointest Surg. 2018;22:1724-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 27. | Liu Q, Liu F, Ding J, Wei Y, Li B. Surgical outcomes and quality of life between laparoscopic and open approach for hepatic hemangioma: A propensity score matching analysis. Medicine (Baltimore). 2019;98:e14485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Dasgupta D, Smith AB, Hamilton-Burke W, Prasad KR, Toogood GJ, Velikova G, Lodge JP. Quality of life after liver resection for hepatobiliary malignancies. Br J Surg. 2008;95:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Rees JR, Blazeby JM, Fayers P, Friend EA, Welsh FK, John TG, Rees M. Patient-reported outcomes after hepatic resection of colorectal cancer metastases. J Clin Oncol. 2012;30:1364-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Wee IJY, Syn N, Lee LS, Tan SS, Chiow AKH. A systematic review and meta-analysis on the quality of life after hepatic resection. HPB (Oxford). 2020;22:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Rincon E, Monteiro-Guerra F, Rivera-Romero O, Dorronzoro-Zubiete E, Sanchez-Bocanegra CL, Gabarron E. Mobile Phone Apps for Quality of Life and Well-Being Assessment in Breast and Prostate Cancer Patients: Systematic Review. JMIR Mhealth Uhealth. 2017;5:e187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |