Published online Apr 28, 2020. doi: 10.13105/wjma.v8.i2.89
Peer-review started: December 27, 2019
First decision: January 19, 2020
Revised: February 8, 2020
Accepted: March 19, 2020
Article in press: March 19, 2020
Published online: April 28, 2020
Processing time: 123 Days and 0.2 Hours
The incidence of gastric cardia cancer is increasing around the world. Since the discovery of Helicobacter pylori (H. pylori), numerous studies have proved that it is a causative factor for many kinds of digestive system tumors. Although the literature on gastric cardia cancer and H. pylori is not scarce, there are still many controversies on the relationship between gastric cardia cancer and H. pylori. Many Western research results showed that there was a negative or no correlation between H. pylori infection and gastric cardia cancer, but in several studies in Asian countries, such as China, H. pylori was demonstrated to be a risk factor for gastric cardia cancer. Therefore, we intended to analyze the related studies to find out the relationship between H. pylori and gastric cardia cancer and find out the causes of the above controversies. We also conducted a meta-analysis of the relationship between cagA positive expression of H. pylori and gastric cardia cancer, to find out whether there is an effect between those two. The primary purpose of this paper was to explore the relationship between gastric cardia cancer and H. pylori. Through analysis, the study showed the reasons for the controversies mentioned above: (1) Geographical factors could affect the relationship between H. pylori and gastric cardia cancer; (2) The definition of gastric cardia cancer in various studies is inconsistent. The result of a meta-analysis about the relationship between H. pylori virulence factor cagA and gastric cardia cancer showed that there was no relationship between these two.
Core tip: The relationship between gastric cardia cancer and Helicobacter pylori (H. pylori) is unclear. Therefore, this article focuses on the relationship between gastric cardia cancer and H. pylori and the reasons for this relationship. This paper also discusses the relationship between cagA and gastric cardia cancer, as well as the influence of different colonization sites of H. pylori on gastric cardia cancer and the influence of H. pylori on the prognosis of gastric cardia cancer, and such.
- Citation: Yin JJ, Duan FJ, Madhurapantula SV, Zhang YH, He G, Wang KY, Ji XK, Wang KJ. Helicobacter pylori and gastric cardia cancer: What do we know about their relationship? World J Meta-Anal 2020; 8(2): 89-97
- URL: https://www.wjgnet.com/2308-3840/full/v8/i2/89.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i2.89
In modern society, cancer has become a significant cause of morbidity and mortality worldwide. At present, the incidence of gastric cardia cancer is increasing around the world[1,2]. Gastric cardia cancer stands out distinctly and is different from gastric cancer and esophageal cancer[3,4]. It is relatively insidious, and the degree of cancer cell differentiation is low. Gastric cardia cancer also has extensive invasion and rapid metastasis. Gastric cardia cancer has seriously endangered human health and has become a significant public health problem[5]. This cancer occurs in the region of the gastric cardia, which is located at the junction of the stomach and esophagus. It is the transitional zone between the distal esophageal mucosa and the proximal gastric mucosa. Gastric cardia cancer always occurs on the lesser curvature side of the gastric cardia (~75%), followed by the posterior and anterior walls, and the greater curvature side is rarely affected.
Gastric cardia cancer is neither esophageal cancer nor gastric cancer. There are many differences among the three. Scholars have found that the incidence of esophageal cancer and gastric cardia cancer increased, while the incidence of distal gastric cancer decreased[6-8]. Many epidemiological, histopathological, and molecular biological studies have showed that there are some similarities between gastric cardia cancer and distal esophageal adenocarcinoma, but gastric cardia cancer is different from distal gastric cancer and esophageal squamous cancer. Gastric cardia cancer and the other two cancers have not only different pathogenesis, but also have different prognostic factors. Besides, esophageal adenocarcinoma mainly spreads to the parastatal lymph nodes and the lower posterior mediastinum, while gastric cardia carcinoma has the characteristic of bilateral metastasis to the chest and abdominal cavity.
For the definition of gastric cardia cancer, there are few international definitions. Gastric cardia cancer is defined as cancer occurring at the anatomic site of the cardia, within 2 cm below the esophagogastric junction[9]. The Siewert[3] classification is another standard classification scheme. It differentiates the following three distinct tumor entities in the area of the esophagogastric junction: Esophageal tumor (type I), true cardia tumor (type II), and subcardial gastric carcinoma (types III). The World Health Organization (WHO) classification of tumors classified gastric cardia cancer as tumors of the esophagogastric junction in 2000. The literature states that “adenocarcinomas that cross the esophagogastric junction are called adenocarcinoma of the esophagogastric junction, regardless of where the bulk of the tumor lies.”
Some scholars believe that the formation of gastric cardia cancer has undergone multi-stage pathological processes such as cardia inflammation, intestinal metaplasia, intraepithelial neoplasia, carcinoma in situ, and invasive cancer[10]. There are many reasons for the formation of gastric cardia cancer, and the development of gastric cardia cancer is the result of multiple factors interacting in various stages.
The age-standardized incidence of gastric cardia cancer (per 100000 cases) in different parts of the world was shown in the study of Colquhoun et al[11]. It showed that Eastern/Southeastern Asia had a higher incidence of gastric cardia cancer than other regions in the world, at 8.7 per 100000 for males and 2.4 per 100000 for females. The incidence of gastric cardia cancer in Sub-Saharan Africa was lower than that in other regions, at 0.2 per 100000 for males and 0.1 per 100000 for females. Gastric cardia cancer was more common in males than in females.
China has a higher incidence of gastric cardia cancer in the world. Epidemiological data showed that the incidence of esophageal and gastric cardia cancer was consistent. China is a high incidence area of esophageal cancer, and many studies suggested that the incidence of gastric cardia cancer is also high in this area, where esophageal cancer has a high incidence. This phenomenon has been observed in China’s Linxian (Henan Province)[12], Cixian (Hebei Province)[13], Chaoshan (Guangdong Province)[14], and other areas with a high incidence of esophageal cancer.
Helicobacter pylori (H. pylori), which colonizes specifically in the human stomach, was first identified from patients with peptic ulcer disease by Barry Marshall and Robin Warren[15]. The prevalence of H. pylori infection in most countries in the world remains high. According to Hooi et al[16], there were 4.4 billion cases of H. pylori infection worldwide in 2015. Africa had the highest percentage of H. pylori infection (70.1%; 95% confidence interval [CI]: 62.6-77.7), while the lowest percentage was observed in Oceania (24.4%; 95%CI: 18.5-30.4). Nigeria had the highest H. pylori infection rate of any country (87.7%; 95%CI: 83.1-92.2). The prevalence of H. pylori in Latin America cannot be underestimated. A meta-analysis in a study by Curado et al[17] suggested that H. pylori infection rates are high in all age groups in Latin America. Differences in social and economic conditions across different countries might also affect the infection rate of H. pylori[18]. It was associated with many diseases[19-21], especially gastric cancer[22-24].
H. pylori colonizes uniquely in the human stomach. Severe diseases caused by H. pylori infection are related to the host, bacteria, and environment, such as some gastrointestinal disorders[25]. There is also a link between gastric cardia cancer and H. pylori. Therefore, the purpose of this paper was to find out the relationship between them through literature review and meta-analysis (Supplementary Figure 1).
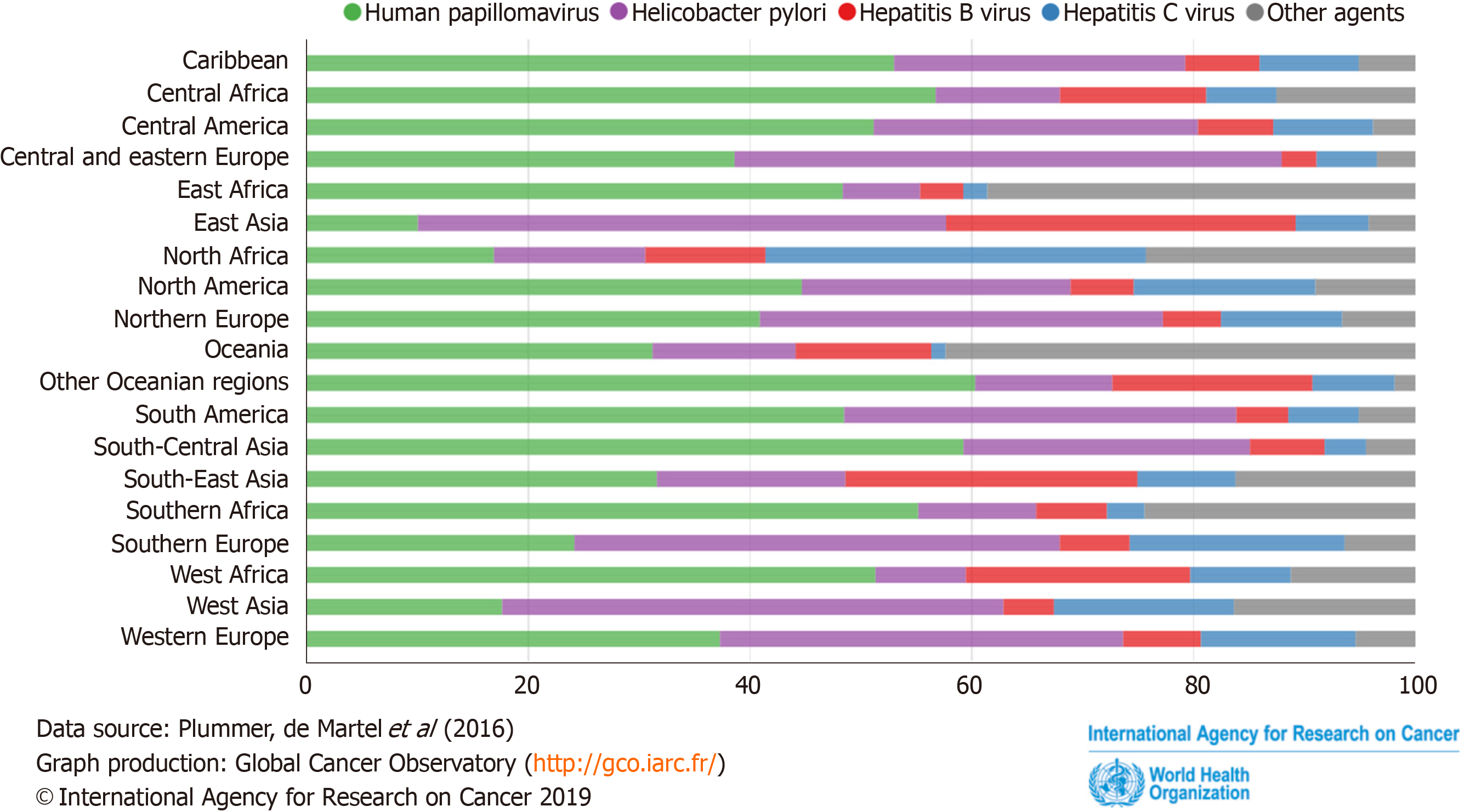
Data from GLOBOCAN 2018 showed that H. pylori infections account for 35.7% of cancers caused by infection-related factors worldwide, ranking first. It showed more details about the proportion of cancers caused by H. pylori infection in various regions of the world (Figure 1). The top three areas were: Central and Eastern Europe (49.3%), East Asia (47.6%), and West Asia (45.2%). These data showed the seriousness of the harm of H. pylori to humans.
Table 1 shows the H. pylori infection rates and age-standardized incidence of gastric cardia cancer (per 100000 cases) in different parts of the world, based on the studies of Colquhoun et al[11] and Hooi et al[16]. The data showed that the infection rate of H. pylori was also high in several regions with a high incidence of gastric cardia cancer. However, although the infection rate of H. pylori was as high as 76.9% in Africa and Western Asia, the incidence of gastric cardia cancer was relatively low. Therefore, these data revealed a correlation between gastric cardia cancer and H. pylori to some extent.
| Region1 | Gastric cardia cancer | H. pylori infection rate (%) | |
| Males | Females | General population | |
| Eastern/Southeastern Asia | 8.7 | 2.4 | 58.1 |
| Eastern Europe | 4.7 | 1.4 | 62.8 |
| Central/Southern America & Caribbean | 4.0 | 1.3 | 63.4 |
| Central Asia | 3.8 | 1.7 | 79.5 |
| Northern & Western Europe | 3.7 | 1.0 | 37.2 |
| Oceania | 3.4 | 1.1 | 24.4 |
| Southern Europe | 3.3 | 0.9 | 55.0 |
| Northern American | 2.7 | 0.7 | 37.1 |
| Northern Africa & Western Asia | 2.5 | 1.2 | 76.92 |
| Sub-Saharan Africa | 0.2 | 0.1 | |
Data from some Western countries showed that H. pylori was a protective factor for gastric cardia cancer, or there was no pathogenic relationship between these two. A nested case-control study of a Norwegian population by Hansen et al[26] and others found that gastric cardia cancer was negatively associated with H. pylori (odds ratio [OR] = 0.27, 95%CI: 0.12-0.59). Ye et al[27] found no correlation between gastric cardia cancer and H. pylori infection based on the native Swedish population who were younger than 80 years.
However, studies in China, Japan, and other Asian countries have shown that H. pylori was the pathogenic factor for gastric cardia cancer. A cohort study by Kamangar et al[28] on 29584 residents in Linxian (Henan Province, China) suggested that H. pylori infection was a risk factor for gastric cardia cancer (hazard ratio [HR] = 1.64; 95%CI: 1.26-2.14). Yasuo et al[29] also found that 75% of Japanese patients with gastric cardia cancer had H. pylori infection, and H. pylori infection was closely associated with gastric cardia cancer.
Marlene[30] and others conducted a meta-analysis of the research on the relationship between H. pylori infection and gastric cardia cancer. The population of this study included people from all over the world. The results of the study showed that for gastric cardia cancer, the pooled relative risk (PRR) was 1.08 (95%CI: 0.83-1.40; I2 = 52.8%), but the difference was not statistically significant. Subsequently, those authors divided the regions into high incidence areas and low incidence areas based on the incidence of gastric cancer. China, Japan, and South Korea were classified as high-risk settings, while Australia, Finland, Germany, United States, etc. were classified as low-risk Settings. The results showed that H. pylori infection was a risk factor for gastric cardia cancer in the high incidence areas of gastric cancer (RR = 0.78, 95%CI: 0.63-0.97; I2 = 11.6%). This result suggested that geographical factors could affect the relationship between H. pylori and gastric cardia cancer.
Also, as mentioned above, although gastric cardia cancer was classified as a type of esophagogastric junction cancer by the WHO in 2000, there are still inconsistencies in the diagnostic criteria for gastric cardia cancer among many current studies. In the study of Hansen et al[26], the diagnosis of gastric cardia cancer was based on International Classification Atom of Diseases for Oncology (second edition). Inconsistencies in the diagnostic criteria for gastric cardia cancer may also lead to a wrong diagnosis, thus affecting the relationship between gastric cardia cancer and H. pylori and leading to inconsistent research results.
The virulence factor genes of H. pylori include vacA, cagA, cagE, oipA, babA2, babB, and iceA, etc.[31,32]. H. pylori virulence factors play an important role in the progression of gastric cardia cancer. Cytotoxin-associated gene A (cagA) is a virulence factor of H. pylori that has been studied most in the world. CagA is located at one end of the cag-PAI (a 40-kb piece of DNA) and is likely to be incorporated into the H. pylori genome through a horizontal transfer process[33]. CagA was only found in H. pylori highly virulent strains. H. pylori cagA protein appears as a bacterial oncoprotein[34]. Lee et al[35] showed that people infected with H. pylori which contains the cagA protein produce more reactive oxygen species and have an increased risk of gastric cancer. CagA protein is the only bacterial oncoprotein identified to date. CagA contains two repeatable protein-binding motifs, the Glu-Pro-Ile-Tyr-Ala (EPIYA) motif and the cagA multimerization (CM) motif. There are two major pathological and biochemical processes that contribute to H. pylori cagA-induced gastric cancer: Abnormal cancer-promoting signals caused by SHP2 imbalance via the EPIYA motif, and gastric epithelial destruction caused by CM-mediated PAR1 inhibition[36]. EPIYA motifs are divided into four categories (EPIYA-A, -B, -C, and -D), depending on the amino acid sequence surrounding each EPIYA motif, and they have different characteristics[37].
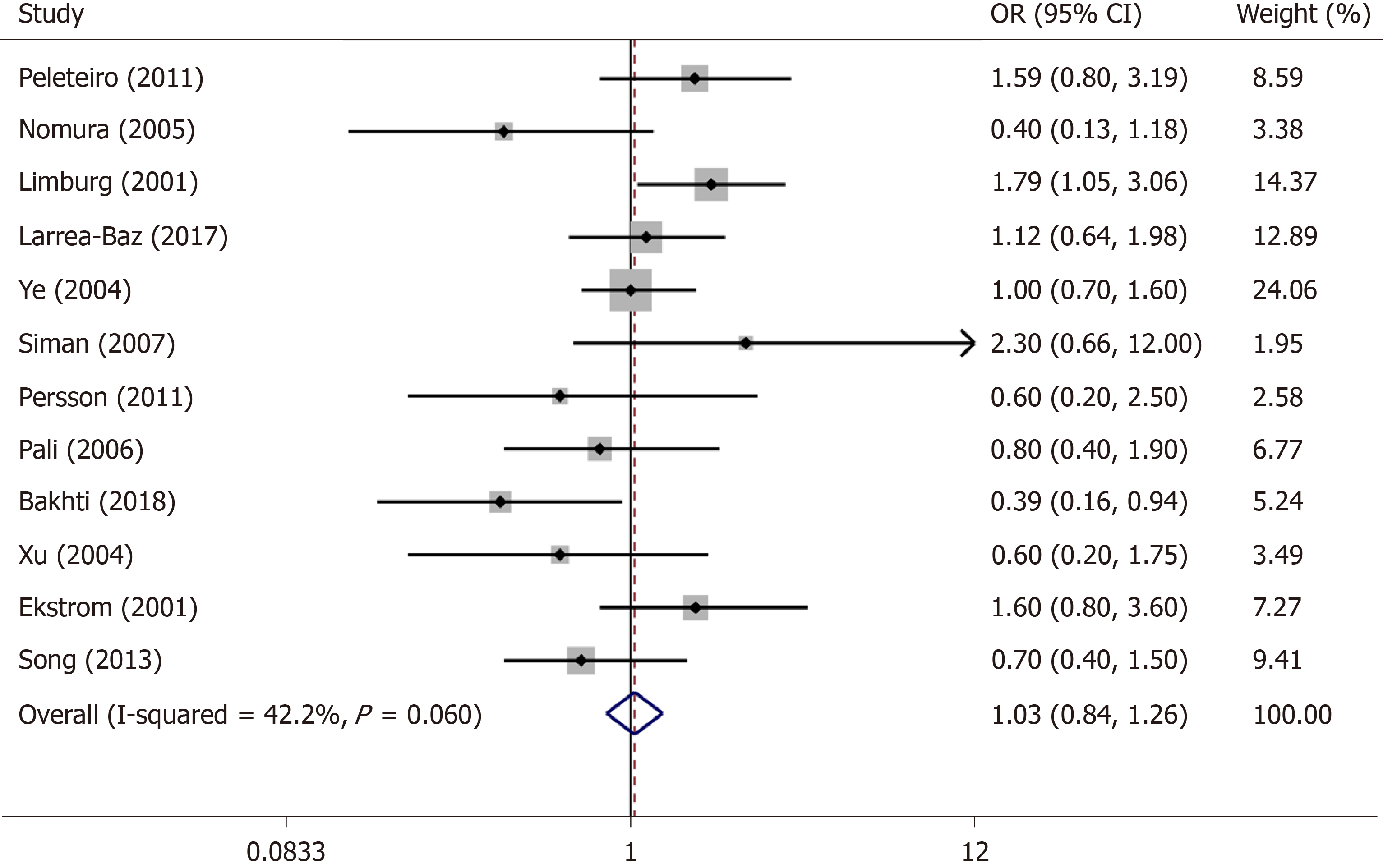
The current research results on the relationship between cagA and gastric cardia cancer are also controversial. In the study by Limburg et al[38], the adjusted OR value of cagA positive gastric cardia cancer patients compared with cagA negative patients was 1.79 (95%CI: 1.05-3.06), indicating that cagA positivity was a risk factor for patients with gastric cardia cancer. Some other studies showed that there was a significant negative correlation between cagA positivity and the development of gastric cardia cancer. Ye et al’s[27] study showed that cagA positivity was not associated with the risk for gastric cardia adenocarcinoma (OR = 1.00, 95%CI: 0.70-1.60). Therefore, we performed a meta-analysis of the relationship between H. pylori cagA and gastric cardia cancer.
The study was based on the guidelines of Meta-analysis of Observational Studies in Epidemiology (MOOSE)[39] and the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA)[40]. PubMed, Web of Science, Embase, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), and Wanfang (China) electronic databases were searched for relevant articles published up to December 2019. The search items were “gastric cardia cancer” and “Helicobacter pylori cagA”.
The quality of the eligible studies (Supplementary file 1) was evaluated according to the Newcastle-Ottawa Scale (NOS)[41] (Supplementary Table 2), and articles with a score higher than six were considered high-quality. The STATA (Version 13.1 MP, Stata Corp, College Station, TX, United States) was used to analyze the data. P < 0.05 or I2 > 50.0% was considered to have significant heterogeneity. A fixed-effects model was used when there was no significant heterogeneity, otherwise a random-effect model was used. A sensitivity analysis was performed to evaluate the stability of the pooled results. Egger’s test[42] and Begg’s test[43] were used to assess the extent of publication bias. P < 0.05 was considered statistically significant, and all statistical tests were two-sided.
After screening, a total of 12 articles were included in the study[27,38,44-53]. The random-effects model (I2 = 42.2%, P = 0.099) and fixed-effects smodel (I2 = 42.2%, P = 0.060) were used for heterogeneity testing, respectively. The results of the heterogeneity test showed no significant difference. The sensitivity analysis showed that the combined OR did not change significantly, indicating that the combined OR was fairly stable. The P values of Egger’s and Begg’s tests were 0.277 and 0.244, respectively. Detailed results are shown in the Supplementary Materials. The fixed-effect model was eventually selected for use (Figure 2). The pooled OR of this study was 1.03 (95%CI: 0.84-1.26). The results could not indicate that H. pylori cagA positivity is a risk factor for gastric cardia cancer (Supplementary Figures 2-4).
H. pylori infection in different parts of the gastric cardia mucosa is different, which is consistent with the difference in the incidence of gastric cardia cancer in different regions. The distribution of H. pylori infection in the cardia mucosa is characterized by the invasion of both sides of the root of mucosal fold in the cardia. The high incidence area of gastric cardia cancer overlap with the high infection area of H. pylori. In the course of gastric cardia cancer, H. pylori infection in the cardia and gastric antrum mainly promotes the occurrence of the tumor. H. pylori infection also affects the prognosis of patients with gastric cardia cancer. H. pylori may be related to the prediction of gastric cardia cancer, but it is not an independent factor.
Gastric cardia cancer is a multi-factorial ailment, which is the result of the interaction of multiple factors, including genetic factors, environmental factors, etc.
Demographic characteristics such as age, gender, and ethnicity are all factors influencing gastric cardia cancer. The incidence of gastric cardia cancer increases in the elderly, and the research by Chen et al[14] showed that the population of 50-80 years had a high incidence of gastric cardia cancer. Several other studies suggested that gastric cardia cancer is more prevalent in males. Colquhoun[11] and others showed that the incidence of gastric cardia cancer was significantly higher in males than in females (male: female = 3:1). Kubo et al[54] and others, through the analysis of five groups of cancer registration data (1992-1998), also found a high incidence of gastric cardia cancer in males.
Current studies have found that many tumors have a family genetic predisposition, and studies on the relationship between gastric cardia cancer and family history have found a correlation between these two. Yang et al[55] investigated 16605 patients with gastric cardia cancer and 26053 patients with non-cardia cancer through questionnaires. And after a long period of follow-up of 2000 patients, they found that positive family history significantly increased the risk of gastric cardia cancer.
Yang et al[55] found that smoking significantly increased the risk of gastric cardia cancer (OR = 1.98, 95%CI: 1.79-2.19). The results of the study by Zendehdel et al[56] also showed that compared to never-users of any tobacco, smokers had an increased risk for gastric cardia cancer (RR = 2.10, 95%CI: 1.50–3.00). Obese subjects (BMI ≥ 30 kg/m2) had a higher risk of gastric cardia cancer than the average population (RR = 2.73, 95%CI: 1.56-4.79), according to the results of a prospective cohort study in the Netherlands[57]. Also, Jansson et al's[58] study showed a correlation between covert coping strategies when maltreated at work and the risk of gastric cardia cancer.
Genetic risk factors, epigenetic risk factors, long noncoding RNAs, and microRNAs are all in the field of molecular biology. For example, a tumor suppressor protein encoded by the p53 gene often mutates in many kinds of cancers and is related to cell proliferation and tumor growth[59]. Shao’s[60] study showed that after Bonferroni correction, the association between TP53BP1 rs560191 G4C and gastric cardia cancer remained significant. The advent of multiple genome-wide association studies has led to the successful identification of many single nucleotide polymorphisms (SNPs), including those associated with gastric cardia cancer. Xiao et al’s[61] study also showed that the interaction between SNPs and H. pylori infection is related to the increased risk of gastric cardia cancer. In Abdi et al’s[62] study, the factors of molecular biology of gastric cardia cancer were studied more specifically, including not only SNPsd but also long noncoding RNAs and microRNAs.
This article discusses the relationship between H. pylori and gastric cardia cancer; however, the relationship between H. pylori and gastric cardia cancer could not be analyzed generally. Accurate classification of gastric cardia cancer and patients' geographic factors can influence the relationship between H. pylori and gastric cardia cancer. Also, H. pylori has a large number of different virulence factors. In this study, only the relationship between the positive expression of cagA and gastric cardia cancer was meta-analyzed, but no correlation between these two was found. The effects of other virulence factors on gastric cardia cancer need to be further studied. Both H. pylori related hosts and the environment may have an impact on cardia cancer, which has not been discussed in depth in our research. In addition, the impact of family history on the relationship between H. pylori and gastric cardia cancer, and even the relationship between eradication of H. pylori and gastric cardia cancer were not included in this study, which need further research.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Melo FF, Shenoy SM, Soriano-Ursúa MA S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Kusano C, Gotoda T, Khor CJ, Katai H, Kato H, Taniguchi H, Shimoda T. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. 2008;23:1662-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Carr JS, Zafar SF, Saba N, Khuri FR, El-Rayes BF. Risk factors for rising incidence of esophageal and gastric cardia adenocarcinoma. J Gastrointest Cancer. 2013;44:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Siewert JR, Stein HJ, Sendler A, Fink U. Surgical resection for cancer of the cardia. Semin Surg Oncol. 1999;17:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Kim JY, Lee HS, Kim N, Shin CM, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Park DJ, Kim HH, Jung HC. Prevalence and clinicopathologic characteristics of gastric cardia cancer in South Korea. Helicobacter. 2012;17:358-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | da Costa DM, Dos Santos Pereira E, de Lima Silva-Fernandes IJ, Ferreira MV, Rabenhorst SH. Characterization of Gastric Cardia Tumors: Differences in Helicobacter pylori Strains and Genetic Polymorphisms. Dig Dis Sci. 2015;60:2712-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 7. | Abrams JA, Gonsalves L, Neugut AI. Diverging trends in the incidence of reflux-related and Helicobacter pylori-related gastric cardia cancer. J Clin Gastroenterol. 2013;47:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Vial M, Grande L, Pera M. Epidemiology of adenocarcinoma of the esophagus, gastric cardia, and upper gastric third. Recent Results Cancer Res. 2010;182:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Wijnhoven BP, Siersema PD, Hop WC, van Dekken H, Tilanus HW. Adenocarcinomas of the distal oesophagus and gastric cardia are one clinical entity. Rotterdam Oesophageal Tumour Study Group. Br J Surg. 1999;86:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Wang LD, Zheng S, Zheng ZY, Casson AG. Primary adenocarcinomas of lower esophagus, esophagogastric junction and gastric cardia: in special reference to China. World J Gastroenterol. 2003;9:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 12. | Wang LD, Zheng S. Cancer mechanisms of esophagus and cardia in populations with high incidence of esophageal cancer in Henan. Zhengzhou Daxue Xuebao (Yixue Ban). 2002;37:717-729. [DOI] [Full Text] |
| 13. | He YT, Hou J, Chen ZF, Qiao CY, Song GH, Meng FS, Jin HX, Chen C. Trends in incidence of esophageal and gastric cardia cancer in high-risk areas in China. Eur J Cancer Prev. 2008;17:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Chen GC, Liu SH, Hong LL. Analysis of 575 cases of gastric cardia pathological changes in Chaoshan gastric cardia cancer high risk area. Zhongguo JIceng Yiyao. 2017;24:801-804. [DOI] [Full Text] |
| 15. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3257] [Article Influence: 79.4] [Reference Citation Analysis (1)] |
| 16. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2031] [Article Influence: 253.9] [Reference Citation Analysis (0)] |
| 17. | Curado MP, de Oliveira MM, de Araújo Fagundes M. Prevalence of Helicobacter pylori infection in Latin America and the Caribbean populations: A systematic review and meta-analysis. Cancer Epidemiol. 2019;60:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Zamani M, Ebrahimtabar F, Zamani V, Miller WH, Alizadeh-Navaei R, Shokri-Shirvani J, Derakhshan MH. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 488] [Article Influence: 69.7] [Reference Citation Analysis (1)] |
| 19. | Kyburz A, Müller A. Helicobacter pylori and Extragastric Diseases. Curr Top Microbiol Immunol. 2017;400:325-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Xie SH, Lagergren J. Risk factors for oesophageal cancer. Best Pract Res Clin Gastroenterol. 2018;36-37:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Kucukazman M, Yeniova O, Dal K, Yavuz B. Helicobacter pylori and cardiovascular disease. Eur Rev Med Pharmacol Sci. 2015;19:3731-3741. [PubMed] |
| 22. | Amieva M, Peek RM. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology. 2016;150:64-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 633] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 23. | Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 880] [Cited by in RCA: 851] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 24. | Cover TL. Helicobacter pylori Diversity and Gastric Cancer Risk. mBio. 2016;7:e01869-e01815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 25. | Passaro DJ, Chosy EJ, Parsonnet J. Helicobacter pylori: consensus and controversy. Clin Infect Dis. 2002;35:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Hansen S, Vollset SE, Derakhshan MH, Fyfe V, Melby KK, Aase S, Jellum E, McColl KE. Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut. 2007;56:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Ye W, Held M, Lagergren J, Engstrand L, Blot WJ, McLaughlin JK, Nyrén O. Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J Natl Cancer Inst. 2004;96:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 28. | Kamangar F, Qiao YL, Blaser MJ, Sun XD, Katki H, Fan JH, Perez-Perez GI, Abnet CC, Zhao P, Mark SD, Taylor PR, Dawsey SM. Helicobacter pylori and oesophageal and gastric cancers in a prospective study in China. Br J Cancer. 2007;96:172-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Egi Y, Ito M, Tanaka S, Imagawa S, Takata S, Yoshihara M, Haruma K, Chayama K. Role of Helicobacter pylori infection and chronic inflammation in gastric cancer in the cardia. Jpn J Clin Oncol. 2007;37:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer Causes Control. 2011;22:375-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Chang WL, Yeh YC, Sheu BS. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J Biomed Sci. 2018;25:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 32. | Dabiri H, Jafari F, Baghaei K, Shokrzadeh L, Abdi S, Pourhoseingholi MA, Mohammadzadeh A. Prevalence of Helicobacter pylori vacA, cagA, cagE, oipA, iceA, babA2 and babB genotypes in Iranian dyspeptic patients. Microb Pathog. 2017;105:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Hatakeyama M, Higashi H. Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis. Cancer Sci. 2005;96:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Hayashi T, Senda M, Morohashi H, Higashi H, Horio M, Kashiba Y, Nagase L, Sasaya D, Shimizu T, Venugopalan N, Kumeta H, Noda NN, Inagaki F, Senda T, Hatakeyama M. Tertiary structure-function analysis reveals the pathogenic signaling potentiation mechanism of Helicobacter pylori oncogenic effector CagA. Cell Host Microbe. 2012;12:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Lee DY, Jung DE, Yu SS, Lee YS, Choi BK, Lee YC. Regulation of SIRT3 signal related metabolic reprogramming in gastric cancer by Helicobacter pylori oncoprotein CagA. Oncotarget. 2017;8:78365-78378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Nishikawa H, Hatakeyama M. Sequence Polymorphism and Intrinsic Structural Disorder as Related to Pathobiological Performance of the Helicobacter pylori CagA Oncoprotein. Toxins (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Chen SY, Zhang RG, Duan GC. Pathogenic mechanisms of the oncoprotein CagA in H. pylori-induced gastric cancer (Review). Oncol Rep. 2016;36:3087-3094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Limburg P, Qiao Y, Mark S, Wang G, Perez-Perez G, Blaser M, Wu Y, Zou X, Dong Z, Taylor P, Dawsey S. Helicobacter pylori seropositivity and subsite-specific gastric cancer risks in Linxian, China. J Natl Cancer Inst. 2001;93:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 16724] [Article Influence: 669.0] [Reference Citation Analysis (0)] |
| 40. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9247] [Cited by in RCA: 8857] [Article Influence: 553.6] [Reference Citation Analysis (0)] |
| 41. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12512] [Article Influence: 834.1] [Reference Citation Analysis (0)] |
| 42. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40242] [Article Influence: 1437.2] [Reference Citation Analysis (2)] |
| 43. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] |
| 44. | Peleteiro B, Cavaleiro-Pinto M, Barros R, Barros H, Lunet N. Is cardia cancer aetiologically different from distal stomach cancer? Eur J Cancer Prev. 2011;20:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Nomura AM, Kolonel LN, Miki K, Stemmermann GN, Wilkens LR, Goodman MT, Perez-Perez GI, Blaser MJ. Helicobacter pylori, pepsinogen, and gastric adenocarcinoma in Hawaii. J Infect Dis. 2005;191:2075-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Fernández de Larrea-Baz N, Pérez-Gómez B, Michel A, Romero B, Lope V, Pawlita M, Fernández-Villa T, Moreno V, Martín V, Willhauck-Fleckenstein M, López-Abente G, Castilla J, Fernández-Tardón G, Dierssen-Sotos T, Santibáñez M, Peiró R, Jiménez-Moleón JJ, Navarro C, Castaño-Vinyals G, Kogevinas M, Pollán M, de Sanjosé S, Del Campo R, Waterboer T, Aragonés N. Helicobacter pylori serological biomarkers of gastric cancer risk in the MCC-Spain case-control Study. Cancer Epidemiol. 2017;50:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Simán JH, Engstrand L, Berglund G, Forsgren A, Florén CH. Helicobacter pylori and CagA seropositivity and its association with gastric and oesophageal carcinoma. Scand J Gastroenterol. 2007;42:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Persson C, Jia Y, Pettersson H, Dillner J, Nyrén O, Ye W. H. pylori seropositivity before age 40 and subsequent risk of stomach cancer: a glimpse of the true relationship? PLoS One. 2011;6:e17404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Palli D, Masala G, Del Giudice G, Plebani M, Basso D, Berti D, Numans ME, Ceroti M, Peeters PH, Bueno de Mesquita HB, Buchner FL, Clavel-Chapelon F, Boutron-Ruault MC, Krogh V, Saieva C, Vineis P, Panico S, Tumino R, Nyrén O, Simán H, Berglund G, Hallmans G, Sanchez MJ, Larrãnaga N, Barricarte A, Navarro C, Quiros JR, Key T, Allen N, Bingham S, Khaw KT, Boeing H, Weikert C, Linseisen J, Nagel G, Overvad K, Thomsen RW, Tjonneland A, Olsen A, Trichoupoulou A, Trichopoulos D, Arvaniti A, Pera G, Kaaks R, Jenab M, Ferrari P, Nesi G, Carneiro F, Riboli E, Gonzalez CA. CagA+ Helicobacter pylori infection and gastric cancer risk in the EPIC-EURGAST study. Int J Cancer. 2007;120:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Bakhti SZ, Latifi-Navid S, Zahri S, Bakhti FS, Hajavi N, Yazdanbod A. Are Helicobacter pylori highly cytotoxic genotypes and cardia gastric adenocarcinoma linked? Lessons from Iran. Cancer Biomark. 2017;21:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Xu XF. Infection of CagA-positive Helicobacter pylor and the risk for cardia and non-cardia gastric cancer in high-risk area of China. Fuzhou: Fujian Yike Daxue, 2004. |
| 52. | Ekström AM, Held M, Hansson LE, Engstrand L, Nyrén O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 287] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 53. | Song H, Michel A, Nyrén O, Ekström AM, Pawlita M, Ye W. A CagA-independent cluster of antigens related to the risk of noncardia gastric cancer: associations between Helicobacter pylori antibodies and gastric adenocarcinoma explored by multiplex serology. Int J Cancer. 2014;134:2942-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Kubo A, Corley DA. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am J Gastroenterol. 2004;99:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Yang X, Wang JP, Cui JL, Lin HL, Hou ZC, Zhu WL, Song X, Li XM, Wang XD, Li JL, Wang LD. Influence of family history, BMI, smoking, and alcohol drinking on risk and prognosis of gastric cardia cancer. Zhengzhou Daxue Xuebao (Yixue Ban). 2013;48:124-127. [DOI] [Full Text] |
| 56. | Zendehdel K, Nyrén O, Luo J, Dickman PW, Boffetta P, Englund A, Ye W. Risk of gastroesophageal cancer among smokers and users of Scandinavian moist snuff. Int J Cancer. 2008;122:1095-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Merry AH, Schouten LJ, Goldbohm RA, van den Brandt PA. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut. 2007;56:1503-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 58. | Jansson C, Johansson AL, Jeding K, Dickman PW, Nyrén O, Lagergren J. Psychosocial working conditions and the risk of esophageal and gastric cardia cancers. Eur J Epidemiol. 2004;19:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Soussi T, Béroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 475] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 60. | Shao A, Zheng L, Chen S, Gu H, Jing H. p21, p53, TP53BP1 and p73 polymorphisms and the risk of gastric cardia adenocarcinoma in a Chinese population. Biomarkers. 2015;20:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Xiao FK, Yang JX, Li XM, Zhao XK, Zheng PY, Wang LD. Interaction of 22 risk SNPs with Helicobacter pylori infection and risk of gastric cardia adenocarcinoma. Future Oncol. 2019;15:3579-3585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Abdi E, Latifi-Navid S, Zahri S, Yazdanbod A, Pourfarzi F. Risk factors predisposing to cardia gastric adenocarcinoma: Insights and new perspectives. Cancer Med. 2019;8:6114-6126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |