Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.2008
Peer-review started: December 4, 2020
First decision: December 30, 2020
Revised: January 12, 2021
Accepted: January 27, 2021
Article in press: January 27, 2021
Published online: March 16, 2021
Processing time: 84 Days and 3.7 Hours
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by systemic involvement and multiple autoantibodies in the serum. Patients with protein C (PC) and protein S (PS) deficiency are prone to thrombosis. In contrast, patients with primary hyperfibrino-lysis tend to bleed.
A 52-year-old female patient with bilateral pleural effusion was diagnosed with "tuberculous pleurisy" and treated with anti-tuberculosis drugs and prednisone. The coagulation-related laboratory results showed decreased fibrinogen, PC activity, PS activity, and antithrombin Ш activity. The immune-related laboratory results showed positive antinuclear antibody, anti-Smith antibody, anticardiolipin antibody (ACL), anti-β2-glycoprotein I antibody (aβ2GPI) and direct Coomb’s test and decreased complement 3 and complement 4. Thoracoscopy was performed and bloody pleural fluid was drained. Pathology of the pleural biopsy showed lymphocytes, plasma cells, and a few eosinophils in adipose and fibrous connective tissue. Results of whole exome sequencing of blood showed no genetic mutations suggesting the presence of hereditary hematological diseases. The patient was finally diagnosed with SLE and primary hyperfibrinolysis, and was treated with prednisolone, hydroxychloroquine, and compound cyclophosphamide.
PC and PS deficiency in SLE might be related to ACL and aβ2GPI. SLE and primary hyperfibrinolysis can coexist in one patient, with both a risk of thrombosis and a risk of bleeding.
Core Tip: Systemic lupus erythematosus (SLE) with both protein C (PC) and protein S (PS) deficiency, and primary hyperfibrinolysis has not been reported in previous literature. We report a patient with SLE presenting with pleural effusion who was found to have both primary hyperfibrinolysis and PC and PS deficiency. The balance between the prevention of thrombosis and hemorrhage should be considered.
- Citation: Liao YX, Guo YF, Wang YX, Liu AH, Zhang CL. Systemic lupus erythematosus combined with primary hyperfibrinolysis and protein C and protein S deficiency: A case report. World J Clin Cases 2021; 9(8): 2008-2014
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/2008.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.2008
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with variable clinical features and multiple autoantibodies. Acute respiratory involvement (ARI) was present in 40% of SLE patients undergoing chest computed tomography (CT)[1]. The most frequent ARI was pleural effusion (33%)[1]. When patients with SLE have antiphospholipid antibodies (APL), they are prone to recurrent arteriovenous thrombosis and pathological pregnancy. Patients with protein C (PC) and protein S (PS) deficiency are prone to thrombosis[2-4], while patients with primary hyperfibrino-lysis tend to bleed[5]. We report a patient with pleural effusion and a diagnosis of SLE with primary hyperfibrinolysis and PC and PS deficiency.
A 52-year-old female patient presented with a history of chest tightness and shortness of breath for six months.
Six months ago, the patient was admitted to a local hospital with bilateral pleural effusion. A left thoracic drainage tube was placed to drain approximately 6000 mL of yellow colored pleural effusion. Investigations of the pleural fluid showed exudative fluid and no acid-fast bacilli. The results of the purified protein derivative of tuberculin test, interferon-γ release assay, autoimmunity antibodies, positron emission tomography-CT, and bronchoscopy were negative. The patient was diagnosed with "tuberculous pleurisy" and was treated with anti-tuberculosis drugs (isoniazid, rifampicin, pyrazinamide), prednisone, and pleurocentesis. Anti-tuberculosis treatment was stopped due to abnormal liver function tests and liver protection treatment was administered.
The patient had no previous medical history.
No smoking and drinking history, and no hereditary family history were noted.
Breath sounds were weak and percussion sound was dull in both lower lungs.
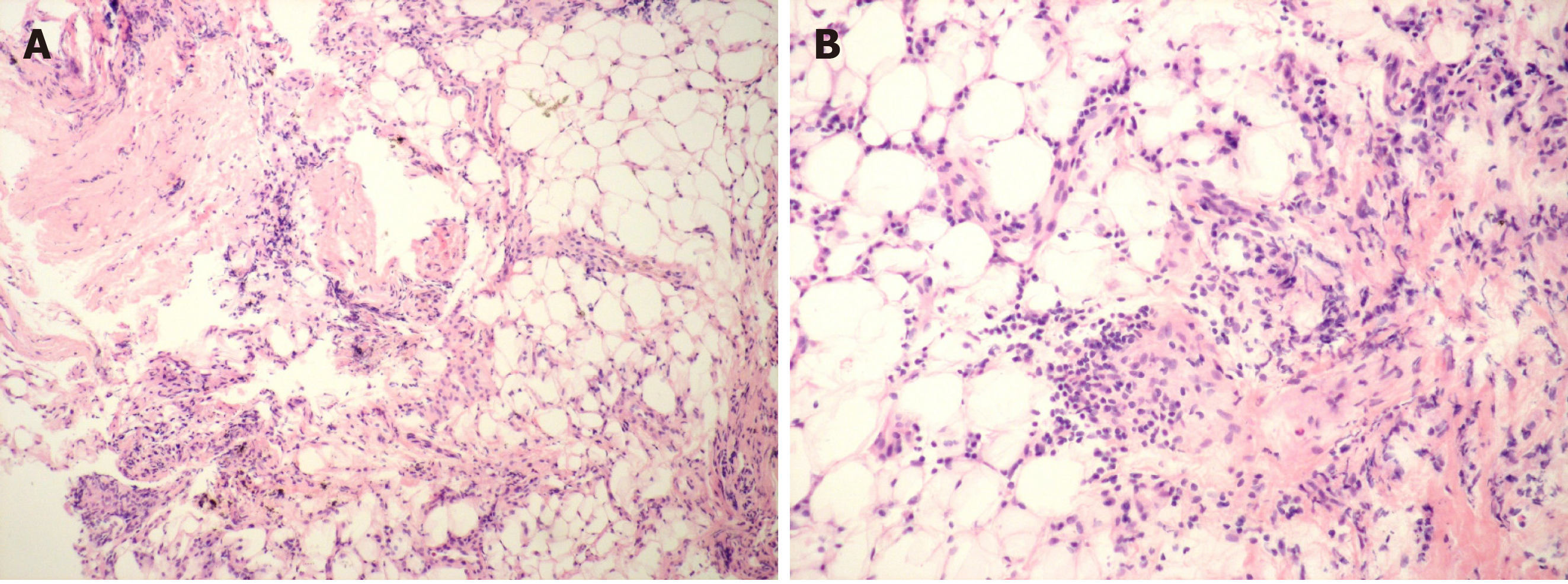
The coagulation-related laboratory results showed: fibrinogen (Fib), 1.2 g/L (normal range: 2.00-4.00 g/L); D-dimer, 263 ng/mL (< 255 ng/mL); thrombin time (PT), 16.1 s (8.8-13.4 s); activated partial thrombin time (APTT), 47.6 s (23.3-38.1 s); PC activity, 30% (70%-140%); PS activity, 43.2% (76%-135%), and antithrombin (AT) Ш activity, 10% (83%-128%). The immune-related laboratory results were as follows: antinuclear antibody (ANA), 1:160; anti-Smith (Sm) antibody, (+); IgG anticardiolipin antibody (ACL), 58.5 U/mL (< 20 U/mL); anti-β2-glycoprotein I antibody (aβ2GPI), 62.04 RU/mL (< 20 RU/mL); complement 3 (C3), 45 mg/dL (79-152 mg/dL); complement 4 (C4), 5 mg/dL (16-38 mg/dL), and direct Coomb’s test (++). Liver function tests showed: alanine transaminase, 44 U/L and aspartate aminotransferase, 46 U/L. Blood gas analysis (in room air) showed PaO2 of 62.4 mmHg. The results of complete blood count, renal function, and thyroid function tests were normal. Thoracoscopy was performed, and 2050 mL of bloody pleural fluid was drained. Pathology of the pleural biopsy showed lymphocytes, plasma cells, and a few eosinophils in adipose and fibrous connective tissue (Figure 1). Results of whole exome sequencing (WES) of blood showed a FCGR2A gene mutation which is related to susceptibility to lupus nephritis and no genetic mutations suggesting the presence of hereditary hematological diseases.
CT pulmonary angiogram, pulmonary ventilation/perfusion scan, and deep venous ultrasound of both lower extremities were normal.
Ai-Hua Liu, MD, Department of Rheumatology and Immunology, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, China: The patient met the revised classification criteria for SLE. Decreased PC and PS might be related to ACL and aβ2GPI. SLE combined with primary hyperfibrinolysis is rare. The patient should be treated with prednisolone 30 mg once a day, hydroxychloroquine 0.2 g twice a day, and compound cyclophospha-mide 50 mg every other day.
Chun-Li Zhang, MD, Department of Hematology, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, China: The patient could be diagnosed with primary hyperfibrinolysis. Whole exome sequencing of blood should be tested to find if she had a hereditary hematological disease.
The final diagnosis of the presented case was SLE and primary hyperfibrinolysis.
The patient was treated with prednisolone 30 mg once a day, hydroxychloroquine 0.2 g twice a day, and compound cyclophosphamide 50 mg every other day.
Following systemic treatment of SLE for 3 mo, the amount of pleural effusion decreased, but Fib did not improve and no bleeding events were observed. PS, PC, AT-III, dsDNA, ACL and aβ2GPI returned to normal with ANA 1:100 and C3 and C4 slightly decreased at 3 mo, 7 mo and 10 mo of treatment.
The patient in this report had bilateral pleural effusion for 6 mo and her condition was misdiagnosed as tuberculous pleurisy. Anti-tuberculous and prednisolone treatment did not improve her condition, and pleural biopsy showed no evidence of tuberculosis. The patient had pleuritis, ANA levels of 1:160, tested positive for anti-Sm antibody, ACL, aβ2GPI, and Coomb’s test, and had reduced C3 and C4 levels; these criteria met the European League Against Rheumatism/American College of Rheumatology revised classification criteria for SLE[6]. A diagnosis of SLE was considered. Although the patient was positive for ACL and aβ2GPI, she had no history of recurrent arteriovenous thrombosis or pathological pregnancy. Therefore, anti-phospholipid antibody syndrome (APS) was not diagnosed. In this patient, Fib was decreased, PT and APTT were prolonged, and D-dimer was normal. Bloody pleural fluid was drained after video-assisted pleural biopsy, coagulation function was abnormal and liver function was normal. Primary fibrinolysis was considered. Due to the presence of PC, PS, and AT III deficiency, combined with primary hyperfibrino-lysis, WES of blood was performed which did not indicate hereditary hematological diseases.
Primary hyperfibrinolysis results from an abnormal increase in fibrinolytic activity that leads to premature, excessive destruction of fibrin and/or degradation of fibrinogen or other coagulation factors which cause bleeding. Primary hyperfibrino-lysis is classified as congenital (caused by e.g., α2-plasmin inhibitor deficiency, plasminogen activator inhibitor type 1 deficiency, increased plasminogen activator) or acquired (caused by e.g., severe liver disease, tumor, surgery and trauma, post-partum hemorrhage)[5]. Secondary hyperfibrinolysis following coagulation in the blood vessels is mainly seen in disseminated intravascular coagulation. SLE patients with positive APL are prone to thromboembolism. The rheological parameters of clots were significantly increased in active SLE patients along with enhanced fibrin crosslinking and hyperfibrinogenemia[7]. Impaired fibrinolysis has been reported in patients with SLE and may contribute to both the development of hypercoagulability and an increased risk of thrombosis[8]. However, this patient had primary hyperfibrinolysis characterized by hemorrhage at the same time, which is very rare.
APL is a heterogeneous group of autoantibodies targeting phospholipid binding proteins, including ACL, aβ2GPI, and lupus anticoagulant (LA). In addition to APS, APL is positive in autoimmune diseases, infections, drugs, and malignant tumors. SLE is the most common rheumatic disease associated with APL. It was found in approximately 30%-40% of patients with SLE[9,10]; LA was present in approximately 34% of patients with SLE[11], ACL was positive in 36% of SLE patients[12], and aβ2GPI was present in 37% of SLE patients[13].
The PC system is composed of PC, PS, and thrombomodulin (TM). TM is a thrombin receptor on the surface of endothelial cells. Thrombin forms a 1:1 complex with TM, cracks PC, and forms activated PC (APC). APC uses PS as a cofactor and exerts an anticoagulant effect by inactivating FV and FⅧ. Inherited PC deficiency is an autosomal dominant disorder with a prevalence of 0.2%-0.5% in the general population and 3% in patients with venous thrombus embolism (VTE)[2]. Inherited PS deficiency is an autosomal dominant disorder with an estimated prevalence of 0.1%-0.7% in the general population and 2% in patients with VTE[2]. Acquired PC and PS deficiency may be caused by decreased synthesis, increased loss, or increased consumption of PC and PS, drugs, or autoimmune antibodies. The patient had no history of familial inheritance, or thromboembolic events. We considered a diagnosis of acquired PC and PS deficiency, and the decline of PC and PS might be related to ACL and aβ2GPI as PC, PS, ACL and aβ2GPI all returned to normal after 3 mo of systemic immunosuppressive treatment of SLE.
Most APL does not bind directly to phospholipids but to phospholipid-binding proteins in the plasma. The main phospholipid-binding proteins in plasma are β2GPI, prothrombin, PS, and PC. The effects of APL on PC and PS pathways include[14]: (1) APL induced acquired APC resistance (APCA)[15]; (2) Antibodies against PC, PS, or endothelial cells. APL has affinity for PC and PS[16]. PC and PS levels are decreased in APS patients[16]. Anti-endothelial antibodies may also be associated with APS[17]. Anti-endothelial antibodies may interfere with the localization of PC on the endothelial PC receptor or have an affinity for TM, thereby preventing the TM binding of thrombin to activate PC. Anti-TM antibodies that interfere with the activation of PC were found in patients with SLE[14]; (3) Low prothrombin levels. Antiprothrombin antibodies have been found in patients with APS and cause phospholipid-dependent coagulation time lengthening. As activation of the PC pathway requires thrombin, low levels of prothrombin may lead to impaired activation of PC[14]. In the study by Belfeki et al[18], APL were positive in 32.1% patients with SLE (LA 16.9%, ACL 13.2%, aβ2GPI 7.5%) and PS deficiency was noted in 32.1% patients with SLE. PC deficiency and acquired APCA showed no significant difference between the SLE patients and controls. A case of SLE presenting with positive APL, acquired APCR and autoimmune hemolytic anemia was reported[19]. However, in the study by Ramirez et al[20], anti-PC was associated with APCR in patients with SLE, independently of APL. Studies showed an association between reduced PS levels and APL in patients with SLE[21,22]; however, another study found no association between decreased PS levels and ACL[23]. Two cases of PS deficiency in patients with SLE with no APL were reported[24,25]. The PS deficiency was possibly aggravated by the presence of C4b-binding protein which may increase in SLE and resulted in a decrease in free PS levels in one case[24], and was caused by oral anticoagulant therapy or deep vein thrombosis in the other case[25]. The results of studies of associations of deficiencies in PC, PS and APL in patients with SLE were conflicting[26]. More clinical and basic trials are needed to verify the association between PC, PS and APL, and explore more mechanisms of PC and PS deficiency in patients with SLE without the influence of APL.
AT, the most important anticoagulant substance, accounts for approximately 75% of the plasma physiologic anticoagulant activity. Its main functions are inactivation of FXa and thrombin, and inactivation of other serine proteases such as FIXa, FXIa, and FXIIa whose anticoagulant activity is closely related to heparin. Inherited AT deficiency is a rare autosomal dominant disorder. The prevalence is approximately 0.02% in the population and 1% in the VTE population. More than 50% of the patients had a history of thromboembolic disease before 50 years old[2]. The causes of acquired antithrombin deficiency include decreased antithrombin synthesis, increased loss, increased consumption, and drugs. AT III deficiency in this patient may be related to abnormal liver function and consumption due to bleeding after thoracoscopy.
For patients with SLE who have positive APL, we should test for PC, PS, and AT levels to assess the risk of thrombosis. SLE combined with primary hyperfibrinolysis is rare, with both a risk of thrombosis and a risk of bleeding. The balance between the two aspects should be taken into consideration.
Manuscript source: Unsolicited manuscript
Specialty type: Rheumatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanaka H S-Editor: Zhang H L-Editor: Webster JR P-Editor: Xing YX
| 1. | Santamaria-Alza Y, Sanchez-Bautista J, Fajardo-Rivero JE, Figueroa Pineda CL. Acute respiratory involvement in Colombian patients with systemic lupus erythematosus undergoing chest computed tomography. Int J Rheum Dis. 2019;22:1825-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Linnemann B, Hart C. Laboratory Diagnostics in Thrombophilia. Hamostaseologie. 2019;39:49-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Wypasek E, Undas A. Protein C and protein S deficiency - practical diagnostic issues. Adv Clin Exp Med. 2013;22:459-467. [PubMed] |
| 4. | Ohga S, Ishiguro A, Takahashi Y, Shima M, Taki M, Kaneko M, Fukushima K, Kang D, Hara T; Japan Childhood Thrombophilia Study Group. Protein C deficiency as the major cause of thrombophilias in childhood. Pediatr Int. 2013;55:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Franchini M, Mannucci PM. Primary hyperfibrinolysis: Facts and fancies. Thromb Res. 2018;166:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen DL, Jayne D, Cervera R, Costedoat-Chalumeau N, Diamond B, Gladman DD, Hahn B, Hiepe F, Jacobsen S, Khanna D, Lerstrøm K, Massarotti E, McCune J, Ruiz-Irastorza G, Sanchez-Guerrero J, Schneider M, Urowitz M, Bertsias G, Hoyer BF, Leuchten N, Tani C, Tedeschi SK, Touma Z, Schmajuk G, Anic B, Assan F, Chan TM, Clarke AE, Crow MK, Czirják L, Doria A, Graninger W, Halda-Kiss B, Hasni S, Izmirly PM, Jung M, Kumánovics G, Mariette X, Padjen I, Pego-Reigosa JM, Romero-Diaz J, Rúa-Figueroa Fernández Í, Seror R, Stummvoll GH, Tanaka Y, Tektonidou MG, Vasconcelos C, Vital EM, Wallace DJ, Yavuz S, Meroni PL, Fritzler MJ, Naden R, Dörner T, Johnson SR. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 890] [Article Influence: 148.3] [Reference Citation Analysis (0)] |
| 7. | Litvinov RI, Nabiullina RM, Zubairova LD, Shakurova MA, Andrianova IA, Weisel JW. Lytic Susceptibility, Structure, and Mechanical Properties of Fibrin in Systemic Lupus Erythematosus. Front Immunol. 2019;10:1626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Dhillon PK, Adams MJ. Thrombosis in systemic lupus erythematosus: role of impaired fibrinolysis. Semin Thromb Hemost. 2013;39:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Taraborelli M, Leuenberger L, Lazzaroni MG, Martinazzi N, Zhang W, Franceschini F, Salmon J, Tincani A, Erkan D. The contribution of antiphospholipid antibodies to organ damage in systemic lupus erythematosus. Lupus. 2016;25:1365-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Savelli SL, Roubey RAS, Kitzmiller KJ, Zhou D, Nagaraja HN, Mulvihill E, Barbar-Smiley F, Ardoin SP, Wu YL, Yu CY. Opposite Profiles of Complement in Antiphospholipid Syndrome (APS) and Systemic Lupus Erythematosus (SLE) Among Patients With Antiphospholipid Antibodies (aPL). Front Immunol. 2019;10:885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Woo KS, Kim KE, Kim JM, Han JY, Chung WT, Kim KH. Prevalence and clinical associations of lupus anticoagulant, anticardiolipin antibodies, and anti-beta2-glycoprotein I antibodies in patients with systemic lupus erythematosus. Korean J Lab Med. 2010;30:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Basiri Z, Gholyaf M, Faridnia M, Nadi E, Bairanvand M. The prevalence of anticardiolipin antibody in patients with systemic lupus erythematosus and its association with clinical manifestations. Acta Med Iran. 2013;51:35-40. [PubMed] |
| 13. | Bruce IN, Clark-Soloninka CA, Spitzer KA, Gladman DD, Urowitz MB, Laskin CA. Prevalence of antibodies to beta2-glycoprotein I in systemic lupus erythematosus and their association with antiphospholipid antibody syndrome criteria: a single center study and literature review. J Rheumatol. 2000;27:2833-2837. [PubMed] |
| 14. | Urbanus RT, de Laat B. Antiphospholipid antibodies and the protein C pathway. Lupus. 2010;19:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Wahl D, Membre A, Perret-Guillaume C, Regnault V, Lecompte T. Mechanisms of antiphospholipid-induced thrombosis: effects on the protein C system. Curr Rheumatol Rep. 2009;11:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Rossetto V, Spiezia L, Franz F, Salmaso L, Pozza LV, Gavasso S, Simioni P. The role of antiphospholipid antibodies toward the protein C/protein S system in venous thromboembolic disease. Am J Hematol. 2009;84:594-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Graham A, Ford I, Morrison R, Barker RN, Greaves M, Erwig LP. Anti-endothelial antibodies interfere in apoptotic cell clearance and promote thrombosis in patients with antiphospholipid syndrome. J Immunol. 2009;182:1756-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Belfeki N, Khanfir MS, Said F, Houman MH. [Thrombophilia in systemic lupus erythematosus: A case-control study]. J Med Vasc. 2018;43:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Staropoli JF, Van Cott EM, Makar RS. Membrane autoantibodies in systemic lupus erythematosus: a case of autoimmune hemolytic anemia, antiphospholipid antibodies, and transient acquired activated protein C resistance. Transfusion. 2008;48:2435-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Ramirez GA, Mackie I, Nallamilli S, Pires T, Moll R, Pericleous C, Isenberg DA, Cohen H, Efthymiou M. Anti-protein C antibodies and acquired protein C resistance in SLE: novel markers for thromboembolic events and disease activity? Rheumatology (Oxford). 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Tomás JF, Alberca I, Tabernero MD, Cordero M, Del Pino-Montes J, Vicente V. Natural anticoagulant proteins and antiphospholipid antibodies in systemic lupus erythematosus. J Rheumatol. 1998;25:57-62. [PubMed] |
| 22. | Matuszewska E, Grygalewicz J, Wygledowska G, Mazurkiewicz H. [The evaluation of natural anticoagulants in systemic lupus erythematosus in children]. Pol Merkur Lekarski. 2003;14:125-129. [PubMed] |
| 23. | Costallat LT, Ribeiro CC, Annichino-Bizzacchi JM. Antithrombin, protein S and protein C and antiphospholipid antibodies in systemic lupus erythematosus. Sangre (Barc). 1998;43:345-348. [PubMed] |
| 24. | Plana-Pla A, Bielsa Marsol I, Ferrandiz Foraster C. Protein S deficiency revealed by skin necrosis in a patient with lupus. Lupus. 2019;28:903-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Saigal R, Goyal L, Agrawal A, Wadhwani D, Mital P, Sharma R. Systemic Lupus Erythematosus with Deep Vein Thrombosis and Cutaneous Ulcer. J Assoc Physicians India. 2015;63:85-86. [PubMed] |
| 26. | Vlachoyiannopoulos PG, Samarkos M, Sikara M, Tsiligros P. Antiphospholipid antibodies: laboratory and pathogenetic aspects. Crit Rev Clin Lab Sci. 2007;44:271-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |