Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1931
Peer-review started: November 10, 2020
First decision: December 13, 2020
Revised: December 25, 2020
Accepted: January 14, 2021
Article in press: January 14, 2021
Published online: March 16, 2021
Processing time: 115 Days and 9.1 Hours
Angiomyolipomas (AMLs), belonging to the family of mesenchymal tumors, are considered benign lesions that occur mostly in the kidney or as a part of tuberous sclerosis. Epithelioid AML (EAML) is a rare type of AML that appears to have malignant potential. Extrarenal AMLs usually occur in the liver according to the retrieved literature reports. There have been only two previous reports of monofocal primary AML of the pancreas; however, no cases of primary monotypic EAML of the pancreas have been reported.
An asymptomatic 59-year-old woman incidentally revealed a tumor during abdominal ultrasound examination. Routine blood tests and physical examination were within normal limits. Abdominal ultrasound revealed a 1.9-cm hypoechogenic mass in the tail of the pancreas, clearly visualized by endoscopic ultrasound. However, contrast-enhanced abdominal computed tomography scans did not demonstrate the lesion. A subsequent gadolinium-enhanced magnetic resonance imaging scan showed that the lesion had some characteristic manifestations. The lesion was initially thought to be a neuroendocrine tumor (asymptomatic PanNET). After surgical resection, histopathology and immunohistochemistry confirmed the diagnosis of EAML. At the 6-mo follow-up, no recurrence, spread, or metastasis was identified on computed tomography or magnetic resonance imaging.
The preoperative diagnosis of pancreatic AML is extremely difficult. Imaging techniques are essential for providing valuable morphological features for differential diagnosis.
Core Tip: We present a rare case of pancreatic epithelioid angiomyolipoma diagnosed after an anatomopathological examination. The patient was asymptomatic. Here are several findings from multiple modalities, including multidetector computed tomography, ultrasound, endoscopic ultrasound, magnetic resonance imaging, and providing a pathologic correlation. Awareness of the characteristic features, including immunoreactivity for the human melanoma black-45 marker, may help in the diagnosis of this rare entity. This report represents, to our knowledge, the first epithelioid angiomyolipoma arising in the pancreas.
- Citation: Zhu QQ, Niu ZF, Yu FD, Wu Y, Wang GB. Epithelioid angiomyolipoma of the pancreas: A case report and review of the literature. World J Clin Cases 2021; 9(8): 1931-1939
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1931.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1931
Angiomyolipoma (AML) is classified into two distinct histological subtypes: Classic triphasic AML and monotypic epithelioid AML (EAML). Classic AML is benign and characterized by proliferation of blood vessels, smooth muscle, and adipose tissue in variable proportions. EAML is composed of purely epithelioid cells with a paucity of adipose tissue components. The fact that immunohistochemical staining for melanocyte and smooth muscle cell markers in these tumors has standardized the histological diagnosis of this entity. The reported characteristics of renal EAML with malignant potential often resemble those of renal cell carcinoma on both radiology and histology[1-3]. In the present report, we present an extrarenal EAML in the pancreas.
A 59-year-old asymptomatic woman underwent abdominal ultrasound (US) in the workflow of health examination, revealing a 1.9-cm hypoechogenic mass in the tail of the pancreas.
The patient used amlodipine besylate for 20 years for the treatment of hypertension. The remarkable medical history was thyroid cancer resection 5 mo prior and treatment with radionuclide therapy.
Neither she nor anyone in her family had a history of tuberous sclerosis complex (TSC).
The patient’s vital signs and physical examination were likewise unremarkable.
Laboratory findings indicated that the patient’s carbohydrate antigen 19-9 level, as well as her carcinoembryonic antigen level, was within the normal range.
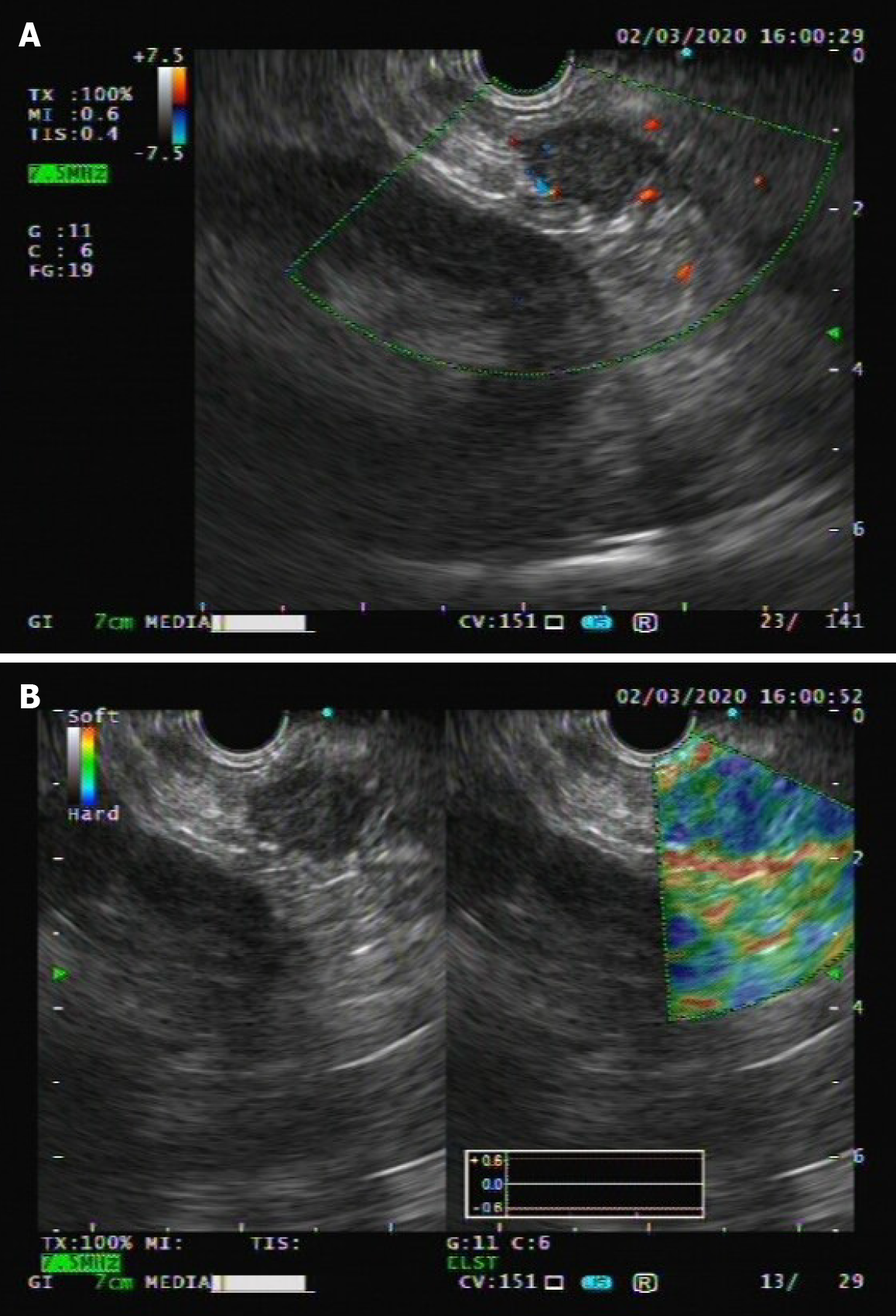
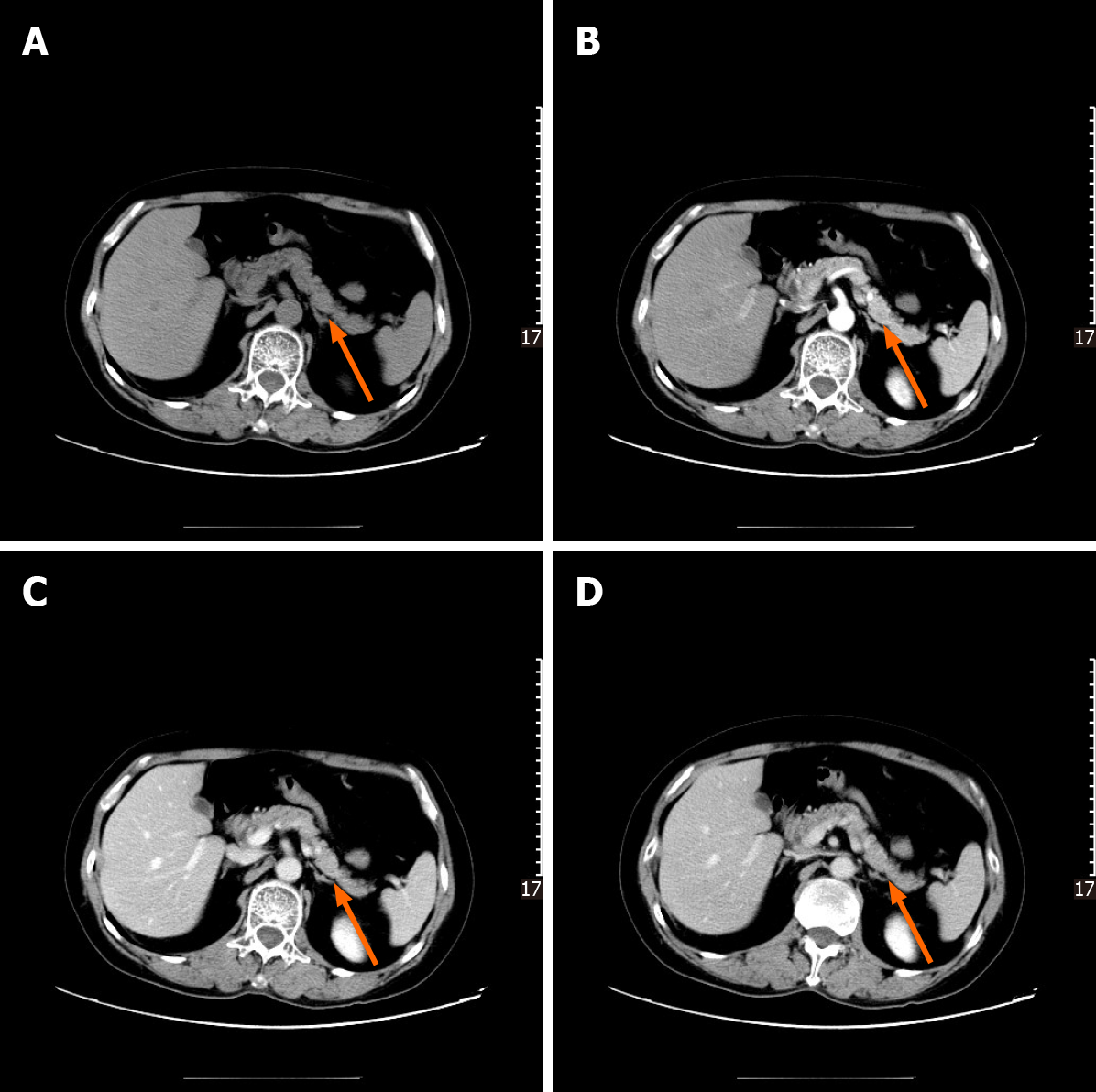
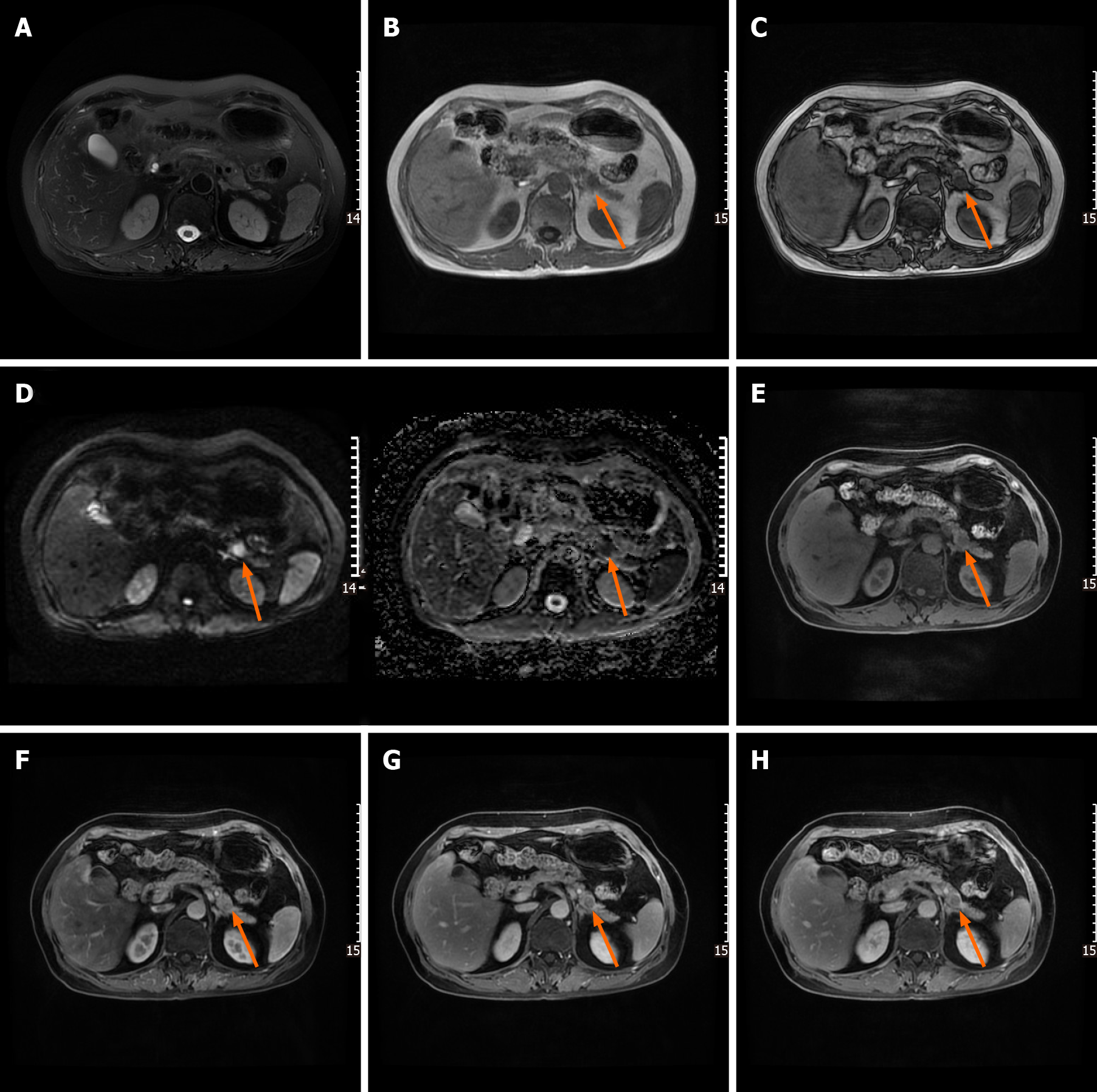
Following abdominal US examination, the patient underwent enhanced multidetector computed tomography (MDCT), magnetic resonance imaging (MRI), and endoscopic US (EUS) for further evaluation. The size and morphology of the pancreas were nearly normal in all imaging examinations. US and EUS revealed a 1.9-cm hypoechogenic mass in the tail of the pancreas that did not display the typical expected adipose echogenicity. No hypervascularity was noted on the Doppler images (Figure 1A). Ultrasonic elastography showed a green-to-blue cold color, indicating soft conditions (Figure 1B). The soft tissue mass was occult on the computed tomography (CT) scan due to its homogeneous isodensity to the background pancreatic parenchyma in the unenhanced, arterial, portovenous, and delayed phases (Figure 2). No other lesions were detected on the abdominal and pelvic CT scan. On MRI, the mass demonstrated a long or equal signal intensity (SI) on LAVA-T1-weighted imaging and slightly long SI on FS-T2-weighted imaging. Chemical shift gradient-echo images demonstrated a slight loss of SI on out-of-phase imaging related to the small amount of microscopic lipid component within the mass (Figure 3A-C). DWI showed the mass-region of hyperintesity. ADC map was dark with an ADC value 0.707 × 10-3 mm2/s, consistent with reduced diffusivity (Figure 3D). Additionally, contrast-enhanced MRI revealed that the mass was slightly hyperenhancing relative to surrounding pancreas on arterial phase imaging. Then, the mass washed out in the portal and delayed phases very quickly (Figure 3E-H).
A neuroendocrine tumor (asymptomatic PanNET) was preoperatively diagnosed based on location and imaging findings. Considering the overall characteristics of the mass and the patients’ health condition, surgical resection should still be re-commended due to the absence of available data on long-term follow-up.
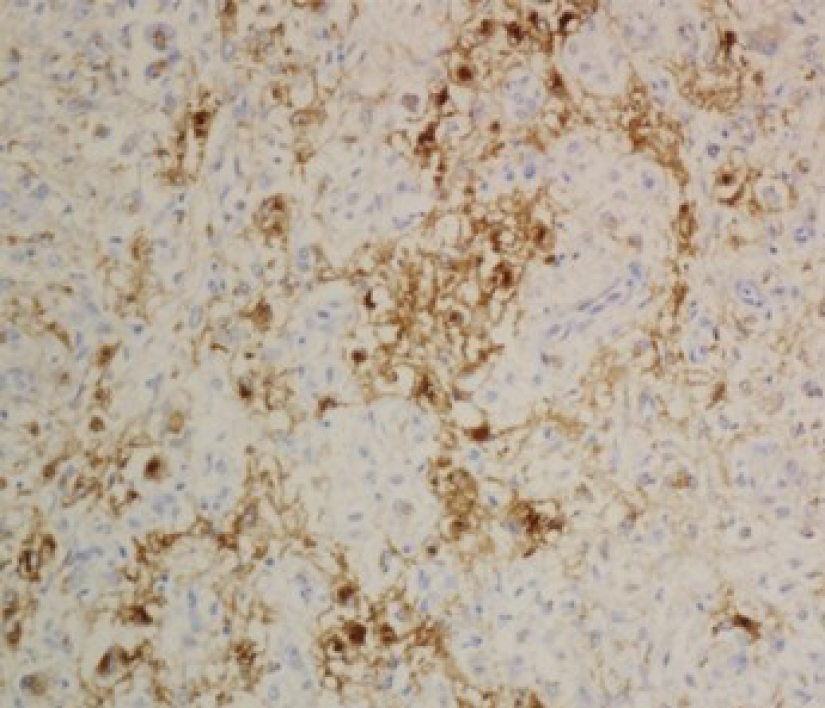
Gross pathological examination revealed a well-defined, brown nodular tumor in the tail of the pancreas measuring 1.7 cm × 1.7 cm. The pathological diagnosis was based on pleomorphic perivascular epithelioid cells (PECs) (Figure 4) and immuno-histochemical staining for melanoma and smooth muscle cell markers. While adipose tissue was scarcely observed, mitotic figures were rare, and atypical mitoses were not detected. The sections were immunostained using monoclonal anti-HMB-45, anti-desmin, anti-melan-A, and anti-SMA antibodies. Typical smooth muscle cells and epithelioid cells were immunoreactive for HMB-45 (Figure 5) and positive for melan-A and SMA. Accordingly, the final pathological diagnosis was primary EAML.
The patient requested surgical intervention. During the operation, it was noted that the lesion was adjacent to splenic vessels. Subsequently, laparoscopic resection of the tail of the pancreas and spleen was performed.
The patient made an uneventful recovery and was discharged from the hospital on the 8th day after surgery. The patient was followed for 6 mo postoperatively, and no signs of recurrence or metastasis were observed on CT and MR examinations.
AML was recognized as a mesenchymal tumor in the 2004 World Health Organization classification of renal tumors[4] and a member of the family of PEComas[5] that may occur sporadically or in association with TSC[6]. Although AML is most common in the kidney, extrarenal sites can also be involved. The liver, with over 100 reported hepatic cases[7-9], is reported to be the most frequently involved extrarenal organ. Rare additional sites of reported AMLs include the lung[10,11], spleen[12,13], colon[14,15], heart[16], skin[17,18], parotid gland[19,20], mediastinum[21], spermatic cord[22], nasal cavity[23,24], and retroperitoneal soft tissue[25,26]. The nature of most AMLs is benign, while examples of renal and extrarenal AMLs have described malignant potential[1-3,27]. EAML is considered a potentially malignant neoplasm since one-third of patients develop metastatic lesions involving extension into the vena cava and metastasis[27]. EAML can occur locally, metastasize, and result in death[28]. No clear criteria have been developed to identify malignant tumors, but most reported examples did not exhibit increased mitotic activity[27,29]. Our patient’s tumor exhibited benign historical characteristics, and follow-up information 6 mo after the operation indicated no evidence of recurrence. The patient remains in continuous follow-up.
Clinical presentation varies and is nonspecific. Adding to the difficulty of a preoperative diagnosis, most extrarenal AMLs are asymptomatic and remain occult unless incidentally detected.
Renal AMLs involve the renal cortex and exhibit diffusely high echogenicity on US. Most renal AMLs demonstrate a fat density of less than -20 HU on a nonenhanced CT scan, although EAMLs show high attenuation. On a CT scan with contrast agent, they show a prolonged enhancement pattern homogeneous with peripheral kidney tissue. On MRI, the signal intensities of AMLs are decided by the fat content[30]. Nevertheless, the majority of renal AMLs can be recognized easily under the technique of fat suppression or chemical shift, except for those with poor lipids. It may be challenging to achieve the accurate diagnosis of EAMLs because adipocytes are scarce in tumors. Renal EAMLs demonstrate a range of MR appearances, with non-specific DWI findings, hypointensity on T2-weighted MR images, and differing degree of enhancement depending on the components of the tumor. It must be recognized that renal EAML can appear adjacent or even within conventional AML and show high attenuation on CT and homogenous enhancement with a prolonged enhancement pattern[31].
Due to advances in US, MDCT, and MR imaging technologies, both symptomatic and asymptomatic extrarenal AMLs that can occur in various parts of the body have been noticed and reported frequently. So far, the most frequent location is the liver. One-half of AMLs occurring in the liver lack an appreciable fat content, which is unlike renal AMLs. Hepatic EAMLs on US appear as hyperechogenic lesions with clear boundaries. Relative hypervascularity may be found in the lesions. MDCT is useful for the evaluation of fat content within a mass, which can be helpful to differentiate lipid-rich and lipid-poor types. On CT scans, lipid-poor hepatic AMLs have a peripheral angiomyomatous component and soft tissue attenuation. On MRI scans, imaging findings of hepatic AMLs are consistent with those of renal AMLs[28]. Fifty percent of hepatic AMLs lacking macroscopic fat content remain a diagnostic dilema[9,32,33].
The primary EAML in the pancreas that we reported in our patient appeared differently on CT and MRI when compared with primary hepatic and renal AMLs. The incidence and general radiographic features of primary EAML of the pancreas are not known. The pancreas can be involved by EAML, and as such, when faced with a pancreatic mass, doctors ought to include EAML in the differential diagnosis. Upon a review of the literature, there are a limited number of imaging studies on AML of the pancreas and no studies on EAML of the pancreas[34]. Imaging features of pancreatic AML have been infrequently reported. The imaging findings associated with the mass in our case were partially similar to those of primary pancreatic AML but differed from those of reported EAMLs in the kidney and liver. The uncommon primary AML of the pancreas was reported for the first time by Heywood et al[34] in 2004 with US and CT examination before surgery. The thick-walled cystic mass, measuring 4.5 cm × 3 cm × 2.5 cm, was located in the uncinate process of pancrease and was accompanied by hemorrhage. In 2017, Kim et al[35] reported the second known case of monofocal primary AML. The mass, measuring 2 cm, was located in the body of the pancreas, suggesting a low-grade malignant or benign tumor. EUS revealed a hyperechogenic mass without hypervascularity. Isodense mass on unenhanced CT images and peripheral enhancement were observed during the arterial, parenchyma, and portovenous phases. On MRI, the mass, without diffusion restriction, displayed heterogeneous peripheral high SI on T2 imaging and homogenous low SI on T1 imaging. Additionally, dynamic MRI revealed that the central part of the mass was poorly enhanced, while the peripheral part was strongly enhanced.
Our case represents a pancreatic EAML whose epithelioid cell component is predominant. Histologically, EAML is composed of a group of PEC tumors with cells of epithelioid morphology, smooth muscle, and a partially melanocyte phenotype[3,5]. These lesions can resemble melanoma or conventional renal tumors in the kidney that have a predominance of sarcomatous elements. Characteristic histology and an immunohistochemical phenotype should aid in the correct diagnosis.
Typical AMLs feature lobular structures and are colored gray-to-yellow on gross examination. Necrosis as well as intratumoral hemorrhage can be observed. Approximately 30% of patients are found to have multiple tumors, and in patients with TSC, the proportion is even higher. Multiple tumors are not equal to metastasis but the indication of multifocality[6,36].
Through microscopic examination, various proportions of components, including vessels with thick walls, smooth muscle, and mature adipose tissue, were observed in AML. Rare cases (i.e., monotypic EAML) with the predominant proportion of epithelioid cells can be extremely difficult to diagnose. In general, EAMLs are considered to originate from smooth muscle and are characterized by abundant eosinophilic cytoplasm as well as nuclear pleomorphism, atypia, and mitotic activity to varying degrees[37,38]. Apits[39] was the first to describe epithelioid features in renal AML, often arranged around vascular spaces, and increasing awareness of the lesion has led to the recognition of monotypic EAML[39]. However, very subtle differences were observed between benign and malignant EAMLs, and there were no clear diagnostic criteria for malignancies. If there is no conclusive evidence of metastasis, the diagnosis of malignant AML is extremely difficult.
Some AMLs have an unusual morphology, including those with one predominant component, such as EAML, which may prompt a misdiagnosis of leiomyosarcoma, liposarcoma, and even carcinoma. Immunohistochemical staining reflects the distinctive cellular differentiation of the tumor. The most instructive finding is the smooth muscle or myoid cells in AMLs that coexpress muscle markers, such as SMA, and human melanocytic markers, such as HMB-45. In our patient, negative staining for cytokeratin, chromogranin A, and synaptophysin argued against carcinoma and neuroendocrine carcinoma, while positive staining for HMB-45, Melan-A, and SMA was added in the correct diagnosis.
In most cases, AMLs are benign in the clinical course, even those with multiple foci, a bizarre morphology, or local invasion. Surgical resection can reach the cure level. EAML, in contrast to classic AML (which is benign), may be associated with distant metastasis and local recurrence. Making a prognosis of malignant EAML is challenging for a limited number of cases. According to the literature, the survival of patients with malignant EAML ranges from several months to 3 years. Cibas et al[31] reported a case of malignant EAML that was treated successfully with single-agent doxorubicin.
Our horizon about the occurrence sites and morphological features of AML has been enlarged to a great extent in recent years. Our report of primary pancreatic EAML prompts our awareness of AML. This report of primary EAML in the pancreas further widens this spectrum. To date, this is the first such case of only a few HMB-45-positive EAMLs outside the kidney and liver and is the first to describe relatively complete imaging findings of primary AML in the pancreas without hemorrhage. Further evaluation, including imaging modalities, imaging features, and prognosis, is urgently necessary to better characterize primary pancreatic AML.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rajan R S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Christiano AP, Yang X, Gerber GS. Malignant transformation of renal angiomyolipoma. J Urol. 1999;161:1900-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | El Jack AK, Tomaszewski JE, Haller DG, Siegelman ES. Metastatic PEComa arising from renal angiomyolipoma: MRI findings. J Magn Reson Imaging. 2007;26:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Lai HY, Chen CK, Lee YH, Tsai PP, Chen JH, Shen WC. Multicentric aggressive angiomyolipomas: a rare form of PEComas. AJR Am J Roentgenol. 2006;186:837-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 622] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 5. | Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008;452:119-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 372] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 6. | Burgueño Gómez B, Lindo Ricce M, Mora Cuadrado N, González de Frutos C. Concurrent hepatic and renal angiomyolipomas in tuberous sclerosis complex. Rev Esp Enferm Dig. 2020;112:412-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Yamasaki S, Tanaka S, Fujii H, Matsumoto T, Okuda C, Watanabe G, Suda K. Monotypic epithelioid angiomyolipoma of the liver. Histopathology. 2000;36:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Ronen S, Prieto VG, Aung PP. Epithelioid angiomyolipoma mimicking metastatic melanoma in a liver tumor. J Cutan Pathol. 2020;47:824-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Huang YM, Wei PL, Chen RJ. Epithelioid Angiomyolipoma of the Liver. J Gastrointest Surg. 2018;22:175-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Guinee DG Jr, Thornberry DS, Azumi N, Przygodzki RM, Koss MN, Travis WD. Unique pulmonary presentation of an angiomyolipoma. Analysis of clinical, radiographic, and histopathologic features. Am J Surg Pathol. 1995;19:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Ito M, Sugamura Y, Ikari H, Sekine I. Angiomyolipoma of the lung. Arch Pathol Lab Med. 1998;122:1023-1025. [PubMed] |
| 12. | Hulbert JC, Graf R. Involvement of the spleen by renal angiomyolipoma: metastasis or multicentricity? J Urol. 1983;130:328-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Asayama Y, Fukuya T, Honda H, Kaneko K, Kuroiwa T, Yoshimitsu K, Irie H, Shinokuma J, Yamaguchi K, Masuda K. Chronic expanding hematoma of the spleen caused by angiomyolipoma in a patient with tuberous sclerosis. Abdom Imaging. 1998;23:527-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Maesawa C, Tamura G, Sawada H, Kamioki S, Nakajima Y, Satodate R. Angiomyolipoma arising in the colon. Am J Gastroenterol. 1996;91:1852-1854. [PubMed] |
| 15. | Oishi K, Fukuda S, Sakimoto H, Eto T, Takahashi M, Nishida T. Angiomyolipoma of the colon: report of a case. Surg Today. 2009;39:998-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Shimizu M, Manabe T, Tazelaar HD, Hirokawa M, Moriya T, Ito J, Hamanaka S, Hata T. Intramyocardial angiomyolipoma. Am J Surg Pathol. 1994;18:1164-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Shim HS, Kim DH, Kwon H, Jung SN. Cutaneous angiomyolipoma in the forehead. J Craniofac Surg. 2014;25:1120-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Ammanagi AS, Dombale VD, Shindholimath VV. Cutaneous angiomyolipoma. Indian Dermatol Online J. 2013;4:255-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Rosado P, Villalain L, De Vicente JC, Vivanco B, Torre A. Angiomyolipoma of the parotid gland: report of a case and review of the literature. J Oral Maxillofac Surg. 2010;68:2609-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Guevara N, Lassalle S, Castillo L, Butori C, Santini J. [Angiomyolipoma of the parotid gland]. Ann Otolaryngol Chir Cervicofac. 2008;125:90-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Knight CS, Cerfolio RJ, Winokur TS. Angiomyolipoma of the anterior mediastinum. Ann Diagn Pathol. 2008;12:293-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Giulianelli R, Albanesi L, Attisani F, Brunori S, Gentile BC, Mavilla L, Mirabile G, Pisanti F, Vincenti G, Shestani T, Schettini M. A case of angiomyolipoma of the spermatic cord and testicle. Arch Ital Urol Androl. 2012;84:165-166. [PubMed] |
| 23. | Pandey V, Khatib Y, Gupte P, Pandey R, Khare MS. Monotypic angiomyolipoma of the nasal cavity: An extremely rare cause of nasal mass with recurrent epistaxis. Indian J Pathol Microbiol. 2020;63:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Hao JY, Liu LP, Pan H, Wang C. [One case of nasal angiomyolipoma]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;53:697-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Wroclawski ML, Baccaglini W, Pazeto CL, Carbajo C, Matushita C, Carneiro A, Pompeo A, Glina S, Pompeo ACL, Cavalcante LB. Extrarenal Angiomyolipoma: differential diagnosis of retroperitoneal masses. Int Braz J Urol. 2018;44:639-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Ali AM, Rizvi SJ, Kanodia KV. Extrarenal retroperitoneal angiomyolipoma with oncocytoma. Indian J Urol. 2018;34:82-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Zhan R, Li YQ, Chen CY, Hu HY, Zhang C. Primary kidney malignant epithelioid angiomyolipoma: Two cases report and review of literature. Medicine (Baltimore). 2018;97:e11805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Lasri A, Touzani MA, Lahyani M, Karmouni T, Elkhader K, Koutani A, Andaloussi AA. [Malignant renal epithelioid angiomyolipoma (EAML): about a rare case]. Pan Afr Med J. 2019;33:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Pea M, Bonetti F, Zamboni G, Martignoni G, Riva M, Colombari R, Mombello A, Bonzanini M, Scarpa A, Ghimenton C. Melanocyte-marker-HMB-45 is regularly expressed in angiomyolipoma of the kidney. Pathology. 1991;23:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 173] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Nguyen TTL, Terris B, Barat M. Hepatic epithelioid angiomyolipoma mimicking hepatocellular carcinoma. Diagn Interv Imaging. 2020;101:501-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Cibas ES, Goss GA, Kulke MH, Demetri GD, Fletcher CD. Malignant epithelioid angiomyolipoma ('sarcoma ex angiomyolipoma') of the kidney: a case report and review of the literature. Am J Surg Pathol. 2001;25:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Sung CK, Kim SH, Woo S, Moon MH, Kim SY, Kim SH, Cho JY. Angiomyolipoma with minimal fat: differentiation of morphological and enhancement features from renal cell carcinoma at CT imaging. Acta Radiol. 2016;57:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Israel GM, Hindman N, Hecht E, Krinsky G. The use of opposed-phase chemical shift MRI in the diagnosis of renal angiomyolipomas. AJR Am J Roentgenol. 2005;184:1868-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Heywood G, Smyrk TC, Donohue JH. Primary angiomyolipoma of the pancreas. Pancreas. 2004;28:443-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Kim HH, Park DH. Imaging Findings of Primary Angiomyolipoma of the Pancreas: A Case Report. J Korean Soc Radiol. 2017;77:9. [DOI] [Full Text] |
| 36. | Aydin H, Magi-Galluzzi C, Lane BR, Sercia L, Lopez JI, Rini BI, Zhou M. Renal angiomyolipoma: clinicopathologic study of 194 cases with emphasis on the epithelioid histology and tuberous sclerosis association. Am J Surg Pathol. 2009;33:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 37. | Lopater J, Daniel L, Akiki A, Boissier R, Lechevallier E, Coulange C. [Renal epithelioid angiomyolipoma]. Prog Urol. 2009;19:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Smentkowski K, Kelly D. Epithelioid angiomyolipoma with tumor thrombus. Can J Urol. 2019;26:9960-9962. [PubMed] |
| 39. | Apits K. Die Geschwulste und Gewebsmissbildungen der Nierenrinde; die adenoma. Virchows Arch. 1943;311:328. |