Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1916
Peer-review started: October 20, 2020
First decision: December 24, 2020
Revised: December 26, 2020
Accepted: January 6, 2021
Article in press: January 6, 2021
Published online: March 16, 2021
Processing time: 135 Days and 23.5 Hours
The standard treatment of transitional cell carcinoma of the upper urinary tract consists of radical nephroureterectomy with bladder cuff removal, which can be performed either in open or laparoscopy or robot-assisted laparoscopy. Treatment of chronic renal insufficiency patients with upper urothelial tumor is in a dilemma. Urologists weigh and consider the balance between tumor control and effective renal function preservation. European Association of Urology guidelines recommend that select patients may benefit from endoscopic treatment, but laparoscopic treatment is rarely reported.
In this case report, we describe a case of 79-year-old female diagnosed with urothelial carcinoma of the renal pelvis and adrenal adenoma with chronic renal insufficiency. The patient was treated with retroperitoneal laparoscopic partial resection of the renal pelvis and adrenal adenoma resection simultaneously.
Retroperitoneal laparoscopic partial resection of the renal pelvis is an effective surgical procedure for the treatment of urothelial carcinoma of the renal pelvis.
Core Tip: For patients with chronic renal insufficiency diagnosed with renal pelvic urothelial carcinoma, retroperitoneal laparoscopic partial resection of the renal pelvis is a new effective surgical method and an alternative method for endoscopic treatment, especially for surgeons with rich experience in laparoscopic surgery and patients with other diseases for which laparoscopic surgery is needed simultaneously.
- Citation: Wang YL, Zhang HL, Du H, Wang W, Gao HF, Yu GH, Ren Y. Retroperitoneal laparoscopic partial resection of the renal pelvis for urothelial carcinoma: A case report. World J Clin Cases 2021; 9(8): 1916-1922
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1916.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1916
The proportion of cases of transitional cell carcinoma of the upper urinary tract (UUT-TCC) is approximately 5% of all urothelial tumors[1], although renal pelvis tumors are relatively rare.
With the increase in many chronic diseases, such as hypertension, diabetes mellitus and medicinal renal injury, the number of patients with chronic renal insufficiency (CRI) is gradually increasing clinically. Overall, treatment of patients with upper urothelial tumors and chronic renal insufficiency remains a dilemma, and urologists must weigh and consider the balance between tumor control and renal function preservation.
The standard treatment for UUT-TCC consists of radical nephroureterectomy (RNU) with bladder cuff removal, which can be performed either in open surgery or laparoscopy, including robot-assisted laparoscopy[2,3].
In patients with organ-confined UUT-TCC, laparoscopic nephroureterectomy has the advantages of minimal invasiveness and oncologic outcomes comparable to those of open nephroureterectomy[4], with all the benefits of minimally invasive surgery including less blood loss, shorter hospital stays, reduced postoperative pain, and shorter convalescence time[3]. In some selected cases of renal insufficiency, solitary kidney, bilateral tumors, high risk of complications associated with nephrourete-rectomy or low grade/low stage tumors, conservative treatment such as endoscopic resection or ablation may also be performed[5,6].
A 79-year-old female with a history of intermittent painless gross hematuria for 15 d.
The patient had intermittent painless gross hematuria but had no other symptoms such as flank or abdominal mass, weight loss, anorexia, fatigue, or bone pain.
The patient had no previous medical history.
Her family history was unremarkable.
The patient’s temperature was 36.7 °C, heart rate was 78 beats per min, respiratory rate was 18 breaths per min, blood pressure was 118/78 mmHg, and oxygen saturation in room air was 97%. Clinical physical examination revealed no abnormalities.
Urinalysis, routine blood tests, coagulation and liver function were normal; however, serum creatinine was 150 µmol/L and blood urea nitrogen (BUN) 10.04 mmol/L, and urinary cytology revealed heterocyte positivity. The level of adrenal gland hormone was in the normal range.
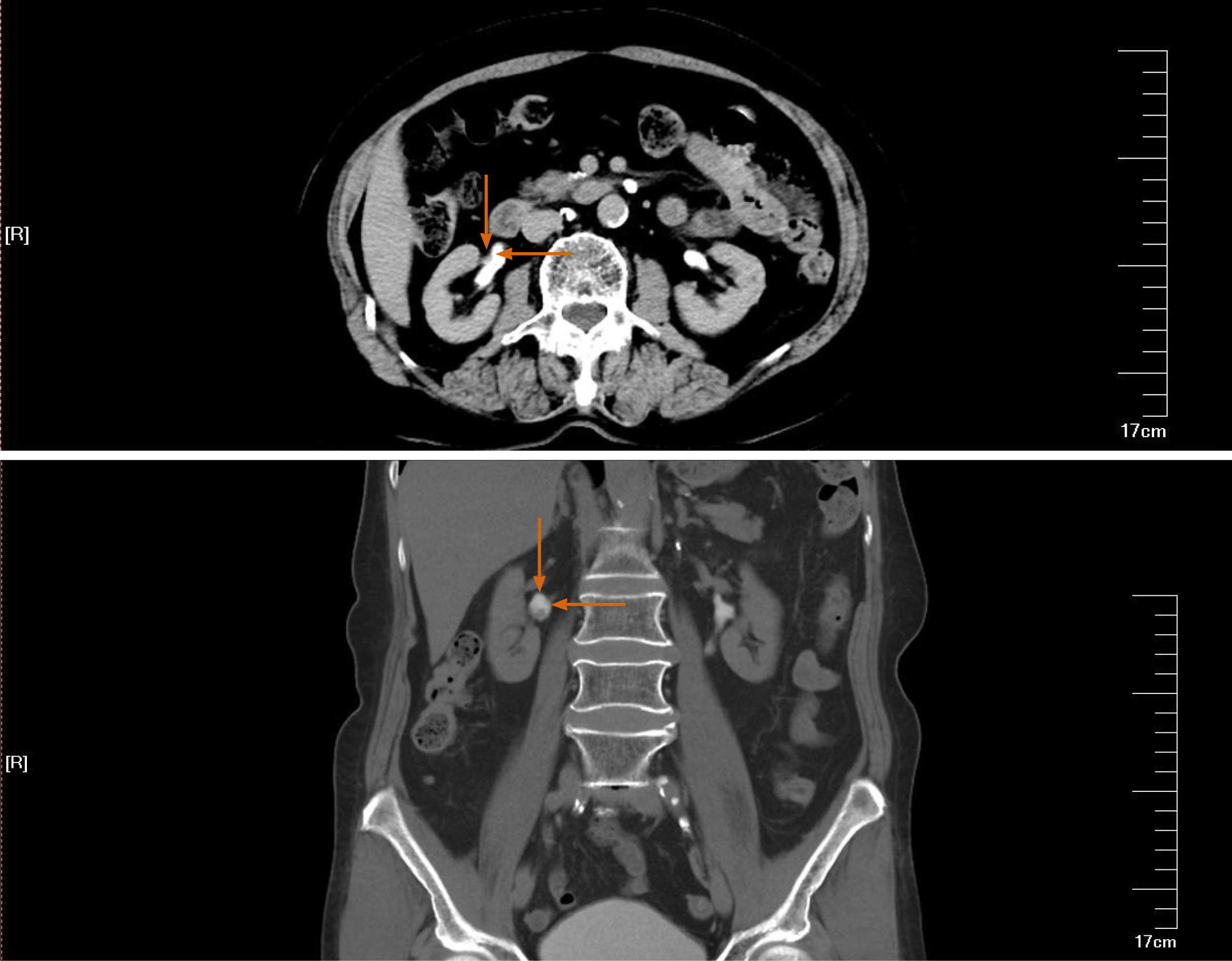
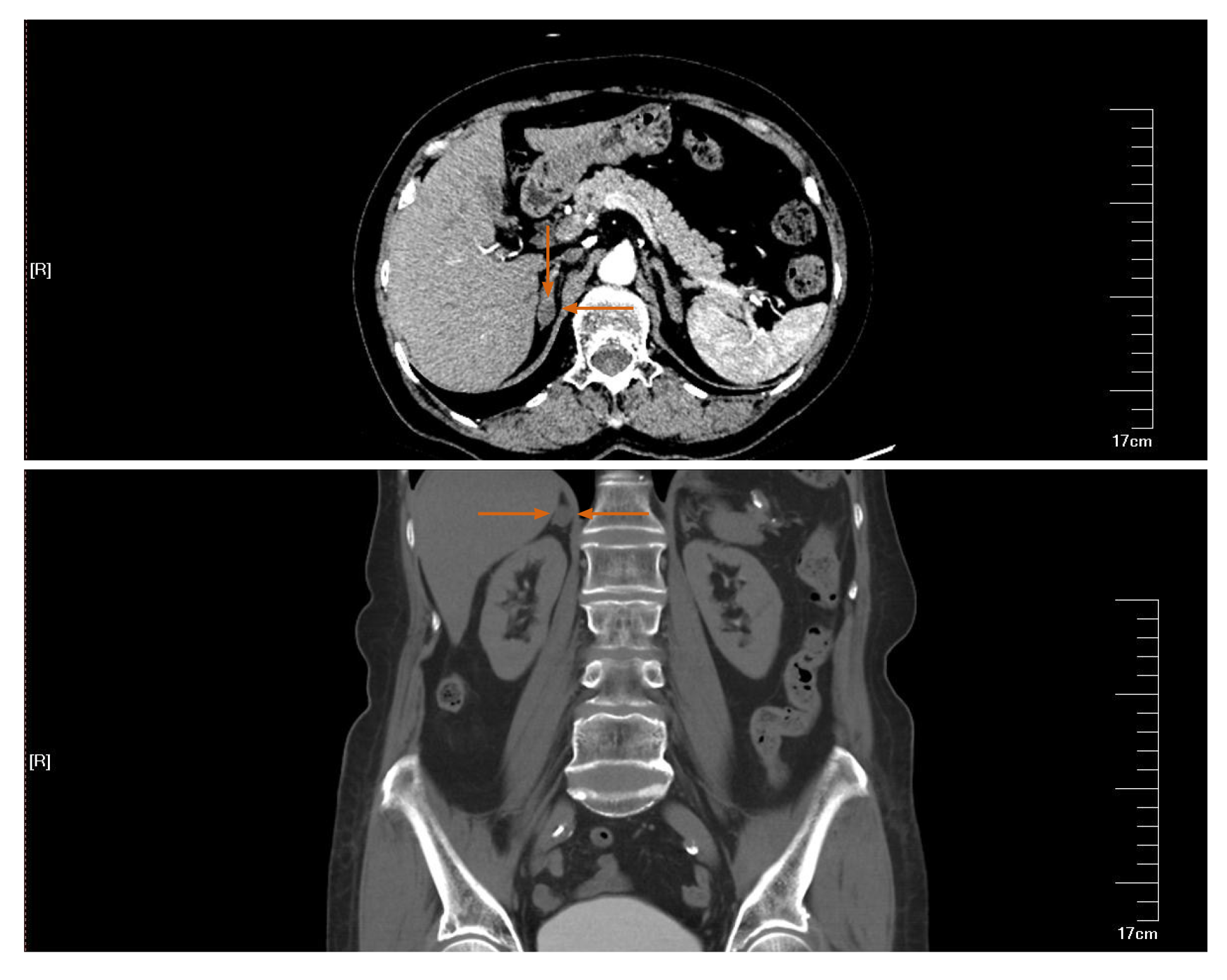
Ultrasound examination revealed a right renal pelvic mass located above the ureteric junction of the renal pelvis. The diameter of the tumor was 7 mm × 7 mm. A right adrenal gland mass was also found, the size of which was 12 mm × 12 mm. A computed tomography (CT) abdominal scan showed a mildly enlarged pelvis–calyceal system and right adrenal gland solitary mass. There was a pathological lesion visible in the right pelvis with dimensions of 10 mm × 8 mm, which was enhanced from 20 to 80 Hounsfield units after injection of contrast medium (Figure 1). The size of the right adrenal gland solitary mass was 14 mm × 14 mm (Figure 2). Preoperative rigid cystoscopy revealed normal mucosa in the bladder.
The patient was diagnosed with UUT-TCC complicated CRI and right adrenal gland adenoma. The patient underwent rigid ureteroscopy, which identified a cauliflower-like mass in the right renal pelvis.
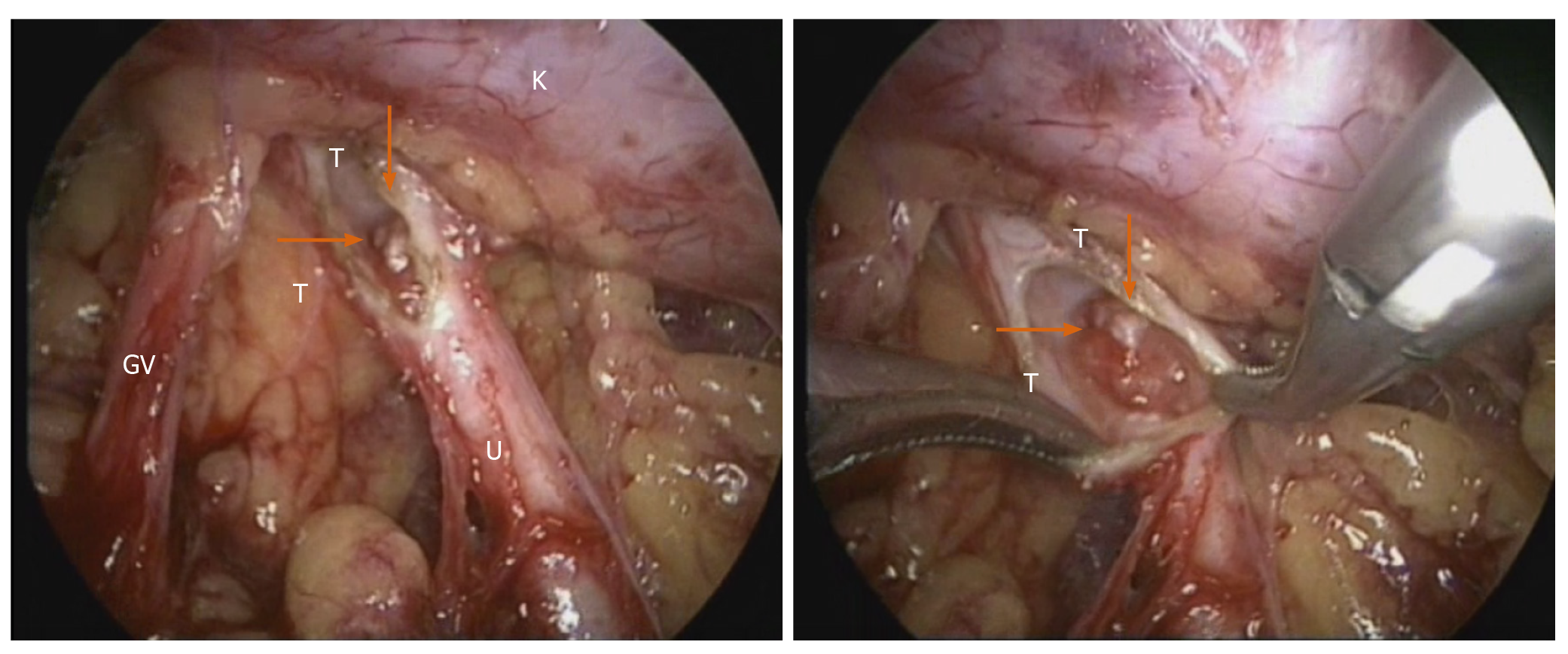
The prognostic indicators of UUT-TCC included tumor diameter, pT stage and tumor necrosis, which were independent predictors of metastasis-free and disease-free survival. The patients with advanced-stage tumors, extensive necrosis, and a tumor diameter of 3 cm were significantly impaired by increasing pT stage[7]. We considered the renal pelvic tumor as a low grade based on ureteroscopy. Based on our medical center’s considerable experience in retroperitoneal laparoscopic surgery, the option of a retroperitoneal laparoscopic approach was offered to the patient. After induction of general anesthesia, the patient was placed in the left lateral position. The retroperitoneal approach was performed using the conventional three-hole approach according to a technique described previously[8]. The right adrenal gland was first explored, and a right adrenal gland solitary mass was found located in the medial branch, consistent with the change in adenoma. Tumor resection was performed. Next, the right ureter, renal pelvis, lower pole of the kidney and genital vein were routinely freed. After releasing the ureter, the right renal pelvis was opened, and a cauliflower-like tumor was visualized (Figure 3). When the tumor was resected, a margin of approximately 0.5 cm of healthy renal pelvis tissue was preserved as much as possible; to avoid tumor implant metastasis, the tumor specimen was placed in a specimen bag immediately after resection. The renal pelvis was reconstructed with interrupted sutures.
Postoperative recovery was good, with no complications. Renal function was stabilized 1 wk after the surgery, with levels of serum creatinine 200 µmol/L and BUN 10.57 mmol/L. The pathology report showed renal pelvis TCC pTaG1 and right adrenal adenoma. Two years after the surgery, the patient underwent ultrasound and CT scan follow-up examinations, revealing no tumor recurrence or right renal hydronephrosis. The treatment was safe and efficient, but longer follow-up is still needed.
Nephro-ureterectomy with bladder cuff removal is the gold standard for the treatment of UUT-TCC. Some patients may benefit from endoscopic treatment (ET), such as holmium laser tumor ablation with a flexible ureteroscope or percutaneous nephrostomy tumor resection. For example, patients with unifocal, < 2 cm, and low-grade lesions without local invasion are considered to have low-risk disease[9]. In the case of low-grade/low-stage TCC of the renal pelvis, open surgery is being replaced by endoscopic surgery[10,11]. However, ureteroscopic tumor ablation remains associated with a high risk of disease recurrence and progression due to inadequate assessment of risk stratification by imaging or tumor biopsy[9]. Moreover, the risk of perioperative bleeding with the percutaneous approach is higher than that with laparoscopic surgery, and the risk of needing dialysis after nephrectomy is extremely high in patients with isolated kidney or renal insufficiency if bleeding occurs. Tumor recurrence or trocar metastasis after percutaneous access has also been reported, as indicated in Table 1.
| Ref. | Number | Operation | Tumor grade |
| Rastinehad et al[16] | 1 | PCN tumor resection | N/A |
| Treuthardt et al[17] | 1 | PCN tumor resection + BCG perfusion therapy | High grade |
| Wang et al[18] | 1 | Radical nephroureterectomy after PCN biopsy 1 wk | High grade |
| Oefelein et al[19] | 1 | PCN tumor resection | Low grade |
| Fuglsig et al[20] | 1 | PCN tumor resection | High grade |
| Sharma et al[21] | 1 | PCN tumor resection | N/A |
European Association of Urology (EAU) guidelines recommend offering kidney-sparing management to patients with solitary kidney and/or impaired renal function. Kidney-sparing surgery for low-risk upper urinary tract urothelial cell carcinoma reduces the morbidity associated with the loss of renal function without affecting oncologic outcomes[12]. In low-risk cancers, kidney-sparing management is the preferred approach, as survival rates are similar to those after RNU[13]. Furthermore, kidney preservation is advantageous if adjuvant or salvage chemotherapy is required. It also eliminates the possibility of overtreatment[14].
Our patient was treated with retroperitoneal laparoscopic partial resection of the renal pelvis. One previous case was reported by Wojtarowicz et al[14] worldwide, but there were no long-term follow-up results. In the present case, the patient was followed up for 2 years and showed stabilized renal function without tumor recurrence and progression. We believe that retroperitoneal laparoscopic partial resection of the renal pelvis is an alternative method for suitable patients and for surgeons with rich experience in laparoscopic surgery.
The advantages of retroperitoneal laparoscopic partial resection of the renal pelvis are as follows. (1) It can ensure the resection depth and scope and reduce residual tumor. (2) Completely resected tissue can offer tumor staging and grading. (3) There is no possibility of tumor implantation and metastasis caused by renal pelvic lymph and venous reflux under high pressure by endoscopic treatment. And (4) The operation does not damage the renal parenchyma, with less bleeding and damage to the kidney.
However, this method does have limitations and shortcomings. It is only suitable for patients whose extrarenal pelvic tumors are confined to the renal pelvis, and it cannot be applied for tumors in the renal calyx. In addition, when the closed collection system is opened, there is concern that the tumor may overflow into the urine, leading to tumor implantation and metastasis. Therefore, the following methods are recommended to reduce the possibility of tumor implantation and metastasis. (1) Dry gauze is placed around the incision of the renal pelvis to reduce pollution of surrounding tissues by the urine after the incision is made. And (2) Direct contact of surgical instruments with the tumor during the operation is avoided. After resection, the tumor should be placed into a specimen bag that is sealed to prevent the tumor from contacting the surrounding tissues. The operation should be carried out under low pneumoperitoneum pressure as much as possible. The incision should be tightly sutured to fix the trocar, and the trocar should be pulled out after deflation to avoid the “chimney effect” and reduce trocar tumor planting. The wound should be washed with effective chemotherapeutic drugs.
When performing a kidney-sparing procedure, the ipsilateral upper urinary tract requires careful follow-up due to the high risk of disease recurrence[15]. Patients undergoing kidney-sparing surgery should be followed more frequently and rigorously than those undergoing RNU, and repeated endoscopic procedures are necessary. EAU guidelines recommend ureteroscopy for low-risk tumors at 3 mo, followed by cystoscopy and CT urography at 3 and 6 mo and then annually for 5 years.
In conclusion, retroperitoneal laparoscopic partial resection of the renal pelvis is an effective surgical procedure for the treatment of urothelial carcinoma of the renal pelvis. It is indicated for patients with isolated kidneys or renal insufficiency, but its safety needs to be further evaluated. The procedure should not be routine but rather used as a supplement to ET, especially for surgeons with extensive experience in laparoscopic surgery and medical centers lacking flexible ureteroscopy equipment.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Simone G S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Freund JE, Liem EIML, Savci-Heijink CD, Baard J, Kamphuis GM, de la Rosette JJMCH, de Bruin DM. Confocal laser endomicroscopy for upper tract urothelial carcinoma: validation of the proposed criteria and proposal of a scoring system for real-time tumor grading. World J Urol. 2019;37:2155-2164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Lee H, Kim HJ, Lee SE, Hong SK, Byun SS. Comparison of oncological and perioperative outcomes of open, laparoscopic, and robotic nephroureterectomy approaches in patients with non-metastatic upper-tract urothelial carcinoma. PLoS One. 2019;14:e0210401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Pathak RA, Hemal AK. Techniques and Outcomes of Robot-assisted Nephro-ureterectomy for Upper Tract Urothelial Carcinoma. Eur Urol Focus. 2018;4:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Simone G, Papalia R, Guaglianone S, Ferriero M, Leonardo C, Forastiere E, Gallucci M. Laparoscopic versus open nephroureterectomy: perioperative and oncologic outcomes from a randomised prospective study. Eur Urol. 2009;56:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Seisen T, Peyronnet B, Dominguez-Escrig JL, Bruins HM, Yuan CY, Babjuk M, Böhle A, Burger M, Compérat EM, Cowan NC, Kaasinen E, Palou J, van Rhijn BW, Sylvester RJ, Zigeuner R, Shariat SF, Rouprêt M. Oncologic Outcomes of Kidney-sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur Urol. 2016;70:1052-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 6. | Guan S, Tang Z, Sun WB, Tian HY, Wang YL, Huang ZM, Yang B, Qian ZM. Percutaneous Resection of Renal Pelvic Neoplasms in Isolated Kidney through a Large Sheath: Report of Two Cases. Zhongguo Weichuang Waike Zazhi. 2012;12:565-568. |
| 7. | Simone G, Papalia R, Loreto A, Leonardo C, Sentinelli S, Gallucci M. Independent prognostic value of tumour diameter and tumour necrosis in upper urinary tract urothelial carcinoma. BJU Int. 2009;103:1052-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Melquist JJ, Redrow G, Delacroix S, Park A, Faria EE, Karam JA, Matin SF. Comparison of Single-docking Robotic-assisted and Traditional Laparoscopy for Retroperitoneal Lymph Node Dissection During Nephroureterectomy With Bladder Cuff Excision for Upper-tract Urothelial Carcinoma. Urology. 2016;87:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Vemana G, Kim EH, Bhayani SB, Vetter JM, Strope SA. Survival Comparison Between Endoscopic and Surgical Management for Patients With Upper Tract Urothelial Cancer: A Matched Propensity Score Analysis Using Surveillance, Epidemiology and End Results-Medicare Data. Urology. 2016;95:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Wang Q, Zhang T, Wu J, Wen J, Tao D, Wan T, Zhu W. Prognosis and risk factors of patients with upper urinary tract urothelial carcinoma and postoperative recurrence of bladder cancer in central China. BMC Urol. 2019;19:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Verges DP, Lallas CD, Hubosky SG, Bagley DH Jr. Endoscopic Treatment of Upper Tract Urothelial Carcinoma. Curr Urol Rep. 2017;18:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Cowan NC, Dominguez-Escrig JL, Gontero P, Hugh Mostafid A, Palou J, Peyronnet B, Seisen T, Soukup V, Sylvester RJ, Rhijn BWGV, Zigeuner R, Shariat SF. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol. 2021;79:62-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 537] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 13. | Jung H, Giusti G, Fajkovic H, Herrmann T, Jones R, Straub M, Baard J, Osther PJS, Brehmer M. Consultation on UTUC, Stockholm 2018: aspects of treatment. World J Urol. 2019;37:2279-2287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Wojtarowicz M, Słojewski M, Gołąb A, Petrasz P. Retroperitoneoscopic renal pelvis resection as treatment of the urothelial tumor in a solitary kidney. Cent European J Urol. 2013;66:299-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Mandalapu RS, Remzi M, de Reijke TM, Margulis V, Palou J, Kapoor A, Yossepowitch O, Coleman J, Traxer O, Anderson JK, Catto J, de la Rosette J, O'Brien T, Zlotta A, Matin SF. Update of the ICUD-SIU consultation on upper tract urothelial carcinoma 2016: treatment of low-risk upper tract urothelial carcinoma. World J Urol. 2017;35:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Rastinehad AR, Ost MC, Vanderbrink BA, Greenberg KL, El-Hakim A, Marcovich R, Badlani GH, Smith AD. A 20-year experience with percutaneous resection of upper tract transitional carcinoma: is there an oncologic benefit with adjuvant bacillus Calmette Guérin therapy? Urology. 2009;73:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Treuthardt C, Danuser H, Studer UE. Tumor seeding following percutaneous antegrade treatment of transitional cell carcinoma in the renal pelvis. Eur Urol. 2004;46:442-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Wang SS, Ho HC, Su CK, Chen WM, Cheng CL, Yang CR. Seeding of malignant renal tumor through a nephrostomy tract. J Chin Med Assoc. 2004;67:308-310. [PubMed] |
| 19. | Oefelein MG, MacLennan G. Transitional cell carcinoma recurrence in the nephrostomy tract after percutaneous resection. J Urol. 2003;170:521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Fuglsig S, Krarup T. Percutaneous nephroscopic resection of renal pelvic tumors. Scand J Urol Nephrol Suppl. 1995;172:15-17. [PubMed] |
| 21. | Sharma NK, Nicol A, Powell CS. Track infiltration following percutaneous resection of renal pelvic transitional cell carcinoma. Br J Urol. 1994;73:597-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |