Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1893
Peer-review started: September 26, 2020
First decision: December 28, 2020
Revised: January 8, 2021
Accepted: January 27, 2021
Article in press: January 27, 2021
Published online: March 16, 2021
Processing time: 160 Days and 8.9 Hours
Synovial sarcoma (SS) accounting for 6%-10% of primary soft tissue malignancies mainly occurs in deep soft tissue adjacent to joints of the limbs. Primary pulmonary SS (PPSS) is rare and has a poor prognosis. Cases of secondary distant metastases of PPSS occur rarely and there is a lack of corresponding imaging reports. We summarized the imaging findings of PPSS with multiple metastases confirmed by surgery and pathology, and shared valuable information on PPSS.
A 43-year-old female patient had a solid space occupying lesion in the right upper lobe of the lung. The results of a hemogram, erythrocyte sedimentation rate (ESR) and tumor markers were all within the normal range, tuberculin skin test (5 TU PPD) was negative (-). Chest computed tomography examination showed similar round soft tissue density in the posterior segment of the right upper lobe. Thoracoscopic-assisted wedge resection of the right upper lobe of the lung, right upper lobe resection and lymph node dissection were performed. Nine months after surgery, ultrasound examination showed multiple metastases on the chest wall and kidney.
PPSS is a rare malignant lung tumor with strong invasiveness, early distant metastasis and poor prognosis. There are very few imaging reports. PPSS is often manifested as irregular tumor and calcification, and the metastases have extremely low echo on ultrasonography. Contrast-enhanced ultrasound indicates that the arterial phase of tumor metastases shows rapid centripetal high enhancement, manifested as “fast forward and fast regression”.
Core Tip: A 43-year-old female patient was diagnosed with primary pulmonary synovial sarcoma (PPSS) and nine months later, metastases were found in the chest wall and both kidneys. There are very few imaging reports on PPSS. This study summarizes the imaging findings of PPSS and its secondary distant metastases. PPSS is often manifested as irregular tumor and calcification, and the metastases have extremely low echo on ultrasonography. Contrast-enhanced ultrasound indicates that the arterial phase of tumor metastases shows rapid centripetal high enhancement, manifested as “fast forward and fast regression”.
- Citation: Li R, Teng X, Han WH, Li Y, Liu QW. Imaging findings of primary pulmonary synovial sarcoma with secondary distant metastases: A case report. World J Clin Cases 2021; 9(8): 1893-1900
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1893.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1893
Primary pulmonary synovial sarcoma (PPSS) is very rare and has no specific clinical manifestations. Distant metastasis occurs early, and is diagnosed mainly based on immunohistochemistry and the SYTSSX fusion gene[1-4]. At present, due to the small number of cases, there is no uniform standard for imaging diagnosis. We report a case of PPSS with secondary multiple metastases and show that the imaging findings of primary lung lesions are mostly shallow lobulated with peripheral calcification. The metastatic lesions show extremely low echo on ultrasonography. Contrast-enhanced ultrasound shows that the tumor metastases have a rich blood supply, and the arterial phase shows rapid centripetal high enhancement, manifested as "fast forward and fast retreat". In the course of the disease, clinicians can carry out early interventions according to the manifestations on ultrasound and contrast-enhanced ultrasound.
The patient, a 43-year-old female was admitted to hospital due to a right upper lobe space occupying lesion. Nine months after lung surgery, she was treated again for painless gross hematuria.
During physical examination, the patient was found to have space occupying lesions in the right lung, and was first admitted to hospital for right lung lesion resection. After 9 mo, there was no obvious inducement for painless hematuria for 5 d, thus she was admitted to hospital again.
Her past history was unremarkable. No other operations were performed during this period.
Her family history was unremarkable.
Nine months after lung surgery, the patient was admitted to hospital again due to sudden, painless hematuria. A mass of 2.0 cm × 1.0 cm × 1.0 cm could be touched under the right chest wall, with fair activity, tough quality and a clear boundary.
The results of a hemogram, ESR, and other tumor markers (alpha fetoprotein, carcinoembryonic antigen, CA-125, CA-199) were all within the normal range, tuberculin skin test (5 TU PPD) was negative.
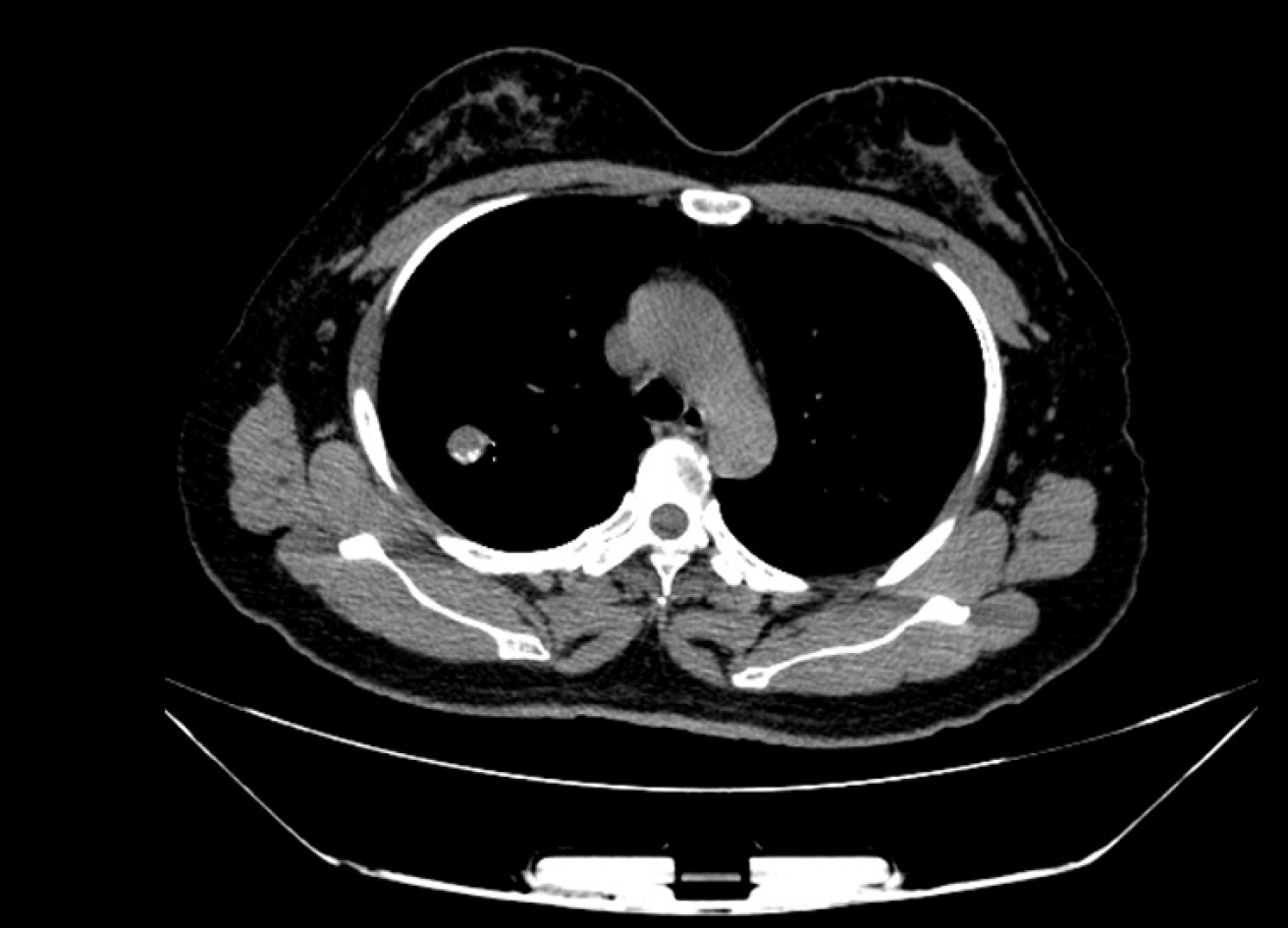
Computed tomography (CT) image showed an irregular dense soft tissue lesion in the posterior upper lobe of the right lung, approximately 3.8 cm × 3.3 cm × 4.0 cm in size, and calcification in the periphery of the tumor (Figure 1).
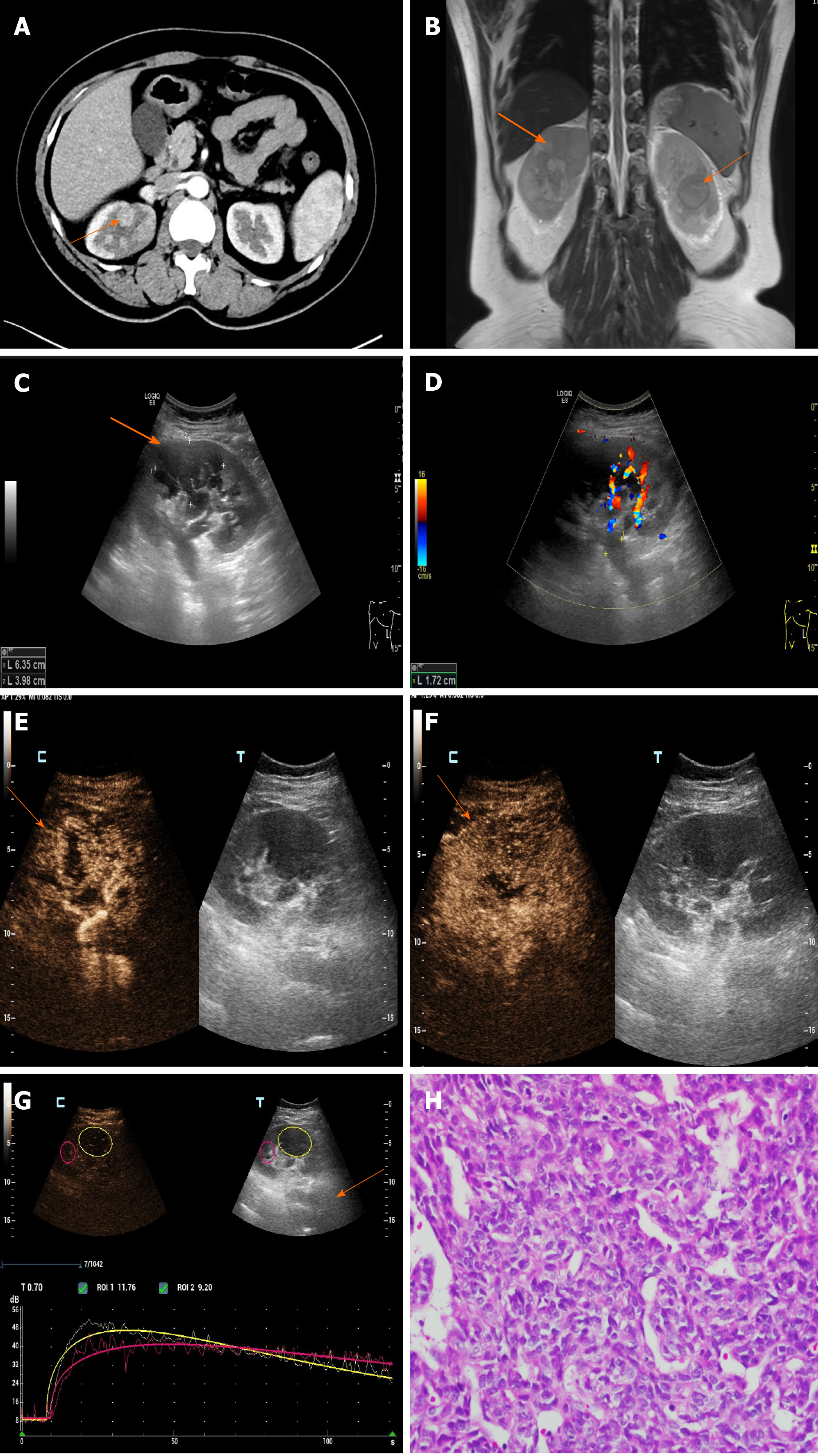
The CT density of both kidneys was uneven, with patchy slightly high-density shadows in the upper and lower pole of the right kidney and the middle parenchyma of the left kidney, and an unclear boundary. Enhanced CT (Figure 2A) showed mild to moderate inhomogeneous enhancement, and the density decreased slightly in the delayed phase. The degree of enhancement was lower than that of the surrounding normal renal parenchyma. Filling defects were found in the bilateral renal veins, which showed mild enhancement. Magnetic resonance imaging (MRI) (Figure 2B) showed multiple long T1, short T2 and diffusion-weighted imaging high signals in both kidneys. Similar signal clusters were found in the left renal pelvis and upper ureter. Two dimensional ultrasonography (Figure 2C and D) images showed that the volume of both kidneys was increased, and several extremely low echo masses were seen in the parenchyma, with unclear and irregular boundaries, the bilateral renal veins were widened and hypoechoic filling was seen inside. Color Doppler flow imaging (CDFI) showed no obvious blood flow signals in both renal veins, and no obvious blood flow signal was found in the renal lesions. The following were seen on contrast-enhanced ultrasonography (CEUS, Figure 2E-G): Multiple solid space occupying lesions were found in both kidneys, the contrast medium filled the heart rapidly during the arterial phase, showing slightly high enhancement, and low enhancement when the contrast agent withdrew in 60 s. Metastasis of synovial sarcoma was considered based on the patient’s medical history and imaging findings.
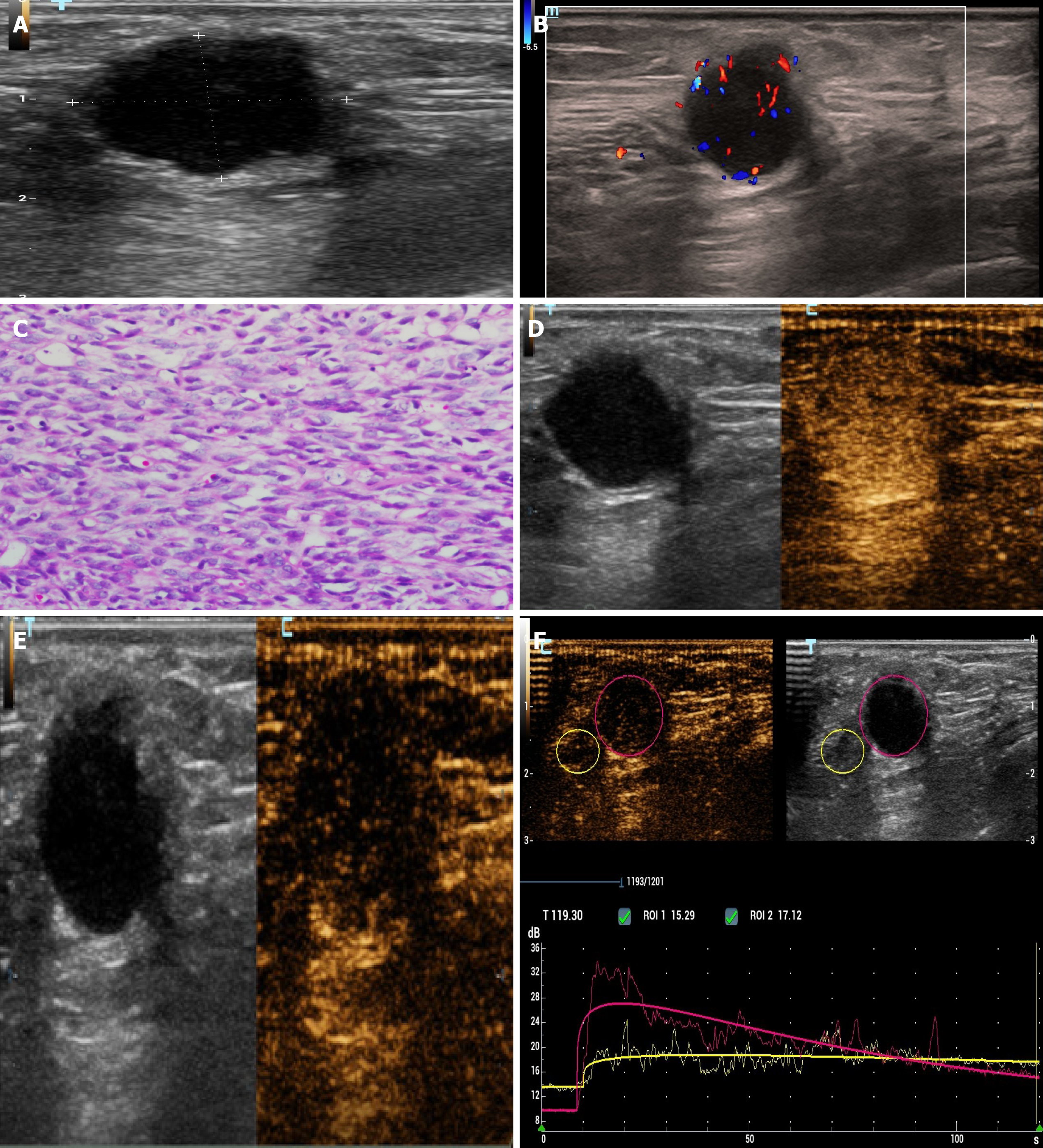
According to chest wall ultrasonography (Figure 3A-C), a very low echo mass approximately 1.5 cm × 1.4 cm × 2.0 cm in size was seen in the superficial subcutaneous fascia layer, with a clear boundary, regular, aspect ratio > 1, and the echo of the surrounding fat layer was increased. On CDFI, a spot strip blood flow signal was seen inside. CEUS (Figure 3D-F) showed that the contrast medium filled the heart rapidly (9 s) during the arterial phase, showing overall high enhancement. After 39 s, the contrast medium quickly withdrew and showed low enhancement.
Combined with the immunohistochemical results, postoperative pathology of pulmonary lesions indicated synovial sarcoma. Pathology of the chest wall mass and kidney mass showed that the nucleus was fusiform or ovoid in shape under the microscope with hyperchromasia, the nucleoli were not obvious, cytoplasm was sparse and unclear, and the mitotic count was rare. Pathological diagnosis was synovial sarcoma metastases (Figures 2H and 3C).
PPSS; nine months later, chest wall metastasis, bilateral renal metastases with bleeding.
Thoracoscopic-assisted wedge resection of the right upper lobe of lung, right upper lobe resection and lymph node dissection were performed for the pulmonary space occupying lesions.
Nine months after lung surgery, selective renal artery embolization was performed for secondary metastases, according to physician and patient preference. After 6 mo of follow-up, the patient had repeated hematuria and the prognosis was poor.
SS mainly occurs in large joints of the limbs. PPSS is very rare, and was diagnosed in 1995[2]. The age of onset is mostly young and middle-aged, with no significant difference between male and female[3] The SYTSSX fusion gene has certain significance in the diagnosis of pulmonary synovial sarcoma which is difficult to identify by immunohistochemistry alone[4]. The typical symptoms are chest pain, cough and hemoptysis; however, patients can also be asymptomatic, and PPSS can be found accidentally during physical examination, and there are few reports on the imaging features of PPSS.
PPSS has a high degree of malignancy, with an overall 5-year survival rate of 50%[3], and is usually seen on CT as a round or irregular heterogeneous dense soft tissue lumpy shadow, with a large diameter, clear boundary, some are shallow lobulated without burrs, with uneven density, visible necrosis, liquefaction and calcification[5,6]. Distant metastasis may occur in a few cases. Lung, lymph nodes and bone are the common sites of metastasis. In this patient, the imaging data on chest wall and kidney metastases were analyzed. The CT scan showed that the renal masses were generally low-density, the enhanced scan showed a gradual uneven enhancement of the masses, and a few lesions around abnormally thickened blood vessels. The delayed phase showed low enhancement. MRI revealed that the signal intensity of the mass was uneven and a low signal capsule was seen. In this case, the lesion signal was slightly lower in the inverse phase. Ultrasonography and CEUS reports on distant metastases in PPSS are rare. In this case, the chest wall metastases showed very low echo with a clear edge, clear boundary, angular edge and expansive growth. The contrast-enhanced ultrasound showed that the contrast medium filled rapidly in the arterial phase and showed high enhancement as a whole, reaching a peak at 11 s, and the contrast medium exited rapidly, showed low enhancement and a “fast forward and fast backward” enhancement mode. Two dimensional ultrasound of bilateral renal metastases showed multiple low echoes with a clear edge, clear boundary and convex growth. Contrast-enhanced ultrasound showed the tumor feeding artery, with rapid centripetal filling of contrast medium in the arterial phase and high enhancement. After reaching a peak in 25 s, the contrast medium rapidly withdrew and showed low enhancement and a "fast forward and fast backward" mode. The contrast-enhanced ultrasound mode of slow progression and fast regression of renal metastases is not exactly the same as that of primary renal SS reported in the literature. It is considered that the tumor may have a rich blood supply, less internal bleeding, cystic degeneration and necrosis. In this case, renal metastases should be differentiated from renal cell carcinoma. The typical contrast-enhanced ultrasound of renal cell carcinoma often showed synchronous enhancement of tumor and renal cortex in the arterial phase, with peritumoral rim enhancement around the tumor, high peak value, heterogeneous enhancement, rapid regression, annular high enhancement at the edge in the later stage of regression[7-9]. The perfusion pattern and degree of contrast-enhanced ultrasound can be used to differentiate tumors[10,11].
The incidence of PPSS is low, but the prognosis is very poor. At present, there is no uniform standard treatment plan for synovial sarcoma. Surgery is still the main treatment method for pulmonary synovial sarcoma with maximum resection of the tumor. The postoperative recovery and follow-up of patients are supplemented with corresponding chemotherapy and radiotherapy, which is expected to improve the survival rate of patients with pulmonary synovial sarcoma. We should be aware of distant metastasis and conduct regular assessments as soon as possible to improve the prognosis. Imaging analyses of PPSS patients with multiple metastases will provide important clinical information for surgery and for monitoring the disease course.
PPSS is extremely rare but the prognosis is very poor, and its diagnosis relies on pathological immunohistochemistry and genetic testing. There is no unified standard therapy for postoperative adjuvant chemotherapy and imaging features play an important role in monitoring the course of the disease.
We thank the patient for permitting us to use her data to complete this article.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ishida H S-Editor: Fan JR L-Editor: Webster JR P-Editor: Wang LL
| 1. | Nuwal P, Dixit R, Shah NS, Samaria A. Primary monophasic synovial sarcoma lung with brain metastasis diagnosed on transthoracic FNAC: Report of a case with literature review. Lung India. 2012;29:384-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Zeren H, Moran CA, Suster S, Fishback NF, Koss MN. Primary pulmonary sarcomas with features of monophasic synovial sarcoma: a clinicopathological, immunohistochemical, and ultrastructural study of 25 cases. Hum Pathol. 1995;26:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Dennison S, Weppler E, Giacoppe G. Primary pulmonary synovial sarcoma: a case report and review of current diagnostic and therapeutic standards. Oncologist. 2004;9:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Kim GH, Kim MY, Koo HJ, Song JS, Choi CM. Primary Pulmonary Synovial Sarcoma in a Tertiary Referral Center: Clinical Characteristics, CT, and 18F-FDG PET Findings, With Pathologic Correlations. Medicine (Baltimore). 2015;94:e1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Duran-Mendicuti A, Costello P, Vargas SO. Primary synovial sarcoma of the chest: radiographic and clinicopathologic correlation. J Thorac Imaging. 2003;18:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Kambo JS, Richardson B, Ionescu DN, Tucker T, Kraushaar G. Primary pulmonary synovial sarcoma: a case report with unique and impressive computed tomography findings. Can Respir J. 2015;22:e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Cai HJ, Cao N, Wang W, Kong FL, Sun XX, Huang B. Primary renal synovial sarcoma: A case report. World J Clin Cases. 2019;7:3098-3103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Xu ZF, Xu HX, Xie XY, Liu GJ, Zheng YL, Lu MD. Renal cell carcinoma and renal angiomyolipoma: differential diagnosis with real-time contrast-enhanced ultrasonography. J Ultrasound Med. 2010;29:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Cao H, Fang L, Chen L, Zhan J, Diao X, Liu Y, Lu C, Zhang Z, Chen Y. The independent indicators for differentiating renal cell carcinoma from renal angiomyolipoma by contrast-enhanced ultrasound. BMC Med Imaging. 2020;20:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Stock K, Kübler H, Maurer T, Slotta-Huspenina J, Holzapfel K. [CEUS-diagnosis of solid renal tumors]. Radiologe. 2018;58:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Rübenthaler J, Negrão de Figueiredo G, Mueller-Peltzer K, Clevert DA. Evaluation of renal lesions using contrast-enhanced ultrasound (CEUS); a 10-year retrospective European single-centre analysis. Eur Radiol. 2018;28:4542-4549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |