Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1853
Peer-review started: August 12, 2020
First decision: December 8, 2020
Revised: December 31, 2020
Accepted: January 23, 2021
Article in press: January 23, 2021
Published online: March 16, 2021
Processing time: 204 Days and 17.8 Hours
Craniometaphyseal dysplasia (CMD) is a rare genetic disorder. Autosomal dominant CMD (AD-CMD) is caused by mutations in the ANKH gene. Affected individuals typically have distinctive facial features including progressive thickening of the craniofacial bones. Treatment for AD-CMD primarily consists of surgical intervention to release compression of the cranial nerves and the brain stem/spinal cord. To alleviate progression of the clinical course and improve the quality of life in children waiting to undergo the necessary surgery, we investigated clinical changes in a diagnosed patient with AD-CMD over three years.
A 17-mo-old boy presented with progressive nasal obstruction, snoring and hearing loss symptoms. Physical examination showed enlargement of the head circumference and clinical features such as wide nasal bridge, paranasal bossing, widely spaced eyes with an increased bizygomatic width, and a prominent mandible. The patient underwent otolaryngological examination, endoscopy, hearing test, laboratory examination of phosphorus and bone metabolism, cranial and femoral computed tomography, X-ray and next-generation sequencing. The patient was diagnosed with AD-CMD due to p.Phe377 deletion (c.1129_1131del) on exon 9 of the ANKH gene. After adherence to a prescribed low-calcium diet, the boy’s alkaline phosphatase (ALP) levels continuously decreased to within the normal range. However, after 14 mo of dietary intervention, his parents altered his diet to an intermittent low-calcium diet to include milk and eggs. The patient’s ALP was slightly higher than normal after the dietary change but remained close to the normal range. His serum osteocalcin changed to within normal levels after dietary regulation for 33 mo. His serum combined beta C-terminal telopeptide of type I collagen also continuously decreased after the nutritional intervention, although still slightly higher than normal levels. Despite fluctuating blood test results, the boy’s nasal symptoms were markedly relieved and steadily improved after dietary intervention. No significant changes were found in the craniofacial bones by cranial radiography. Close monitoring of clinical features is still ongoing. Calcitriol treatment is currently under consideration and a surgical procedure is planned as necessary in the future.
We herein report the first Chinese case of AD-CMD with heterozygous mutation of p.Phe377 deletion (c.1129_1131del) on the ANKH gene. Biochemical alterations were significantly improved after dietary intervention indicating that a low-calcium diet may be applied in pediatric AD-CMD patients with ANKH mutations to help alleviate phenotypic manifestations and improve the quality of life before surgical intervention. Further large scale studies are needed to replicate these findings and to establish the appropriate timing for nutritional and surgical interventions
Core Tip: Craniometaphyseal dysplasia is a rare genetic disorder. It has been found to be caused by mutations in the ANKH gene with an autosomal dominant inheritance pattern. If untreated, hyperostosis and sclerosis of the skull may lead to cranial nerve compressions resulting in deafness, blindness and facial palsy. Surgical interventions would usually be needed in severe cases such as these. We herein report the first Chinese case with heterozygous mutation of p.Phe377 deletion (c.1129_1131del) on the ANKH gene. We investigated the clinical changes in this patient who underwent nutritional intervention between 3 and 33 mo. Biochemical alterations were significantly changed after dietary intervention. Phenotypic manifestations were markedly alleviated. A low-calcium diet may help slow the clinical course in children and improve the quality of life before the necessary surgery. Further large scale studies are needed to establish the appropriate timing of nutritional and surgical interventions.
- Citation: Wu JL, Li XL, Chen SM, Lan XP, Chen JJ, Li XY, Wang W. A three-year clinical investigation of a Chinese child with craniometaphyseal dysplasia caused by a mutated ANKH gene. World J Clin Cases 2021; 9(8): 1853-1862
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1853.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1853
Craniometaphyseal dysplasia (CMD) is a rare disorder characterized by progressive hyperostosis and sclerosis of the craniofacial bones and metaphyseal flaring of the long bones[1]. Patients with CMD often suffer from neurological symptoms due to obstruction of the cranial foramina. CMD is usually inherited in an autosomal dominant (AD-CMD) mode caused by mutations in the ANKH gene encoding a pyrophosphate-transporting membrane protein[2]. This protein plays a key role in the development and function of bone-regulating osteoblasts and osteoclasts. Specifically, the ANKH protein transports pyrophosphate out of cells which helps control bone formation by preventing mineralization. Approximately 10 mutations in the ANKH gene have been identified in patients with AD-CMD and their families[3,4].
Affected individuals typically have distinctive facial features such as a wide nasal bridge, paranasal bossing, widely spaced eyes with an increase in bizygomatic width, and a prominent mandible. Excess bone formation in the jaw can delay the development of dentition or result in non-erupting teeth. Progressive thickening of craniofacial bones may also lead to narrowing of the cranial foramina including the foramen magnum. If untreated, hyperostosis and sclerosis of the skull in severe cases may lead to cranial nerve compressions resulting in deafness, blindness and facial palsy[1,5-7]. Treatment of AD-CMD includes management of feeding and respiratory issues in newborns and infants, hearing and vision aids for affected children, and surgical intervention for optic nerve impaction and malocclusion in severe cases[8].
A 17-mo-old boy presented with progressive nasal obstruction, snoring and hearing loss symptoms when referred to the hospital.
The patient’s medical history was first reviewed before the diagnosis. His head circumference was 45.5 cm, 46.5 cm and 49.5 cm at age 3 mo, 6 mo and 12 mo, respectively. When he was 6 mo old, fiber nasopharyngoscopy revealed a double choanal stenosis. The patient was found to have a serious nasal obstruction at the age of 12 mo due to a wide nasal bridge. Occasionally, he resorted to mouth breathing, especially at night. The patient was examined at the local hospital, showed low bone mineral density and commenced oral calcium supplements. His head circumference increased to 51 cm (standard value 45.2 cm). Consequently, the patient developed a prominent forehead, prognathism and occipital protuberance. At the age of 16 mo, the patient presented with mild hearing loss. He had been receiving calcium and vitamin D supplementation for 4 mo prior to examination at other hospital; however, the patient’s symptoms developed progressively.
The patient had no other previous medical history.
The patient had no relevant family medical history.
A wide nasal bridge, paranasal bossing, widely spaced eyes with an increased bizygomatic width, and prominent mandible (Figure 1) were noted. However, hypertelorism was not obviously discernible. Additionally, the patient’s frontal and maxillary sinuses were severely obstructed. He had 20 teeth with wide spacing between the teeth. His teeth appeared small. He exhibited no facial nerve palsy or limb muscle tension. His pain perception and muscular strength appeared normal. Nasal laryngeal mirror showed serious choanal stenosis on both sides. The bottom of the patient’s nose exhibited bossing and his palatine bone appeared thickened. The patient’s parents and his elder brother had completely normal features.
The patient’s blood test results are shown in Table 1. On the patient’s first visit to the hospital, he underwent laboratory tests of serum alkaline, calcium and others. After genetic diagnosis, more related tests were performed. Given that the diagnosis of AD-CMD was established, related biochemical tests were followed up (Table 1). The results showed that the patient’s serum concentration of alkaline phosphatase (ALP) decreased after 3 mo of dietary intervention. After 14 mo of low-calcium diet at the age of 2 years and 7 mo, his ALP continuously decreased to within the normal range. His parents then changed his diet to an intermittent low-calcium diet to include milk and eggs. At the age of 4 years and 2 mo, his ALP was slightly higher than normal but still close to the normal range. His serum osteocalcin (OC) was higher than normal even after 8 mo of low-calcium diet. However, it began to continuously drop after dietary restrictions for 14 mo and then reached normal levels. His serum combined beta C-terminal telopeptide of type I collagen (β-CTX) also decreased after 33 mo of the nutritional intervention, but still remained slightly higher than normal. Other biochemical test results were normal.
| Age | 17 mo | 20 mo | 25 mo | 2 yr and 7 mo | 4 yr and 2 mo | Reference value (0-18 years old) |
| Parathyroid hormone (pmol/L) | NA | 3.48 | 3.94 | 4.61 | 3.72 | 1.58-6.83 |
| Serum osteocalcin (ng/mL) | NA | 95 | 166 | 60 | 33. 34 | 14-46 |
| Combined β-CTX (ng/mL) | NA | 1.76 | 4.37 | 1.30 | 1.09 | 0.04-0.78 |
| 25-hydroxyvitamin D (ng/mL) | NA | 22. 3 | 29.67 | 26.78 | 24.66 | 30-100 |
| Serum alkaline phosphatase (U/L) | 884 | 673 | 642 | 243 | 362 | 104-345 (1-3 years old)93-309 (4-6 years old) |
| Calcium (mmol/L) | 2.44 | 2.43 | 2.30 | 2.18 | 2.37 | 2.2-2.75 |
| Phosphorus (mmol/L) | 1.67 | 1.36 | 1.5 | 1.16 | 1.20 | 1-2.15 (1m-3 years old), 0.84-1.85 (> 4 years old) |
| 5’-Nucleotidase (U/L) | 3 | 2 | 4 | 3 | 3 | 0-11 |
Cranial radiography showed that he had increased thickness of the craniofacial bones with obstructed sinuses and narrowing ears and other cavities (Figure 1A-C). Specifically, the middle ear cavities appeared narrowed, while the lumen of the labyrinth (the vestibule, semicircular canal and cochlea) appeared sclerotic. His mastoid cavity disappeared without tympanic cavity effusion. The width of left bony optic canal was 3.93 mm and the width on the right side was 4.17 mm. Prominent thickening of the calvaria was apparent and sclerosis of the skull base and maxilla was noted. The patient’s frontal and maxillary sinuses were severely obstructed with marked thickening of the cranial walls. His forehead bone was 14.22 mm thick. Thickening of his middle turbinate and inferior turbinate was also obvious. The patient’s nasal septum thickness was 8.44 mm, causing his nose and choanae to become significantly narrowed. His frontal sinus and maxillary sinus were invaded by dysplastic tissue. Marked thickening of the cranial walls was also noted.
Sclerosis of the clavicles and ribs was observed by computed tomography (CT) and X-ray (Figure 1E). Metaphyseal flaring in the distal femora due to a modeling defect was the most pronounced abnormality. “Flask deformation” of the proximal metaphysis on both sides (Erlenmeyer flask configuration) (Figure 1F) was also noted. When the patient was four years and 2 mo, he complained of pain in the legs; thus, long bone examination was performed again. CT and X-ray did not demonstrate significant changes compared to his previous image (Figure 2). CT of the middle ears showed the absence of effusions. The patient’s ossicular chain was large.
Acoustic impedance examination showed that he had binaural "B". Otoacoustic emission was not extracted from either of his ears. Auditory brainstem response demonstrated that the 1-v wave in both of ears was slightly delayed and the V wave of 50 dBHL was extracted.
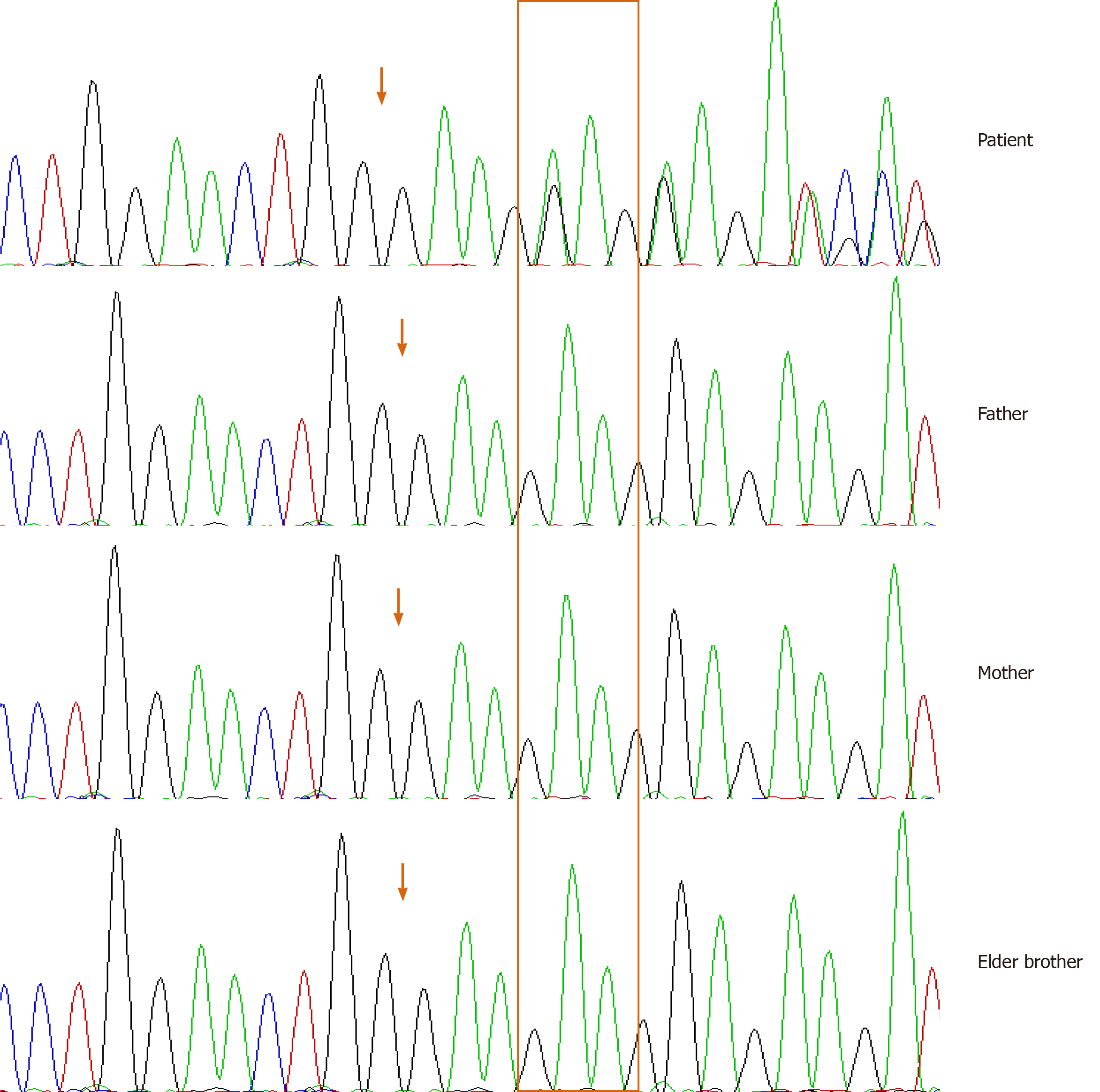
Clinical features and cranial and bone radiographs suggested the diagnosis of CMD. A next-generation sequencing panel of bone disorders was subsequently performed on the proband and the patient’s parents. A heterozygous mutation c.1129_1131del (NM_054027) was found on exon 9 of the ANKH gene leading to an in-frame deletion of p.Phe377del on the encoded protein. This variant was not detected on the ANKH gene in either of his parents or his elder brother. Sanger sequencing was further performed to confirm this finding (Figure 3). We searched the mutation database and found that the same codon mutation was not previously identified[2,3].
After careful evaluation of the child’s symptoms and diagnosis of AD-CMD, considering the future progression of the clinical course, we decided not to immediately offer surgical intervention. A nutritional intervention with a low-calcium diet was first initiated to control the disease progression by limiting calcium rich foods such as milk, beans or bean soup, oysters, shrimp and broccoli etc. With the expectation of approximately 150 mg/day at the beginning of the intervention and subsequent increases with age, his daily diet was managed and monitored by his mother according to the dietician’s guidance but not with careful calculation. Although his diet was not fully complied with or well measured, both his blood tests results and symptoms significantly improved. Other medical surveillance including neurologic signs, hearing and ophthalmologic assessment was evaluated.
The child’s symptoms were relieved after administration of a low-calcium diet. His nasal obstruction and snoring were markedly alleviated, although his hearing loss did not improve significantly. Following a low-calcium diet for 14 mo, his ALP significantly decreased to within the normal range. When his mother changed his diet to an intermittent low-calcium diet to introduce milk and eggs, his ALP remained close to the normal range. His serum OC was higher than normal even after low-calcium diet, but began to drop continuously after undertaking a restricted diet for 8 mo until normal levels were achieved. His serum β-CTX also decreased after the nutritional intervention of 33 mo, but remained slightly higher than normal. Other biochemical test results were all within normal ranges. Close monitoring of the child’s clinical features is ongoing. Calcitriol treatment is being considered. Surgical procedures will also be considered if necessary in the future.
CMD (MIM #123000) is a rare genetic disorder that progressively affects the skeletal system. The typical features of CMD include hyperostosis of craniofacial bones and abnormal modeling of tubular bones. Craniofacial abnormalities commonly include wide-set eyes, wide nasal bridge, paranasal bossing and a prominent mandible. These main features in severe individuals can lead to increased intracranial pressure and narrowing of the neural foramina[3]. Dentition development may also be delayed in some affected children. Teeth may fail to erupt in infants due to hyperostosis and sclerosis of the alveolar bone. Thickening of craniofacial bones continually progresses throughout the life of affected patients, which often results in severe neurological problems. If untreated, compression of cranial nerves can lead to severe conditions such as facial palsy, blindness, or deafness usually as conductive hearing loss. In individuals with typical AD-CMD without severe complications, the life expectancy is usually normal. However, in those individuals with severe AD-CMD due to compression of the foramen magnum, the patient’s life expectancy can be reduced. In view of the foregoing, slowing the clinical course and avoidance of severe conditions are critical for appropriate management of AD-CMD.
The pathogenesis of this disease is thought to be due to excessive skeletal mineralization which is mainly induced by decreases of the mineralization inhibitor pyrophosphate. ANKH is the only gene currently known to be associated with AD-CMD. Pathogenic variants of other genes, as-yet unknown, may also be causative. The ANKH gene encodes a multiple-pass transmembrane protein associated with pyrophosphate transport[4,5]. Many cases of AD-CMD are described as inherited in an autosomal dominant manner. In agreement with those reports, individuals with AD-CMD caused by a pathogenic variant in the ANKH gene have an affected parent, but de novo mutations are frequently observed in simplex cases[6,7]. In the reported case, the child was found to possess a de novo mutation of the ANKH gene, as his parents and his elder brother did not test positive for the mutation. These results were confirmed by Sanger sequencing.
The most important clinical manifestations of AD-CMD are a bony “lion face” syndrome and cranial nerve damage. Overgrowth of the skull causes the forehead to protrude forward and the teeth to become disordered. The prominent protuberance on both sides of the nose present a facial distinction in infancy, namely the “lion face” syndrome. These may be alleviated with age. Excessive hyperplasia of the ethmoid bone and turbinate usually cause narrowing of the nasal cavity and undesirable paranasal sinus vaporization. Obstacles of ventilation function can lead to obstructed nasal breathing, and consequently shortness of breath or forced open mouth breathing. The affected patient reported herein visited the hospital due to these symptoms including shortness of breath and open mouth breathing with gasping during the night. During his examination, it was noted that he had a square skull, frontal bossing, hypertelorism, an extremely wide nasal bridge, prognathism and hypodontia. The patient’s hearing examination additionally showed that he had conductive hearing loss. We then examined his cranial and facial bones by CT. The CT scan revealed that the child had thickening of cranial and facial bones, hypoplastic maxillary sinuses, agenesis of the frontal sinus and middle ear ossicle fixation. His facial nerves, auditory nerves, and optic nerves were not oppressed.
Treatment for severe AD-CMD primarily relies on surgical intervention to alleviate compression of the cranial nerves and the brain stem/spinal cord at the level of the foramen magnum. However, in this case, the patient has not presented any obvious indications of cranial nerve compression nor any of the associated complications. Medical treatments with calcitriol or a low-calcium diet have been investigated to inhibit hyperplasia by regulation of bone metabolism and homeostasis; however, these have shown a restrictive effect. With high doses of calcitriol and low-calcium diet, partial relief of a patient’s facial nerve paralysis and increase in size of the cranial nerve foramina, and demineralization of the base of skull have been observed but did not change metaphyses deformities[8]. In some cases, calcitriol therapy with a low-calcium diet did not alter the clinical progression of the disease[9]. In consideration of both his clinical features and biochemical results, we first initiated a dietary intervention for this child according to his blood test results for bone metabolism.
ALP produced by skeletal osteoblasts can be transported via the blood to the liver where it participates in biliary drainage. High levels of ALP can therefore indicate possible active bone deposition. It commonly occurs that ALP activity is generally 1-2 times higher than adult levels during the pediatric period of bone development. Serum OC is a non-collagen bone protein secreted by osteoblasts. ALP and OC combined can be a sensitive biochemical indicator of bone growth and development in children. While ALP is a marker of early differentiation of osteoblasts, OC can directly reflect the status of bone formation. Serum OC mainly appears during the mineralization and formation stage of bone development, making OC a marker of osteoblast maturation. The expression level of ALP and OC can therefore together reflect the early and later stages of new bone formation as the expression of ALP and OC parallel osteoblast development, increasing with the differentiation of osteoblasts and decreasing with the maturation of osteoblasts. The reciprocal status between the expression pattern of ALP and OC and the development of osteoblasts help to maintain homeostasis. ALP has been shown to be elevated in CMD[10,11]. Serum β-CTX is a valuable approach for evaluating osteoclast activity and bone resorption. A Phe377del mutation of ANKH in a mouse model has been shown to cause impaired osteoblastogenesis and osteoclastogenesis resulting in hypomineralization and a high bone mass phenotype[12]. Supplementation with calcium and calcitriol was shown to alleviate biochemical abnormalities including ALP and parathyroid hormone and mild hypocalcemia[9]. However, in some cases, calcitriol with low-calcium diet has been reported to improve facial paralysis but had no effect on metaphyseal deformity[8].
In this case, a low-calcium diet trial with approximately 150 mg/day at the beginning and increased intake with age was monitored by his mother according to the dietician’s guidance. Although his diet was not carefully calculated, his nasal obstruction and snoring were continuously relieved. Even after his mother changed his diet to an intermittent low-calcium diet to include eggs and milk, his clinical manifestations improved steadily. His blood tests result including ALP, serum OC and β-CTX were significantly ameliorated. With the implementation of the nutritional intervention, observed biochemical changes in ALP, OC and β-CTX indicated that the regulation of homeostasis was initiated, which may explain the improvement in the child’s symptoms. After 33 mo of dietary intervention, no obvious changes on his cranial and femoral radiographs were observed. The mechanism of functional changes in osteogenesis and osteoclasts should be further explored in this disorder. A large scale study is needed for further elucidation.
Our investigation has shown that a low-calcium diet could help alleviate phenotypic manifestations in children and improve their quality of life. In particular, the child’s nasal symptoms improved. However, his hearing loss was not significantly changed. Amelioration of biochemical changes indicates that the homeostasis of bone metabolism was regulated after dietary intervention. The type of calcium diet was determined according to his laboratory results and calcitriol is under consideration for treatment in the near future. However, despite slowing the clinical progression of AD-CMD, surgery may still be necessary. Close monitoring of the clinical course is ongoing to identify the appropriate timing of subsequent nutritional and surgical interventions.
We herein report the first Chinese case of AD-CMD caused by heterozygous mutation of p.Phe377del on the ANKH gene. After a low-calcium intervention for three years, our investigation showed that a simple nutritional intervention could significantly help alleviate phenotypic manifestations in the child and improve the quality of life before surgery if necessary. Biochemical changes suggest the possible regulation of bone homeostasis due to the dietary intervention. Large scale studies are needed to establish the appropriate timing and type of nutritional and surgical interventions. Further exploration of the mechanism of the disorder would be helpful for the development of precise treatment for AD-CMD.
We thank our colleagues at the Department of Otorhinolaryngology and Head and Neck Surgery and Department of Molecular Diagnostic Laboratory, Shanghai Children Hospital, Shanghai Jiaotong University School of Medicine.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reichenberger E S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LYT
| 1. | Carnevale A, Grether P, del Castillo V, Takenaga R, Orzechowski A. Autosomal dominant craniometaphyseal dysplasia. Clinical variability. Clin Genet. 1983;23:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Reichenberger E, Tiziani V, Watanabe S, Park L, Ueki Y, Santanna C, Baur ST, Shiang R, Grange DK, Beighton P, Gardner J, Hamersma H, Sellars S, Ramesar R, Lidral AC, Sommer A, Raposo do Amaral CM, Gorlin RJ, Mulliken JB, Olsen BR. Autosomal dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein ANK. Am J Hum Genet. 2001;68:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Nürnberg P, Thiele H, Chandler D, Höhne W, Cunningham ML, Ritter H, Leschik G, Uhlmann K, Mischung C, Harrop K, Goldblatt J, Borochowitz ZU, Kotzot D, Westermann F, Mundlos S, Braun HS, Laing N, Tinschert S. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat Genet. 2001;28:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Chen IP, Tadinada A, Dutra EH, Utreja A, Uribe F, Reichenberger EJ. Dental Anomalies Associated with Craniometaphyseal Dysplasia. J Dent Res. 2014;93:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Kim YH, Roh DH, Choi BY, Oh SH. Craniometaphyseal dysplasia. Acta Otolaryngol. 2005;125:797-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Puri P, Chan J. Craniometaphyseal dysplasia: ophthalmic features and management. J Pediatr Ophthalmol Strabismus. 2003;40:228-231. [PubMed] |
| 7. | Kornak U, Brancati F, Le Merrer M, Lichtenbelt K, Höhne W, Tinschert S, Garaci FG, Dallapiccola B, Nürnberg P. Three novel mutations in the ANK membrane protein cause craniometaphyseal dysplasia with variable conductive hearing loss. Am J Med Genet A. 2010;152A:870-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Key LL Jr, Volberg F, Baron R, Anast CS. Treatment of craniometaphyseal dysplasia with calcitriol. J Pediatr. 1988;112:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Richards A, Brain C, Dillon MJ, Bailey CM. Craniometaphyseal and craniodiaphyseal dysplasia, head and neck manifestations and management. J Laryngol Otol. 1996;110:328-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Sheppard WM, Shprintzen RJ, Tatum SA, Woods CI. Craniometaphyseal dysplasia: a case report and review of medical and surgical management. Int J Pediatr Otorhinolaryngol. 2003;67:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Wu B, Jiang Y, Wang O, Li M, Xing XP, Xia WB. Craniometaphyseal dysplasia with obvious biochemical abnormality and rickets-like features. Clin Chim Acta. 2016;456:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Chen IP, Wang L, Jiang X, Aguila HL, Reichenberger EJ. A Phe377del mutation in ANK leads to impaired osteoblastogenesis and osteoclastogenesis in a mouse model for craniometaphyseal dysplasia (CMD). Hum Mol Genet. 2011;20:948-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |