Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1580
Peer-review started: September 20, 2020
First decision: December 4, 2020
Revised: December 22, 2020
Accepted: January 8, 2021
Article in press: January 8, 2021
Published online: March 6, 2021
Processing time: 162 Days and 2.5 Hours
Percutaneous radiofrequency ablation (RFA) is an effective treatment for unresectable hepatocellular carcinoma (HCC) and a minimally invasive alternative to hepatectomy for treating tumour recurrence. RFA is often performed using contrast-enhanced computed tomography (CECT) and/or ultrasonography. In recent years, angiographic systems with flat panel image detectors and advanced image reconstruction algorithms have broadened the clinical applications of cone-beam computed tomography (CBCT), including RFA. Several studies have shown the effectiveness of using CBCT for immediate treatment assessments and follow-ups.
To assess the treatment response to RFA for HCC using CBCT.
Forty-eight patients (44 men; aged 37-89 years) with solitary HCC [median size: 3.2 (1.2-6.6) cm] underwent RFA and were followed for 25.6 (median; 13.5-35.2) mo. Image fusion of CBCT and pre-operative CECT or magnetic resonance imaging (MRI) was used for tumour segmentation and needle path and ablation zone planning. Real-time image guidance was provided by overlaying the three-dimensional image of the tumour and needle path on the fluoroscopy image. Treatment response was categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Disease progression, death, time to progression (TTP), and overall survival (OS) were recorded. Kaplan-Meier and Cox regression analyses were performed.
Initial post-RFA CECT/MRI showed 38 cases of CR (79.2%), 10 of PR (20.8%), 0 of SD, and 0 of PD, which strongly correlated with the planning estimation (42 CR, 87.5%; 6 PR, 12.5%; 0 SD; and 0 PD; accuracy: 91.7%, P < 0.01). Ten (20.8%) patients died, and disease progression occurred in 31 (35.4%, median TTP: 12.8 mo) patients, resulting in 12-, 24-, and 35-mo OS rates of 100%, 81.2%, and 72.2%, respectively, and progression-free survival (PFS) rates of 54.2%, 37.1%, and 37.1%, respectively. The median dose-area product of the procedures was 79.05 Gy*cm2 (range 40.95-146.24 Gy*cm2), and the median effective dose was 10.27 mSv (range 5.32-19.01 mSv). Tumour size < 2 cm (P = 0.008) was a significant factor for OS, while age (P = 0.001), tumour size < 2 cm (P < 0.001), tumour stage (P = 0.010), and initial treatment response (P = 0.003) were significant factors for PFS.
Reliable RFA treatment planning and satisfactory outcomes can be achieved with CBCT.
Core Tip: This is a prospective study to assess the treatment response to radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC) using cone-beam computed tomography (CBCT) for treatment planning and image guidance. Satisfactory 1-, 2-, and 3-year overall survival and disease progression outcomes were achieved in patients with solitary HCC when treated by RFA under CBCT. Image fusion of pre-operative CT/magnetic resonance imaging with CBCT allows precise RFA treatment planning and real-time image guidance. The initial treatment response strongly correlates with RFA planning and is an independent predictor of short-term outcomes, implying the necessity of reliable treatment planning.
- Citation: Yao XS, Yan D, Jiang XX, Li X, Zeng HY, Li H. Short-term outcomes of radiofrequency ablation for hepatocellular carcinoma using cone-beam computed tomography for planning and image guidance. World J Clin Cases 2021; 9(7): 1580-1591
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1580.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1580
Percutaneous radiofrequency ablation (RFA) is an effective treatment for unresectable hepatocellular carcinoma (HCC) and a minimally invasive alternative to hepatectomy for treating tumour recurrence[1-3]. In particular, clinical evidence has shown that RFA can be considered a curative therapy for nodules ≤ 5 cm and can achieve comparable long-term survival outcomes to that of surgical resection in patients with lesions ≤ 3 cm[1,3-4].
RFA requires an operator to have a substantial understanding of the anatomy and hands-on experience with ablation techniques and instruments. During the procedure, the operator relies on non-invasive imaging to visualize the tumour and the ablation needle while gradually progressing the needle on a well-planned path. As tumours cannot be visualized under fluoroscopy and the spatial relationship between the tumour and the needle cannot be sufficiently assessed using conventional two-dimensional imaging, RFA is often performed using contrast-enhanced computed tomography (CECT) and/or ultrasonography. However, physical restrictions around a CT scanner often result in a cumbersome workflow, involving several re-scans of the patient during the treatment to confirm the ablation needle’s position while the operator plans or progresses and adjusts the needle towards the tumour. Ultrasound, on the other hand, is limited in its field of view and depth of view, making it challenging to reach deep tumours in critical locations. Moreover, a lack of access to CT and/or ultrasound for therapeutic purposes remains a common resource issue for interventional radiologists and interventional oncologists in many centres.
In recent years, angiographic systems with flat panel image detectors and advanced image reconstruction algorithms have broadened the clinical applications of cone-beam computed tomography (CBCT), including RFA. A CBCT scan acquires a set of images while rotating the X-ray generator and the image detector around the patient’s body to produce CT-like (axial) images as well as three-dimensional (3D) reconstructions. This allows tumour detection, (semi-)automatic segmentation, and real-time image fusion between CBCT, and fluoroscopy–all can be applied using a stand-alone angiographic system. Morimoto et al[5] reported one of the first pilot studies in which CBCT was used for RFA[5]. Abi-Jaoudeh et al[6] described early clinical experience with tumour ablation navigation software for needle path planning, ablation zone marking, and image fusion[6]. Several other studies have shown the effectiveness of using CBCT for immediate treatment assessments and follow-ups[7-10]. Kato et al[11] demonstrated in a comparative CBCT vs ultrasound-guided RFA study that “CBCT guidance contributed to improved rates of complete ablation and decreased rates of cumulative local progression”[11]. Nevertheless, additional evidence of short- and long-term RFA treatment outcomes associated with the standardized use of CBCT for treatment planning and image guidance should be collected and reported to serve relevant clinical communities.
In the present study, we systematically and comprehensively incorporated CBCT into the routine RFA procedure workflow for tumour segmentation, needle path and ablation zone planning, real-time image guidance, and treatment assessment. We prospectively collected treatment responses in HCC patients and evaluated their short-term outcomes.
Approval from the authors’ Hospital Independent Ethics Committee was obtained for this single-centre, single-arm prospective study. HCC patients with solitary lesions, no presence of extrahepatic metastasis, refusing to undergo operation, and older than 18 years of age were included in this study after obtaining informed consent. Patients with breath hold difficulty were excluded due to probable motion artefacts and poor CBCT image quality. Between June 2014 and December 2015, 48 inoperable or elective HCC patients subjected to RFA treatments were consecutively enrolled. HCC was diagnosed by alpha-fetoprotein test and image findings on CECT (LightSpeed VCT, GE Healthcare, Milwaukee, WI, United States) or magnetic resonance imaging (MRI; Signa 1.5 HDxt, GE Healthcare, Milwaukee, WI, United States). Patient age, sex, Barcelona Clinic Liver Cancer (BCLC) stage, Child-Pugh score, tumour size and tumour adjacency to major vessels, the diaphragm, or other organs were recorded and analysed.
RFA was performed using a radiofrequency generator (RITA 1500, AngioDynamics, Mountain View, CA, United States) and two models of ablation needles with multiple electrode arrays (Xli and Talon Semi Flex, AngioDynamics, Mountain View, CA, United States). RF energy was generated at 460 kHz and delivered by the electrodes at the needle tip for the coagulation and ablation of target lesions at 105 °C and 250 W. The Xli 14 G needle contains nine electrode arrays and creates a spherical ablation zone of 4-7 cm (i.e., 2-5 cm lesion size). The Talon Semi Flex 14 G needle contains four electrode tines for a 4 cm ablation zone. Depending on tumour size, proximity to the liver margin, and adjacency to other critical structures, the operators selected between the two models of ablation needles, planned the needle path, and set the appropriate ablation time. CBCT was performed during each procedure prior to RFA for needle path and ablation zone planning and post-RFA for an immediate assessment of the treatment response. Real-time image fusion of CBCT and fluoroscopy was used during percutaneous needle puncture and insertion. Two interventional radiologists (XY and DY, each with over 10 years of experience) jointly performed all the enrolled procedures.
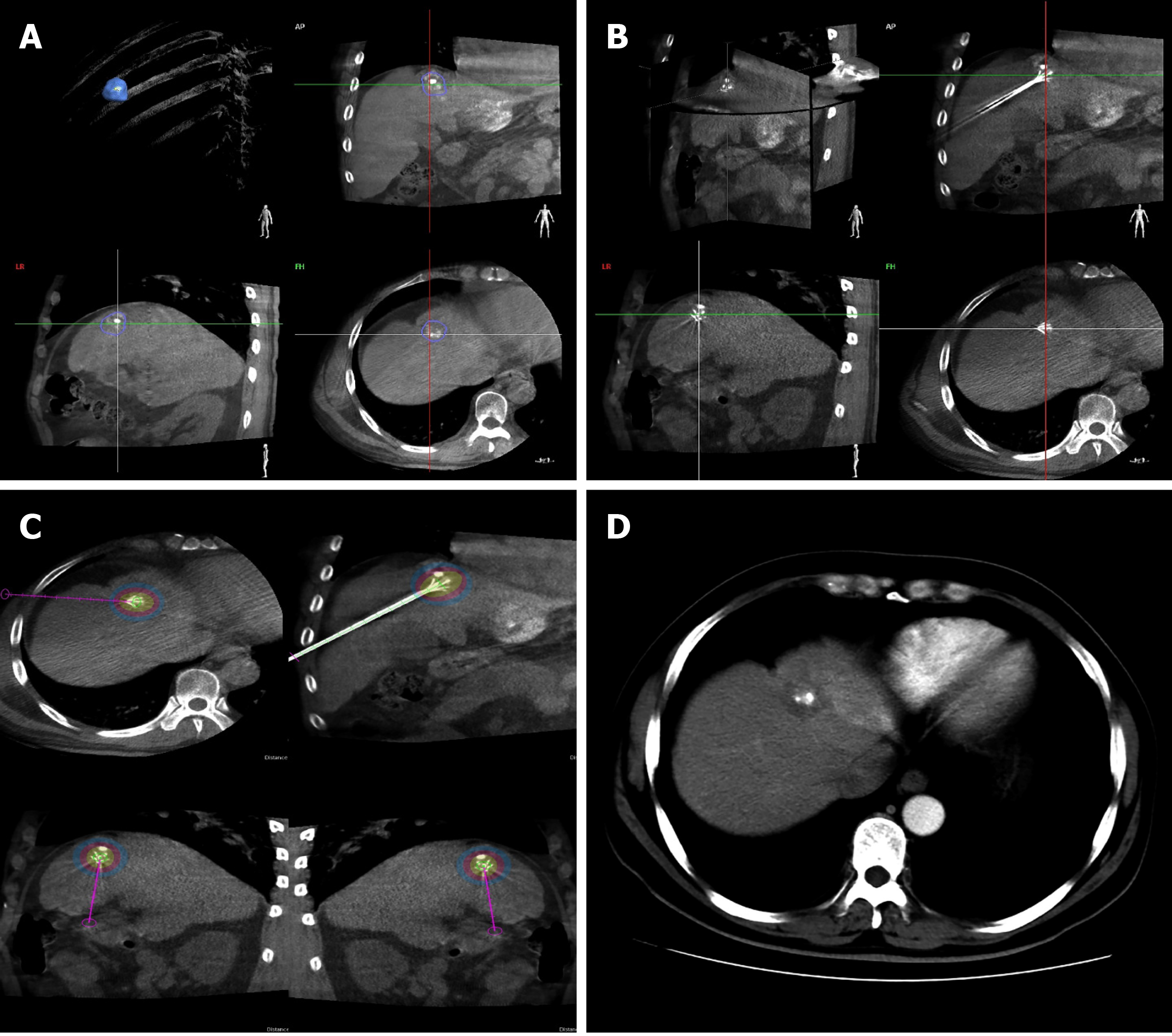
All treatments were performed on an angiographic system (Allura Xper FD20, Philips Healthcare, Best, the Netherlands) equipped with an XperCT (Philips) and software for tumour segmentation and ablation treatment planning (XperGuide Ablation, Philips). Technical parameters of the XperCT abdominal image acquisition protocol and related patient radiation dose have been reported in a previous study[12]. Prior to RFA, a 5-second, low-radiation, non-contrast-enhanced CBCT image was acquired for each patient. HCC was automatically detected and segmented on the reconstructed CBCT image. Operator verification of the target tumour was subsequently carried out on the images in the coronal, sagittal, and transverse planes where tumour boundaries were automatically outlined and manually adjusted (Figure 1A). Upon confirming the tumour location, shape, and volume, the operators selected the percutaneous puncture entry point(s) and virtual needle path(s) to plan ablation needle insertion. Real-time image guidance was achieved by superimposing the CBCT image with the virtual needle path (including an entry point, a marked progression line, and a target point) onto the live fluoroscopy image. As the CBCT image was acquired using the same C-arm gantry as the fluoroscopy image, the system was able to register the two imaging modalities and allow the CBCT overlay to be displayed at the same fluoroscopic projection angle in real time. After the ablation needle was inserted, another CBCT image was acquired to confirm the needle position and the opening of the electrode arrays and to plan the intended ablation zone (Figure 1B and C). The ablation zone was colour coded based on the operator’s input of the ablation needle’s technical configurations.
The treatment response to RFA was assessed using the Modified Response Evaluation Criteria in Solid Tumors (mRECIST)[13] and was categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The initial post-RFA treatment response was assessed days to one week after the procedure using CECT or MRI. Clinical follow-ups were conducted every 4 to 6 wk to diagnose recurrence and tumour progression. Supplementary treatments, including transarterial chemoembolization, hepatoprotection, and symptomatic treatment, were provided to patients who achieved PR and PD, while patients who achieved CR and SD continued to be monitored. Disease progression (defined as tumour recurrence and/or progression), death, time to progression (TTP), and overall survival (OS) were recorded until May 2017 for the short-term outcome study.
Unlike conventional CT, the CT dose index is not suitable for CBCT since the scan field of view of the CBCT image is smaller than that of the conventional CT scan[14]. Suzuki et al[15] reported that the effective dose during CBCT imaging can be evaluated using the dose-area product (DAP)[15]. According to the Monte Carlo effective dose conversion formula from the International Commission on Radiological Protection (ICRP)[16], effective dose (ED) is calculated as kDAP*DAP, and kDAP is calculated as 0.13 mSv/Gy*cm2. The DAP was recorded with a digital subtraction angiography (DSA) dose monitoring system.
Kaplan-Meier analysis was performed to determine OS and progression-free survival (PFS) in the study population. Factors that may be associated with disease progression and/or death (i.e., age, sex, BCLC stage, CP score, tumour size and tumour adjacency to the aorta, diaphragm, or digestive tract) were analysed using Cox regression tests. Quantitative variables are described using the median and range (minimum to maximum). Qualitative variables are presented as numbers and percentages. The standard error (SE), hazard ratio (HR), and 95% confidence interval (CI) are also reported. Statistical significance was defined as a P value less than 0.05. Statistical analyses were performed using SPSS 14.0 software.
Forty-eight HCC patients (44 men and 4 women, aged 37 to 89 years) with solitary tumours and no extrahepatic metastases underwent CBCT-guided RFA successfully without major complications (Table 1). The median tumour size was 3.2 cm (range 1.2-6.6 cm). Eighteen patients had BCLC stage B, while 30 patients had stage C. All of 48 patients had good liver function (Child-Pugh score was A). The median DAP of the procedures was 79.05 Gy*cm2 (range 40.95-146.24 Gy*cm2), and the median ED was 10.27 mSv (range 5.32-19.01 mSv). Sixteen of the enrolled patients who presented with a tumour size less than 3 cm underwent transarterial embolization prior to RFA with the primary purpose of enhancing tumour visualization using lipiodol. A case example of a 37-year-old man who underwent intraprocedural CBCT and 3-mo follow-up CECT is shown in Figure 1.
| Number of patients | Total patients | Disease progression | Deaths |
| 48 | 31 (64.5%) | 10 (20.8%) | |
| Sex | |||
| Male | 44 | 28 | 6 |
| Female | 4 | 3 | 4 |
| Age (years): 58 (37-89) | |||
| < 58 | 23 | 12 | 3 |
| ≥ 58 | 25 | 19 | 7 |
| Tumour size (cm): 3.2 (1.2-6.6) | |||
| < 2 | 11 | 2 | 0 |
| ≥ 2 and < 5 | 26 | 21 | 2 |
| ≥ 5 | 11 | 8 | 8 |
| Tumour adjacent to the aorta, diaphragm, or digestive tract | |||
| Yes | 6 | 5 | 2 |
| No | 42 | 26 | 8 |
| Barcelona Clinic Liver Cancer stage | |||
| A | 18 | 8 | 2 |
| B | 30 | 23 | 8 |
| C | 0 | - | - |
| D | 0 | - | - |
| Child-Pugh score | |||
| A | 48 | 31 | 10 |
| B | 0 | - | - |
| C | 0 | - | - |
| mRECIST–Post-RFA | |||
| CR | 38 (79.2%) | 22 | 6 |
| PR | 10 (20.8%) | 9 | 4 |
| SD | 0 | - | - |
| PD | 0 | - | - |
| mRECIST–final follow-up | |||
| CR | 16 (33.3%) | - | - |
| PR | 1 (2.1%) | - | - |
| SD | 0 | - | - |
| PD | 31 (64.6%) | 31 | 10 |
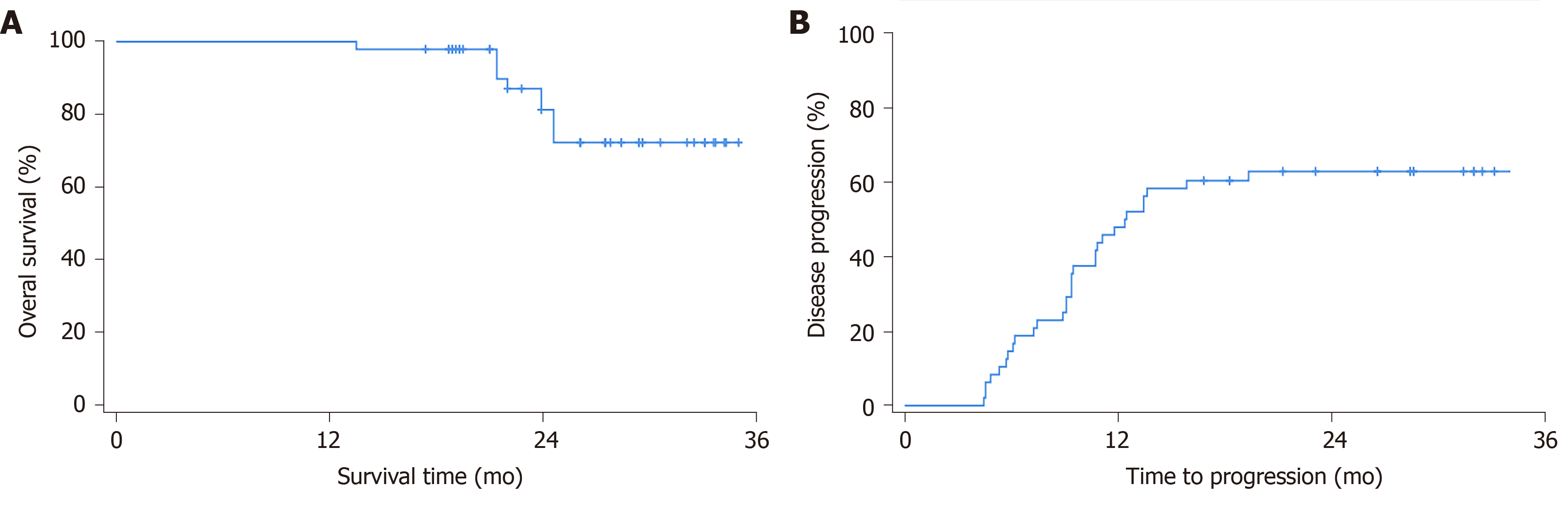
Pre-RFA treatment planning estimated 42 cases of CR (87.5%), 6 of PR (12.5%), 0 of SD, and 0 of PD. The post-RFA initial clinical assessment using contract-enhanced CT or MRI showed 38 cases of CR (79.2%), 10 of PR (20.8%), 0 of SD, and 0 of PD, which strongly correlated with the planning estimation before treatment (accuracy 91.7%; Pearson correlation 0.737, P < 0.01). Ten Patients with PR were treated by transarterial chemoembolization sequentially. The patients were subsequently followed for a median period of 25.6 mo (range 13.5-35.2 mo), during which ten (20.8%) patients died of causes related to liver disease. The median survival time was not reached. Kaplan-Meier analysis estimated 12-, 24-, and 35 mo OS rates of 100%, 81.2%, and 72.2%, respectively (Figure 2A). During the follow-up period, disease progression occurred in 31 (65.6%) patients. Disease progression rates were 54.2%, 62.9%, and 62.9% at 12, 24, and 35 mo, respectively, according to Kaplan-Meier analysis (Figure 2B). The median TTP (mTTP) was 12.8 mo (SE = 1.6, 95%CI: 9.7-15.9). At the end of follow up (median period of 25.6 mo, range from 13.5 to 35.2 mo), final mRECIST assessments indicated 16 (33.3%) cases of CR, 1 (2.1%) of PR, 0 of SD, and 31 (64.6%) of PD.
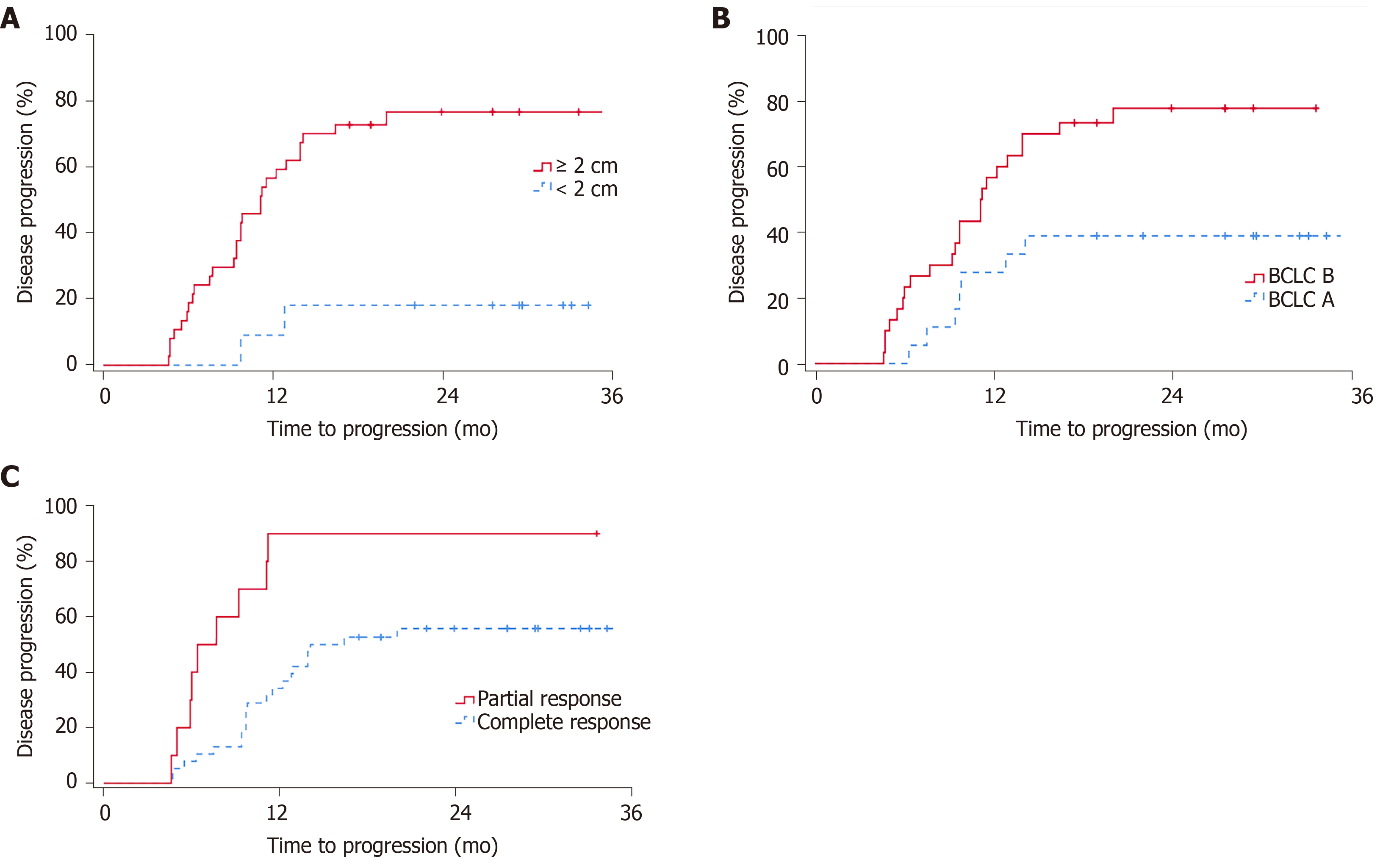
The univariate Cox regression analysis showed that female sex (P = 0.019) and tumour size < 2 cm (P = 0.008) were statistically significant factors for OS (Table 2), while no independent OS predictors were identified using Cox proportional hazards models. Prognosis factors that significantly influenced PFS were age (P = 0.001, mTTP of 35.2 mo for patients under 58 years vs 11.1 mo for patients ≥ 58 years), tumour size < 2 cm (P < 0.001, mTTP not reached), BCLC stage (P = 0.010, mTTP of 35.2 mo for stage A vs 11.1 mo for stage B), and initial post-RFA mRECIST (P = 0.003, mTTP of 14.1 mo for CR vs 6.4 mo for PR) (Table 3 and Figure 3). Cox proportional hazards models showed that age (P = 0.036, HR = 1.052, 95%CI: 1.003-1.103) and post-RFA mRECIST (P = 0.021, HR = 4.080, 95%CI: 1.231-13.524) were independent predictors of PFS.
| Variable | P value |
| Sex | 0.019 |
| Age | 0.676 |
| Tumour size | |
| < 2 cm | 0.008 |
| ≥ 5 cm | 0.853 |
| Tumour adjacent to the aorta, diaphragm, or digestive tract | 0.662 |
| Barcelona Clinic Liver Cancer stage | 0.157 |
| mRECIST–Post-RFA | 0.090 |
| Variable | mTTP (mo) | Univariate analysis | Cox proportional hazards model (multivariate analysis) | ||
| P value | HR | P value | 95%CI | ||
| Sex | 0.246 | ||||
| Male | 12.9 | ||||
| Female | 6.0 | ||||
| Age (years) | 0.001 | 1.052 | 0.036 | 1.003-1.103 | |
| < 58 | 35.2 | ||||
| ≥ 58 | 11.1 | ||||
| Tumour size (cm) | |||||
| < 2 | - | < 0.001 | |||
| ≥ 5 | 9.7 | 0.468 | |||
| Tumour adjacent to the aorta, diaphragm, or digestive tract | 0.189 | ||||
| Yes | 13.9 | ||||
| No | 9.7 | ||||
| Barcelona Clinic Liver Cancer stage | 0.010 | ||||
| A | 35.2 | ||||
| B | 11.1 | ||||
| mRECIST–Post-RFA | 0.003 | 4.080 | 0.021 | 1.231-13.524 | |
| CR | 14.1 | ||||
| PR | 6.4 | ||||
CBCT is a new imaging modality that provides 3D images in addition to conventional 2D images (such as those obtained via DSA and fluoroscopy) during interventional radiology procedures. Its most characteristic feature is the rotating flat panel detector around the patient to obtain a series of data that can be used for CT image reconstruction. Compared with conventional CT, the use of CBCT during the interventional procedure can provide real-time guidance, thus reducing not only the operation time but also therapy-associated complications[16].
We systematically utilized CBCT in RFA to inoperable or elective patients with solitary HCC and assessed the short-term outcomes. CBCT was used for ablation needle path and ablation zone planning using image fusion with pre-operative diagnostic CECT or MRI. Real-time image guidance for needle progression was achieved by overlaying the tumour volume (created from CBCT tumour segmentation) on the planned needle path on the live fluoroscopy image. Technical success was achieved in all 48 procedures without major complications. The median DAP of the procedures was 79.05 Gy*cm2 (range 40.95-146.24 Gy*cm2), and the median ED was 10.27 mSv (range 5.32-19.01 mSv). This finding is similar to those in other reports in the literature[17].
Notably, the treatment response estimation in pre-RFA planning strongly correlated with the post-RFA mRECIST assessment, with high accuracy and statistical significance. This demonstrates satisfactory consistency between CBCT-based treatment planning and the operators’ manual needle manipulation under the real-time image guidance employed in this study. This finding confirms the usefulness and efficacy of CBCT in RFA procedures, as it allows 3D visualization of the tumour and needle path, as well as multimodality image fusion for tumour segmentation and image navigation during needle progression.
Previous studies of the long-term survival outcomes of HCC patients who underwent CT- or ultrasound-guided RFA reported 1-, 3-, and 5-year survival rates of 86%-97%, 51%-81%, and 38%-64%, respectively[3,18-24]. Kato et al[11] demonstrated the survival benefit of CBCT tumour visuali-zation during ultrasound-guided RFA, with 1-, 2-, and 3-year OS rates of 92%, 84%, and 84%, respectively[11]. Our study obtained better 1-, 2-, and 3-year survival outcomes than the aforementioned studies and is highly consistent with two studies on Chinese HCC patients, which reported 1- and 3-year OS rates of 91.4% and 71.3%[3] and 90.0% and 70.8%[19], respectively. The median survival time was understandably not reached in the present short-term outcomes study, as it is typically observed 3 to 5 years post-RFA treatment[3,11,19-24]. Statistical analysis indicated that in our patient group, female sex significantly influenced mortality. However, this result might be due to the small sample size (i.e., 4 patients and 3 deaths) in conjunction with the large tumour sizes (1 tumour > 3 cm, 1 tumour > 4 cm, and 2 tumours > 6 cm) and because PR was achieved in all four patients. Indeed, the multivariate Cox regression analysis did not confirm female sex as an independent predictor of OS.
Our results showed more prominent post-RFA disease progression than previous studies (1- and 3-year PFS rates of 66%-83% and 29%-59%, respectively)[3,19,21-23]. This inconsistency may be due to a significant proportion of the study population with a tumour size ≥ 3 cm (30/48, 62.5%), which influenced the cohort’s prognosis. Moreover, the present study indeed showed that tumour size < 2 cm, while not an independent predictor, was a significantly positive factor for post-RFA PFS and OS outcomes in patients with solitary HCC (Figure 3A). This finding is in line with guidelines and clinical literature with regard to the efficacy of RFA for small HCC[1,2,4,23,25]. In addition, our results indicated that age (negative independent predictor) and BCLC stage were significant factors for PFS (Figure 3B).
Cox proportional hazards models revealed that the initial post-RFA mRECIST assessment was an independent prognostic predictor for patients with solitary HCC (Figure 3C). Despite having supplementary treatment options, 90% of the present study’s population who achieved PR after RFA developed disease progression at an mTTP of 6.4 mo. In contrast, prognosis in the CR subgroup was significantly better (i.e., 57.9% of patients experienced disease progression at an mTTP of 14.1 mo). This observation agrees with Lencioni’s hypothesis of objective responses as surrogate survival endpoints based on statistical analysis[26]. The implication of our finding is threefold. First, it is apparent that well-targeted needle insertion with an adequate ablation technique will help achieve immediate CR and a good prognosis. Second, considering the strong correlation between the planning-based response estimation and the post-RFA assessment, precise planning using CBCT and pre-operative diagnostic CT/MRI in conjunction with real-time image guidance, as described in the present student, can be beneficial to ensure predictable RFA treatment outcomes. Last, CBCT-guided RFA procedures may benefit from an immediate CBCT assessment following treatment to provide a reliable initial prognosis estimation and allow the operator to consider additional ablation procedures to improve the treatment response (if necessary and feasible) while the patient is in situ. While the current study did not use contrast-enhanced CBCT to conduct the post-RFA immediate response assessment, further investigations, particularly those incorporating intravenous contrast injection, might be clinically meaningful.
The comprehensive utilization of CBCT during RFA and the incorporation of image fusion with pre-operative diagnostic CT/MRI for semi-automatic tumour segmentation and reliable treatment planning, as well as real-time image guidance for the operator, could enhance tumour visualization and contribute to satisfactory treatment responses. This is particularly beneficial for interventional oncologists who have limited access to dedicated CT and/or ultrasound units for therapeutic purposes.
Percutaneous radiofrequency ablation (RFA) is an effective treatment for unresectable hepatocellular carcinoma (HCC). In recent years, angiographic systems with flat panel image detectors can use cone-beam computed tomography (CBCT) to guide RFA and show great effectiveness.
To assess the short-term response, radiation dose, and time to progression associated with CBCT guided RFA in HCC patients.
To assess radiation dose during operation, short-term response, and time to progression in HCC patients undergoing CBCT guided RFA.
Our prospective research studied HCC patients who underwent CBCT guided RFA. The basic status of the patients, including age, sex, Barcelona Clinic Liver Cancer stage, Child-Pugh score, tumour size and tumour adjacency to major vessels, the diaphragm, or other organs, was recorded. The radiation dose during RFA, short-term response, and time to progression were recorded during the follow-up.
There were 48 patients (44 males and 4 females) who underwent RFA guided by CBCT without major complications. The median tumour size was 3.2 cm (range 1.2-6.6 cm). The median dose area product of the procedures was 79.05 Gy*cm2 (range 40.95-146.24 Gy*cm2), and the median effective dose was 10.27 mSv (range 5.32-19.01 mSv). The post-RFA initial clinical assessment using contract-enhanced CT or magnetic resonance imaging showed 38 (79.2%) cases of complete response, 10 (20.8%) of partial response, 0 of stable disease, and 0 of progressive disease. The median time to progress was 12.8 mo.
CBCT guided RFA is a reliable treatment for HCC patients. It could enhance tumour visualization and contribute to satisfactory treatment responses. This is particularly beneficial for interventional oncologists who have limited access to dedicated CT and/or ultrasound units for therapeutic purposes.
The current study did not use contrast-enhanced CBCT to conduct the post-RFA immediate response assessment, and further investigations, particularly those incorporating intravenous contrast injection, might be clinically meaningful.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Fogli L, Kamimura K, Mizuguchi T, Sitkin S, Ueda H S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6066] [Article Influence: 866.6] [Reference Citation Analysis (3)] |
| 3. | Zhang L, Ge NL, Chen Y, Xie XY, Yin X, Gan YH, Zhang BH, Zhang JB, Chen RX, Wang YH, Ye SL, Ren ZG. Long-term outcomes and prognostic analysis of radiofrequency ablation for small hepatocellular carcinoma: 10-year follow-up in Chinese patients. Med Oncol. 2015;32:77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, Rajan DK. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol. 2009;20:S342-S347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Morimoto M, Numata K, Kondo M, Nozaki A, Hamaguchi S, Takebayashi S, Tanaka K. C-arm cone beam CT for hepatic tumor ablation under real-time 3D imaging. AJR Am J Roentgenol. 2010;194:W452-W454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Abi-Jaoudeh N, Venkatesan AM, Van der Sterren W, Radaelli A, Carelsen B, Wood BJ. Clinical experience with cone-beam CT navigation for tumor ablation. J Vasc Interv Radiol. 2015;26:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Bapst B, Lagadec M, Breguet R, Vilgrain V, Ronot M. Erratum to: Cone Beam Computed Tomography (CBCT) in the Field of Interventional Oncology of the Liver. Cardiovasc Intervent Radiol. 2015;38:1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Solbiati M, Passera KM, Goldberg SN, Rotilio A, Ierace T, Pedicini V, Poretti D, Solbiati L. A Novel CT to Cone-Beam CT Registration Method Enables Immediate Real-Time Intraprocedural Three-Dimensional Assessment of Ablative Treatments of Liver Malignancies. Cardiovasc Intervent Radiol. 2018;41:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Abdel-Rehim M, Ronot M, Sibert A, Vilgrain V. Assessment of liver ablation using cone beam computed tomography. World J Gastroenterol. 2015;21:517-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Iwazawa J, Ohue S, Hashimoto N, Mitani T. Ablation margin assessment of liver tumors with intravenous contrast-enhanced C-arm computed tomography. World J Radiol. 2012;4:109-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Kato K, Abe H, Ika M, Yonezawa T, Sato Y, Hanawa N, Shimizu S, Endo S, Matsuo R, Tsubota A. C-Arm Cone Beam Computed Tomography Guidance for Radiofrequency Ablation in Hepatocellular Carcinoma. Oncology. 2017;92:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Yao X, Yan D, Jiang X, Li X, Zeng H, Liu D, Li H. Dual-phase Cone-beam CT-based Navigation Imaging Significantly Enhances Tumor Detectability and Aids Superselective Transarterial Chemoembolization of Liver Cancer. Acad Radiol. 2018;25:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3304] [Article Influence: 220.3] [Reference Citation Analysis (36)] |
| 14. | Suzuki S, Yamaguchi I, Kidouchi T, Yamamoto A, Masumoto T, Ozaki Y. Evaluation of effective dose during abdominal three-dimensional imaging for three flat-panel-detector angiography systems. Cardiovasc Intervent Radiol. 2011;34:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Suzuki S, Furui S, Yamaguchi I, Yamagishi M, Watanabe A, Abe T, Kobayashi I. Effective dose during abdominal three-dimensional imaging with a flat-panel detector angiography system. Radiology. 2009;250:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Morant JJ, Salvadó M, Hernández-Girón I, Casanovas R, Ortega R, Calzado A. Dosimetry of a cone beam CT device for oral and maxillofacial radiology using Monte Carlo techniques and ICRP adult reference computational phantoms. Dentomaxillofac Radiol. 2013;42:92555893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Schegerer AA, Lechel U, Ritter M, Weisser G, Fink C, Brix G. Dose and image quality of cone-beam computed tomography as compared with conventional multislice computed tomography in abdominal imaging. Invest Radiol. 2014;49:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Yuan H, Li X, Tian X, Ji K, Liu F. Comparison of Angio-CT and cone-beam CT-guided immediate radiofrequency ablation after transcatheter arterial chemoembolization for large hepatocellular carcinoma. Abdom Radiol (NY). 2020;45:2585-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Yang W, Yan K, Goldberg SN, Ahmed M, Lee JC, Wu W, Zhang ZY, Wang S, Chen MH. Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J Gastroenterol. 2016;22:2993-3005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto T, Yoshida H, Omata M, Koike K. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-77; quiz 578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 577] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 21. | Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Gwak GY, Yoo BC. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 22. | Sucandy I, Cheek S, Golas BJ, Tsung A, Geller DA, Marsh JW. Longterm survival outcomes of patients undergoing treatment with radiofrequency ablation for hepatocellular carcinoma and metastatic colorectal cancer liver tumors. HPB (Oxford). 2016;18:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Francica G, Saviano A, De Sio I, De Matthaeis N, Brunello F, Cantamessa A, Giorgio A, Scognamiglio U, Fornari F, Giangregorio F, Piscaglia F, Gualandi S, Caturelli E, Roselli P, Rapaccini GL, Pompili M. Long-term effectiveness of radiofrequency ablation for solitary small hepatocellular carcinoma: a retrospective analysis of 363 patients. Dig Liver Dis. 2013;45:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Morimoto M, Numata K, Kondo M, Moriya S, Morita S, Maeda S, Tanaka K. Radiofrequency ablation combined with transarterial chemoembolization for subcapsular hepatocellular carcinoma: a prospective cohort study. Eur J Radiol. 2013;82:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Hyun D, Cho SK, Shin SW, Rhim H, Koh KC, Paik SW. Treatment of Small Hepatocellular Carcinoma (≤ 2 cm) in the Caudate Lobe with Sequential Transcatheter Arterial Chemoembolization and Radiofrequency Ablation. Cardiovasc Intervent Radiol. 2016;39:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Lencioni R, Montal R, Torres F, Park JW, Decaens T, Raoul JL, Kudo M, Chang C, Ríos J, Boige V, Assenat E, Kang YK, Lim HY, Walters I, Llovet JM. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol. 2017;66:1166-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |