Published online Feb 26, 2021. doi: 10.12998/wjcc.v9.i6.1367
Peer-review started: September 22, 2020
First decision: December 3, 2020
Revised: December 15, 2020
Accepted: December 26, 2020
Article in press: December 26, 2020
Published online: February 26, 2021
Processing time: 137 Days and 7.1 Hours
In the clinical scenario, adult patients with periodontal diseases and dental malformation, characterized by dental crowding in lower anterior teeth with the thin biotype, often require orthodontic treatment. This case report aimed to evaluate the clinical and radiographic outcomes of periodontally accelerated osteogenic orthodontics (PAOO) combined with autologous platelet-rich fibrin (PRF) in an adult patient with class I malocclusion along with dental crowding, a thin periodontal biotype, and buccal plate deficiency.
A 32-year-old female complaining of dental crowding and gingival bleeding was referred to the orthodontic clinic. The patient underwent periodontal risk assessment prior to orthodontic treatment. She was diagnosed with a high risk of gingival recession due to dental crowding, root prominence, loss of buccal plates, and a thin gingival tissue biotype. The treatment regimen included PAOO combined with autologous PRF for alveolar augmentation and interproximal enamel reduction for moderate dental crowding. Clinically, PAOO-assisted orthodontic tooth movement in this case showed enhanced periodontium remodeling. Radiographic outcomes also showed statistically significant improvements (P < 0.01) in the mandibular buccal alveolar bone.
This case report suggests the combination of autologous PRF with PAOO to enhance bone augmentation and long-term tissue support in adult orthodontic patients with periodontal disease.
Core Tip: Periodontally accelerated osteogenic orthodontics (PAOO) has been widely used in the treatment of patients with periodontal diseases and dental malformation. However, the combined therapy with autologous platelet-rich fibrin and PAOO in orthodontic practice has been rarely reported. The aim of this case report was to evaluate clinical and radiographic outcomes of PAOO combined with autologous platelet-rich fibrin in an adult patient with class I malocclusion with thin periodontal biotype and buccal plate deficiency in order to provide guidance for future clinical diagnosis and treatment.
- Citation: Xu M, Sun XY, Xu JG. Periodontally accelerated osteogenic orthodontics with platelet-rich fibrin in an adult patient with periodontal disease: A case report and review of literature. World J Clin Cases 2021; 9(6): 1367-1378
- URL: https://www.wjgnet.com/2307-8960/full/v9/i6/1367.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i6.1367
A large number of studies have documented in the clinical scenario that patients with dental crowding are often predisposed to thin labial plate thickness[1]. Orthodontic tooth movement (OTM) in these patients is often challenged with potential side effects, such as decreased responsiveness to orthodontic force[2,3], consequently prolonged treatment course, and root resorption thereupon, as well as aggravated bone and soft tissue loss along with tooth movement. Periodontally accelerated osteogenic orthodontics (PAOO)[4] is a delicate conjugation combining clinical periodontal treatment and orthodontic movement, involving surgical decortication, bone grafting, and orthodontic force application and has the advantages of sustainable alveolar bone augmentation, stable periodontium improvements, accelerated tooth movement, and abridged treatment duration[4,5].
Autologous platelet-rich fibrin (PRF) can be obtained from human sources (e.g., from the blood samples of a patient per se)[6], and it is capable of accelerating wound healing and promoting tissue regeneration[7]. PRF has been widely applied in medical therapy and plastic surgery, particularly in oral and maxillofacial surgeries[8,9] such as implantology, guided bone regeneration operations, and exodontia[10]. Nonetheless, to our knowledge, the combined therapy with autologous PRF and PAOO in orthodontic practice has been rarely reported.
The aim of this case report was to evaluate the clinical and radiographic outcomes of PAOO combined with autologous PRF in an adult patient with class I malocclusion along with a thin periodontal biotype and buccal plate deficiency to provide guidance for future clinical diagnosis and treatment.
A healthy 32-year-old female presented to the Stomatological Hospital of Anhui Medical University for orthodontic treatment of tooth crowding and gingival bleeding.
Patient’s tooth crowding started many years ago. However, the gingival bleeding started a few months ago and had gradually worsened recently.
The patient had a free previous dental history.
The patient had a non-contributory personal and family history.
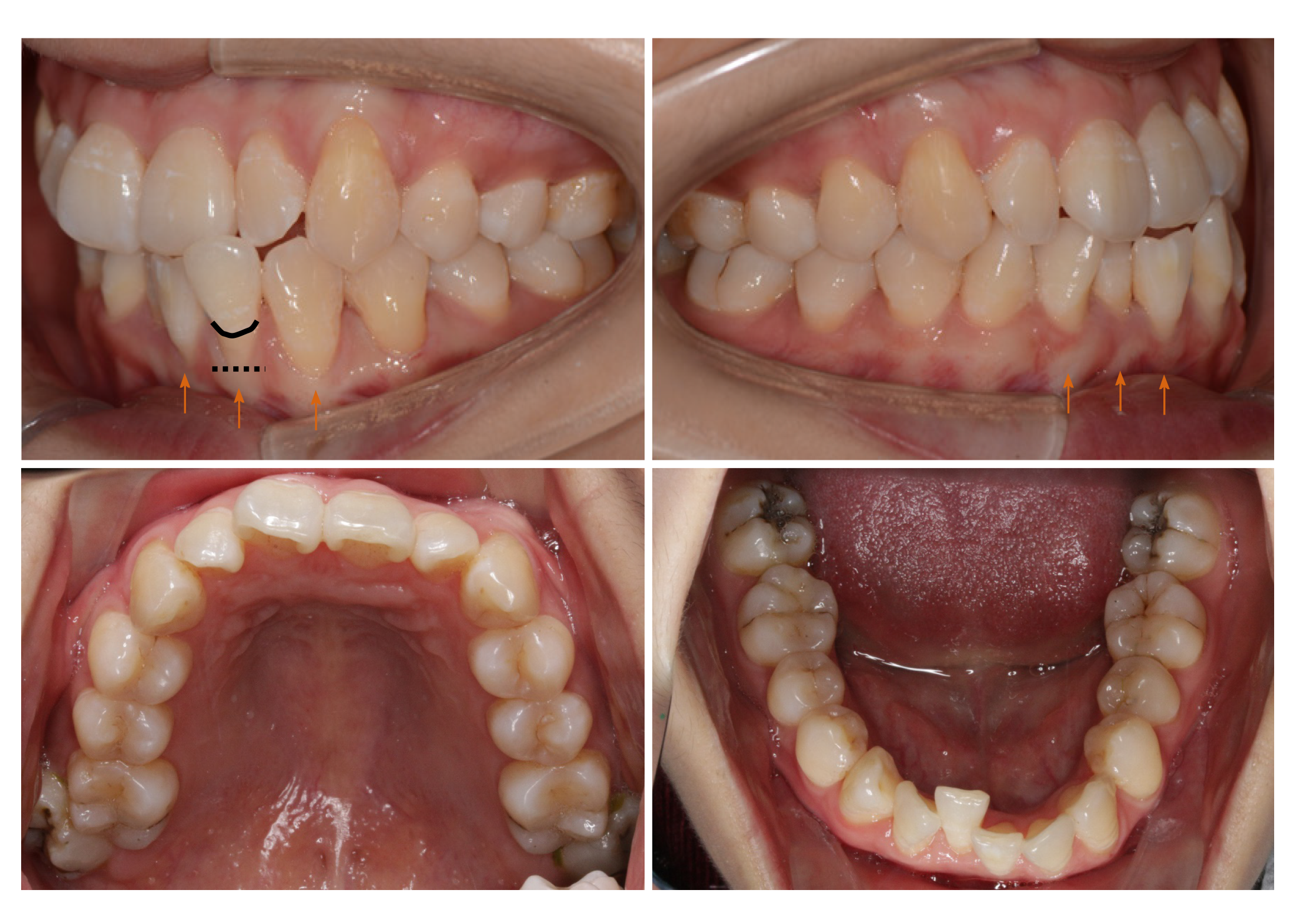
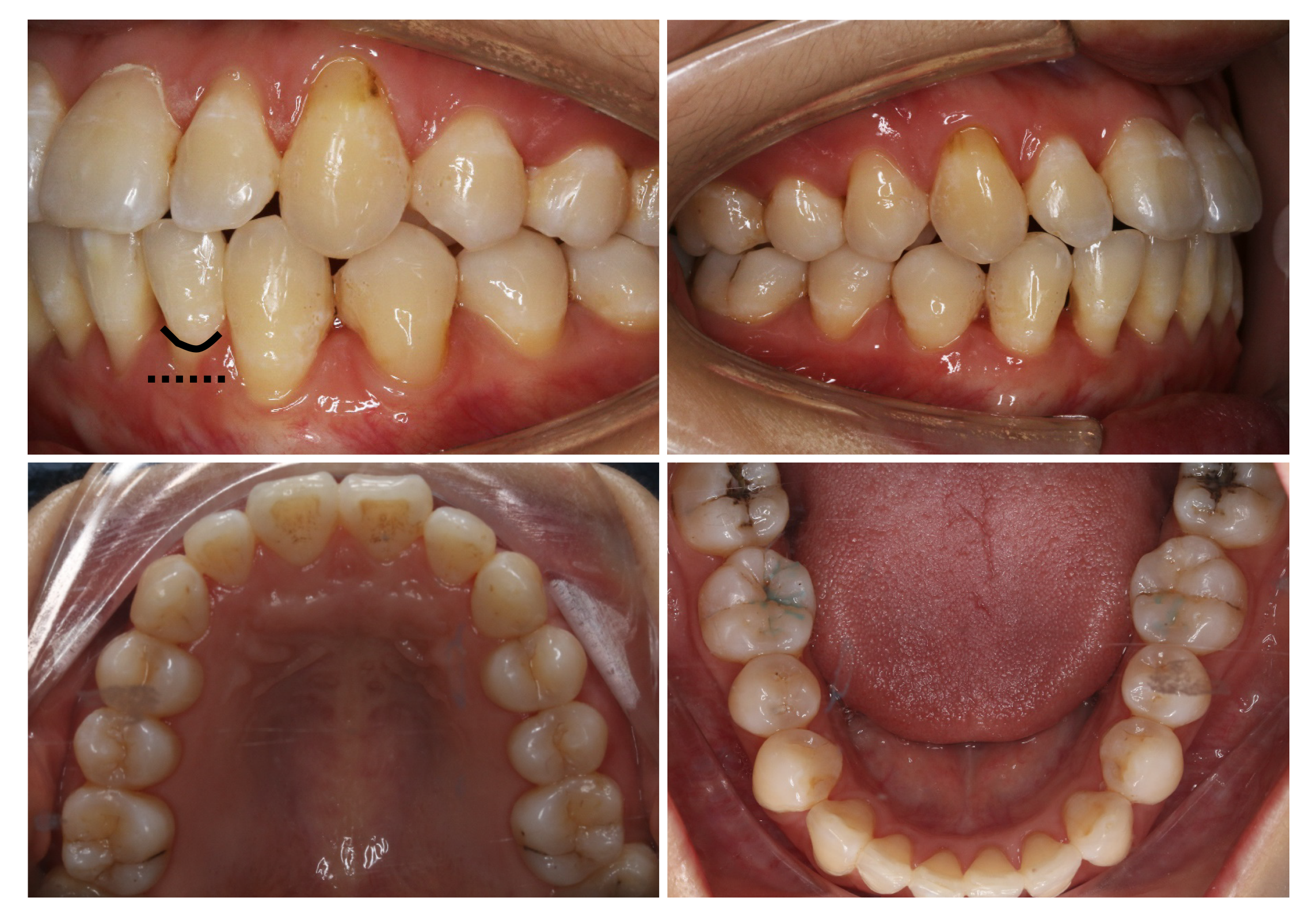
Periodontal examination revealed bleeding on probing percentage (BOP%) of 48.6%, bleeding index of 2-3, and probing depth exceeding 4 mm at some sites. Assessment of soft tissues revealed a thin gingival biotype and severe gingival recession (GR) in some teeth, such as #32 (Figure 1). Orthodontic examination revealed molar class I malocclusion with mild (the maxillary arch) and moderate (the mandibular arch) dental crowding.
The patient's blood test results were all normal.
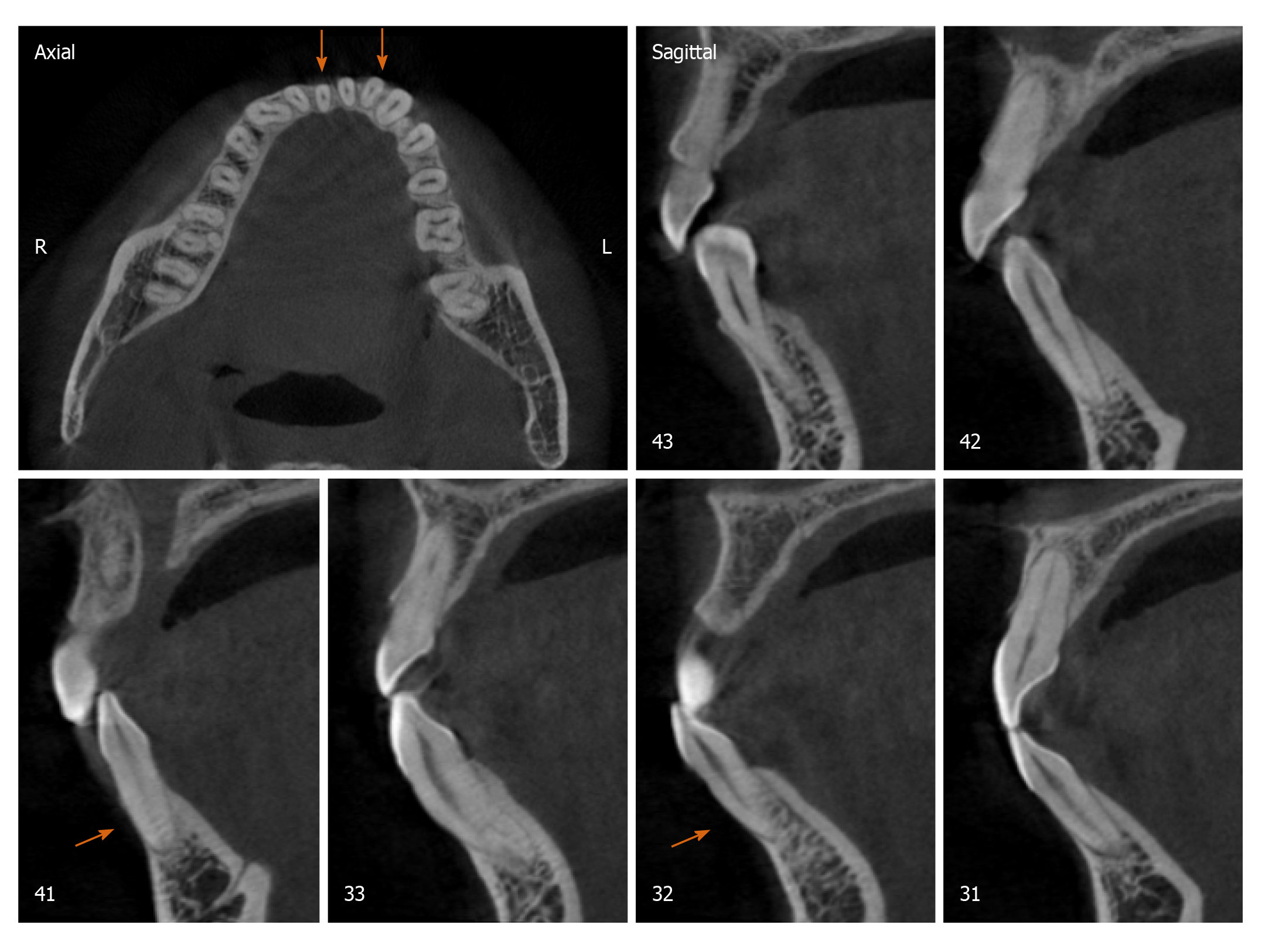
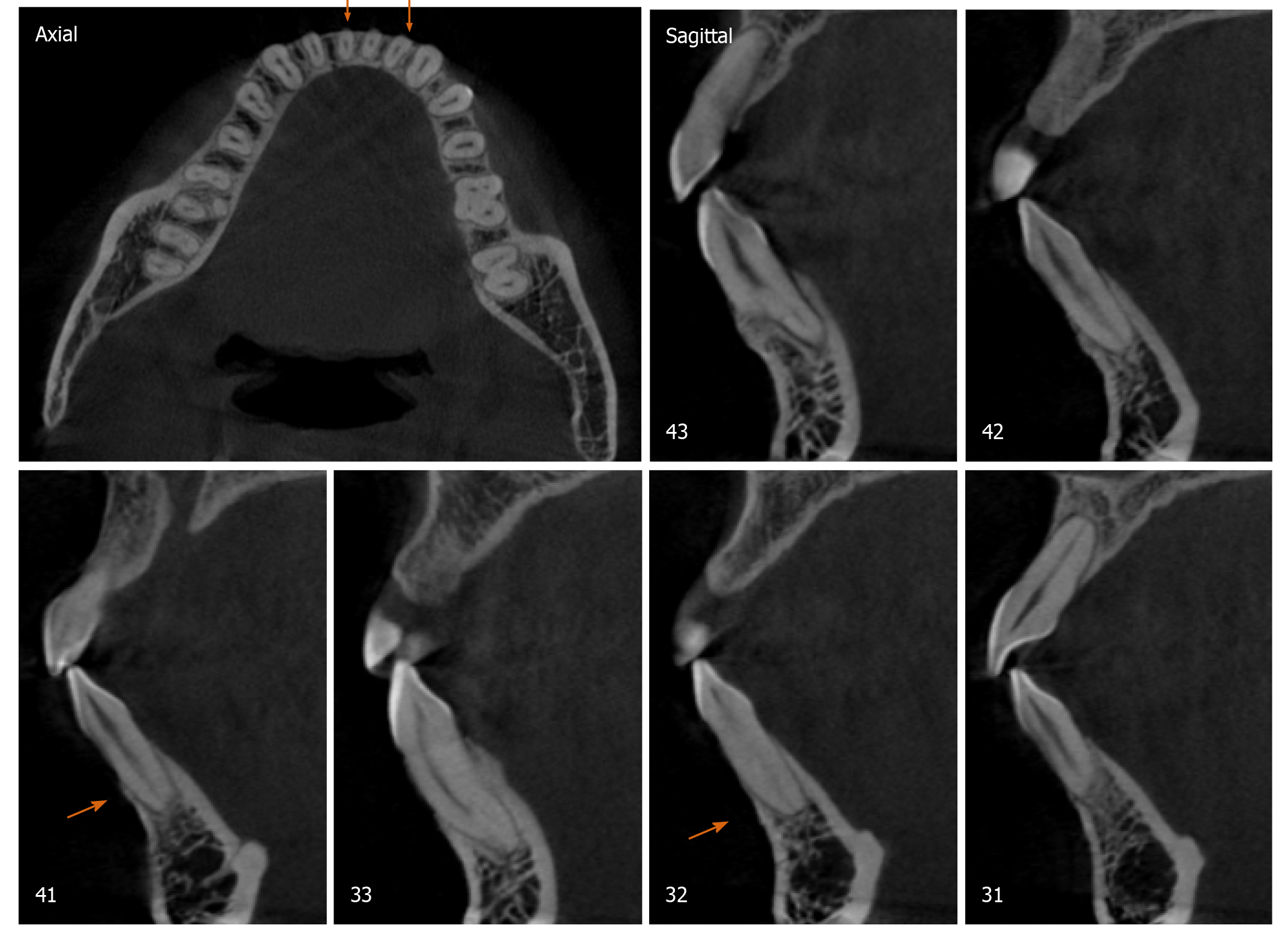
Radiographically, results from cone beam computed tomography (CBCT) demonstrated different levels of bony dehiscence in the mandibular anterior teeth.
The final diagnosis of the presented case is molar class I malocclusion with mild (the maxillary arch) and moderate (the mandibular arch) dental crowding (Figure 1). Radiographically, results from CBCT demonstrated different levels of bony dehiscence in the mandibular anterior teeth (Figure 2).
The patient was diagnosed as having a high risk of GR in case of routine orthodontic treatment. Hence, the orthodontic regimen consisted of PAOO combined with autologous PRF (incorporated into the graft and as a coating membrane) and interproximal enamel reduction. The periodontal treatment scheme included initial therapy, professional mechanical tooth cleaning, and regular consultation. In addition, the patient was instructed to brush her teeth as appropriate. This interdisciplinary regimen was aimed at alignment of teeth, stabilization of occlusion, promotion of the microenvironment of soft and hard tissues, and relief of GR. All therapeutic procedures were performed with written informed consent from the patient.
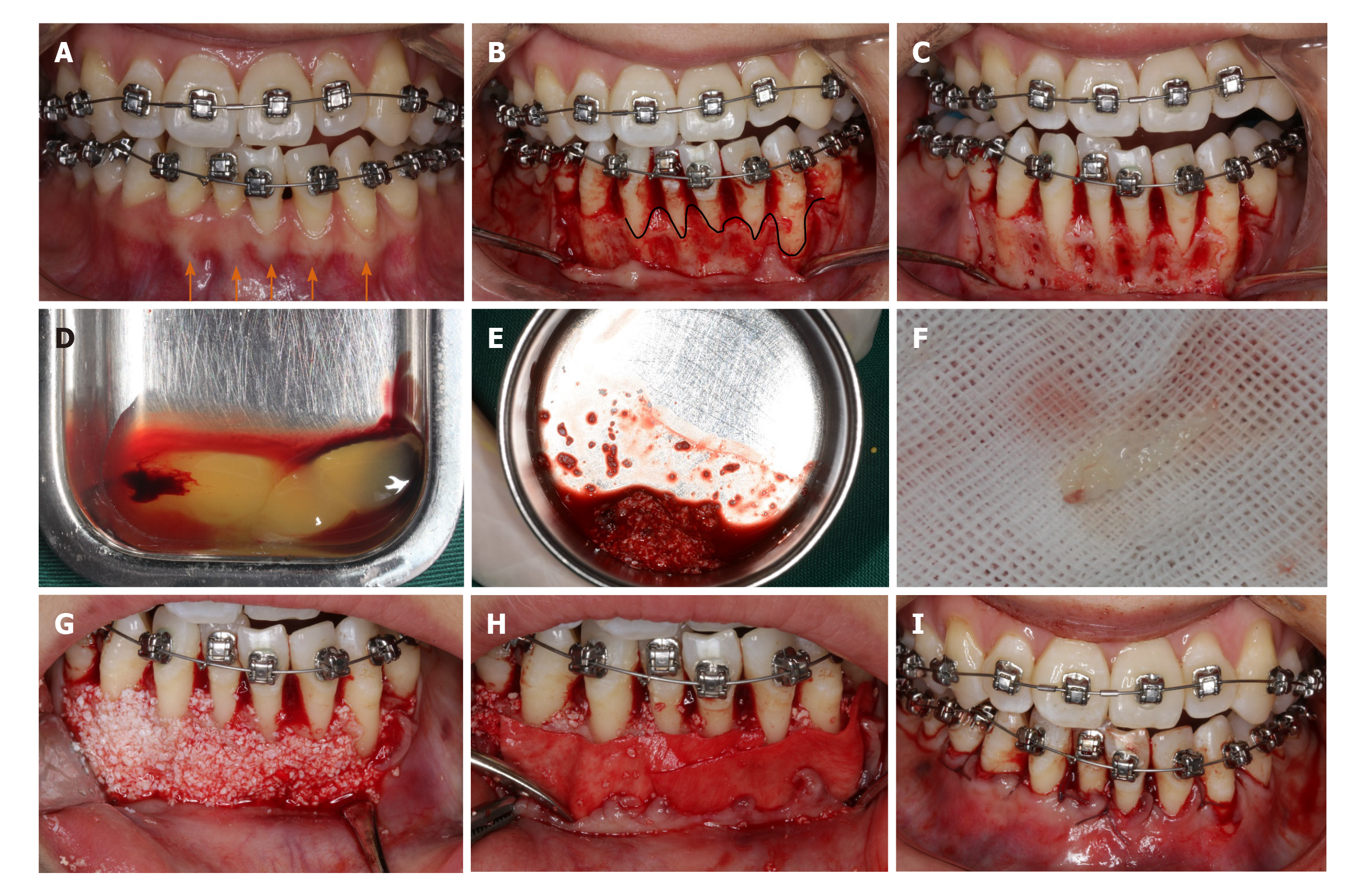
At the end of initial periodontal therapy, orthodontic brackets (Damon Q, Ormco, Brea, CA, United States) were fixed, and interproximal enamel reduction was performed, followed by application of 0.013-Cu-Ni-Ti wires (Ormco) to activate tooth movement (Figure 3A). One week later, PAOO surgery was performed on the mandibular anterior labial side (Figure 3B-I). The patient was followed up at an interval of 2-3 wk, coupled with the placement of orthodontic arch wires of increasing sizes. The patient underwent professional mechanical tooth cleaning for a 3-mo duration to safeguard the health and stability of periodontal tissues during OTM.
Blood samples were taken from the median cubital vein into two 10 mL glass-coated tubes without the addition of anti-coagulants, followed by immediate centrifugation at room temperature at 3000 rpm for 10 min. Thereafter, the clots were carefully isolated with scissors from precipitated red blood cells (Figure 3D). Clots from one tube were minced and incorporated into xenograft (Bio-Oss, Geistlich, Wolhusen, Switzerland) particles, whereas clots from the other tube were prepared as an absorbable membrane (Figure 3E and F). PRF was prepared at the onset of the PAOO surgery.
According to the protocol proposed by Murphy et al[11], a full-thickness flap was performed under local anesthesia (Figure 3B). Given the insufficient thickness of the alveolar bone, cortical perforation was performed in the interradicular areas (Figure 3C), followed by placement of bone xenografts (Bio-Oss, Geistlich) and autologous PRF mixture (Figure 3G) coated with a collagen membrane (Bio-Gide, Geistlich) together with self-prepared PRF membrane (Figure 3H). Then flaps were closed with an interrupted interdental suture and a single sling suture method (Figure 3I).
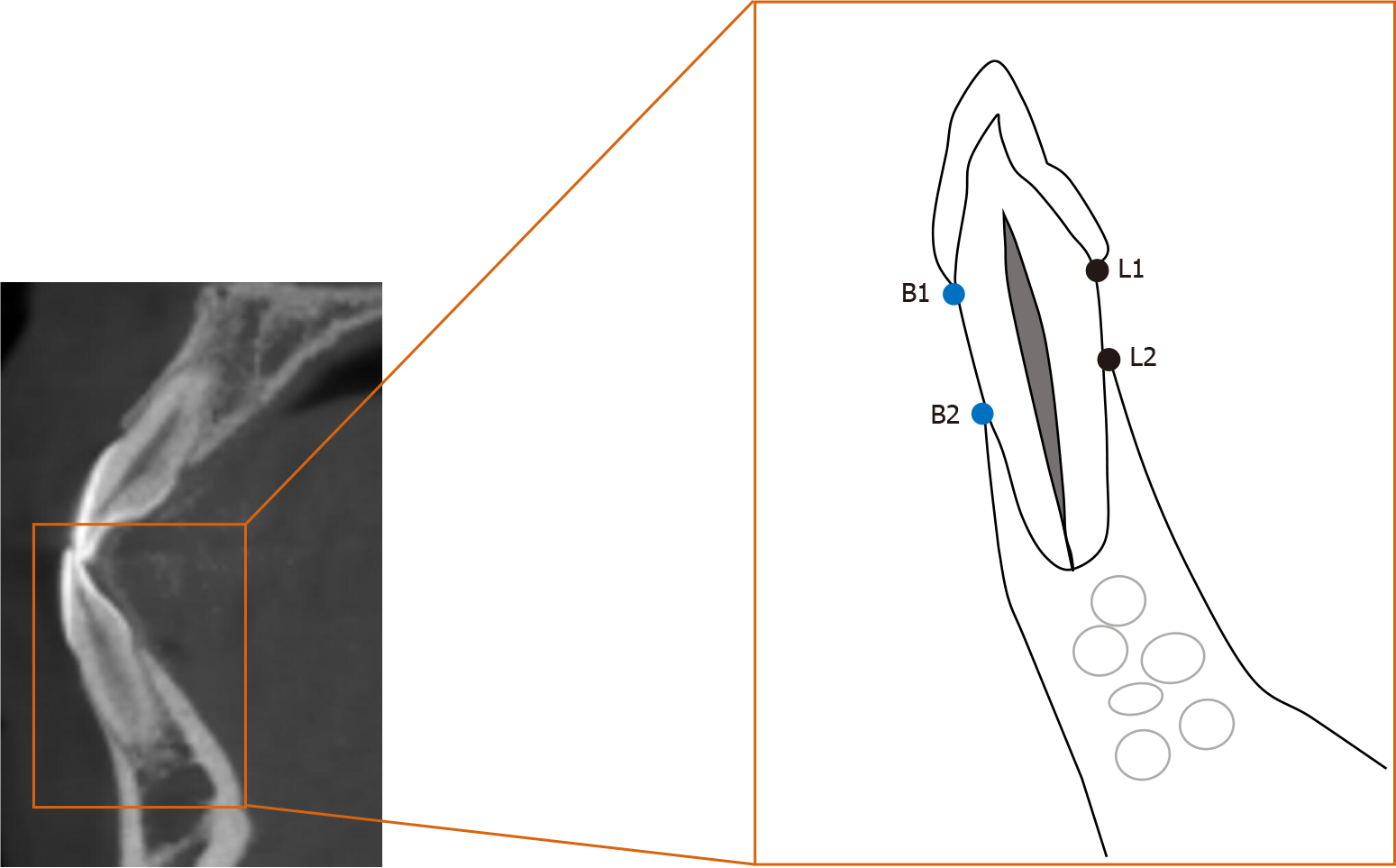
The distance between the enamel-cemental junction to the top of the alveolar ridge (Figure 4) was measured in CBCT sagittal sections with Dolphin Software (Ver. 11.9 Premium; Oslo, Norway). The measurements were performed by a single examiner who was blind to the details of the study or the patient. The measurements were repeated by the same examiner 30 d later, with the average of the results to ensure accuracy and reliability. Both buccal and lingual distances were measured. Independent t test was used to compare the differences in bone height between pre-treatment and post-treatment profiles. A difference of P < 0.05 was considered statistically significant.
Subsequent to surgery, healing was clinically favorable, and crowded teeth were rapidly corrected within the first month. During the treatment, no evident reduction of soft tissues was observed or gingival bleeding was identified. With the treatment, the gingival margin of the mandibular anterior teeth was coronally displaced, the periodontal tissue was stabilized (Figure 5), and probing depth was ≤ 3 mm at all sites. Radiographic studies confirmed augmented alveolar bone (Figure 6) and significantly elevated height of the buccal bone ridge, particularly at #32 and #41 (P < 0.05) (Table 1). In addition, both soft tissue and hard tissue augmentation remained stabilized at 6 mo after the treatment (figures not shown).
PAOO has been proven to broaden the scope of OTM and expand the indications of orthodontics[12,13]. In this case, the alveolar ridges in some lower anterior teeth were significantly elevated, and the total treatment duration was abridged. These outcomes were supported by the findings of prior clinical case reports[4,14-19] and animal studies[20-22]. PAOO can be considered as an integrative surgery for achieving periodontal regeneration and dentoalveolar bone construction[23], and it is capable of optimizing dentoalveolar relationships and creating an equilibrium between hard and soft surrounding tissues in patients with multiple dental malocclusions[24-26], decompensation of mandibular incisors[17-19], and different degrees of crowding[15,16], with either fixed appliances or clear aligners.
The biological mechanism underpinning PAOO has been referred to as the regional acceleratory phenomenon (RAP)[27,28], which is defined as accelerated bone remodeling and decreased regional bone density caused by noxious stimuli[27] (e.g., surgery or fracture), whereby bone metabolism is two to 10 times faster than the normal healing process, attributable to the bursts of bone turnover and soft tissue reconstruction[29]. “Regional” refers to osteopenia at the decortication site and the adjacent bone, and “acceleratory” refers to rapid bone metabolism. The role of RAP is to raise temporarily the biological bone response and systemically recruit growth factors at the decortication area[30]. RAP is actually a transitional process that starts a few days after the trauma, peaks in the first month, and then gradually decreases till the fourth month[31]. Thus, RAP could potentiate tissue reorganization and healing[32], and it has a direct impact on the quality and quantity of OTM[33,34]. In this case, correction of the crowded lower teeth was accomplished in the first month, or rather the most effective period of RAP (i.e. the rate of OTM might be two to three times higher with PAOO than with conventional orthodontic techniques)[35]. Substantially less time in a fixed appliance means less conversion of cytotoxic plaque biofilms into local periodontal inducible stimuli and achievement of more intact periodontium.
As another key factor in PAOO, bone grafts are always contributory to the surgery. Brugnami et al[36] stated that when the tooth was moved outside the bony envelope, corticotomy with concomitant bone grafting technique could maintain the alveolar bone volume around the teeth, whereas corticotomy without concomitant bone grafting could not maintain the alveolar bone volume around the teeth. Bone grafting in PAOO actually abolished the traditional limitation of tooth movement by predictable malleability of the position of the dental root[37]. There are many types of bone grafts, such as autograft, allograft, xenograft, and synthetic bone grafts. Bone grafts mainly feature in the combination of mechanical support and osteoconduction, embedded with another two crucial biological properties: Osteoinduction and osteogenesis. Osteoconduction involves the attachment of osteoblasts and osteoprogenitor cells, and the subsequent migration and ingrowth of these cells within the three-dimensional architecture of the graft[38]. Osteoinduction requires induction of the unspecialized, undifferentiated, and pluripotent cells in the development of the osteoprogenitor lineage, thereby further inducing osteogenesis[39], or rather differentiation and subsequent formation of new bone tissues from donor cells derived from either the host or grafts[40]. Generally, autogenous bone grafts are the gold standard. Nowzari et al[24] first reported a combined application of PAOO with autogenous bone from the mandible in a 41-year-old man. Although this technique has crucial superiority in biocompatibility and viability of transferred osteogenic cells, the harvest is associated with unavoidable problems, such as secondary operation and quantity limitation. Alternatively, other biomaterials, such as allografts, xenografts, and synthetic bone substitutes, are also valuable options for bone regeneration.
In PAOO-assisted OTM, Bio-Oss is widely used as a bone graft. Bio-Oss is a substitute with high osteoconductivity derived from bovine origin. Bio-Oss acts as a scaffold to provide an interface for cells to attach, migrate, and proliferate. Yu et al[18] and Ahn et al[19] used Bio-Oss to correct labial alveolar defects and augment the regional bone. In theory, bone substitutes simultaneously employing osteoconduction, osteoinduction, and osteogenesis are more suitable for bone augmentation in PAOO. However, there is a paucity of literature with respect to the usage of other types of bone grafts, which encourages more research work to improve the properties of materials.
In this case, PRF was employed to enhance the regeneration of bone and soft tissues. PRF was labeled by Choukroun et al[41] in 2000 as a “second-generation” platelet concentrate with the advantage of accumulated growth factors. Essentially, it is anteceded by platelet-rich plasma, which is a suspension rich in leukocytes and plasma components after centrifugation of fresh autologous whole blood. PRF plays a reliable role in optimizing wound healing, reducing pain and swelling[41-44], promoting neovascularization and cell migration, and inducing angiogenesis[45] in the alveolar preservation of an extraction socket, sinus lift, and various bone grafting procedures[7]. When mixed with bone substitutes and covering the surface of bone wound, PRF increases graft survival, bone regeneration, and wound healing owing to the “sticky bone” consistency and release of autogenous growth factors[10,46]. When shaped as a membrane coating the surface of bone grafts, PRF not only averts displacement and leakage of bone particulates[47] but also serves as a porous scaffold. In addition, cell growth on PRF is reported to be significantly quicker than that on bovine collagen membrane (Bio-Gide, Geltish)[48]. Shawky et al[49] described a significant increase in new bone formation with the use of PRF plus autogenous bone graft in patients with maxillary clefts despite the modest contribution to bone density improvements compared with application of autogenous bone graft alone.
Despite the practical advantages of PRF, such as convenient preparation, enhancement of stability of grafting mixture, and protection against exposure, the clinical combination of PRF with PAOO in accelerated OTM has been rarely used. Only one cohort observational study[42] applied PRF in PAOO surgery to observe the clinical effects on edema and pain. Notably, this case report was the first clinical presentation concerning the combined application of PRF with PAOO in an orthodontic patient with a thin biotype and labial plate deficiency. In this case, the combination of PRF with PAOO in the mandibular anterior region accelerated OTM and provided additional gain in the height of the gingival margin, which was similar to the findings presented by Wilcko et al[50]. Although not measured precisely, the thickness of the gingiva was considered to have been enhanced by clinical experience on the labial side of the mandibular incisors, which is also in agreement with the research by Jing et al[51]. PAOO improved soft tissue augmentation, which actually lays the periodontal foundation for successful orthodontics.
However, drawbacks are still inevitable. Extra cost and post-operative risks, such as possible bone loss, resorption of bone grafts, and susceptible infections, can be additional concerns in such patients. Moreover, a follow-up for 6 mo in this case is relatively short, and we are actually performing a large-scale observation and an increased length of follow-up period to evaluate the effectiveness and long-term stability of PAOO in adult orthodontic patients with a thin biotype and buccal plate deficiency.
At present, the main confounders of bone augmentation remain indefinite and uncertain. In this case, the effect of bone augmentation was negative in some dehiscence sites, such as #31; Thereby, approaches to ensure the effectiveness and certainty of bone augmentation in PAOO-assisted OTM bring novel prospects to clinicians. In summary, the first step rests on a meticulous examination of the gingival biotype and buccal bone thickness and proper case assessment prior to regimen implementation, and CBCT analysis is necessary. CBCT is capable of providing more clear and detailed images to show the relationship of target teeth with the adjacent anatomical structures and corresponding bone reconstruction, while orthopantomogram could be beneficial in extensive measurements, including localization, angular, linear, and possible root resorption evaluation, especially in OTM of impacted maxillary canines. Second, standardized and unified clinical guidelines of PAOO surgery should be instituted. Third, prevention of bone substitutes from undergoing displacement and leakage should be emphasized. The last but the most important point, continuous and steady release of growth factors (such as PRF) should be encouraged to enhance bone formation. Despite the discrepancy in technical proficiency among the clinicians and idiosyncratic sensitivity in patients, PAOO combined with PRF is a promising treatment scheme to accelerate OTM and improve bone regeneration in adult patients with periodontal disease.
This case report demonstrated that the combination of autologous PRF with PAOO could reduce the therapeutic duration with rapid OTM plus augmented alveolar bone and soft tissues in adult patients with dental crowding, thin biotype, and buccal bone defects.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kannan S S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Kaya Y, Alkan Ö, Keskin S. An evaluation of the gingival biotype and the width of keratinized gingiva in the mandibular anterior region of individuals with different dental malocclusion groups and levels of crowding. Korean J Orthod. 2017;47:176-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Misawa Y, Kageyama T, Moriyama K, Kurihara S, Yagasaki H, Deguchi T, Ozawa H, Sahara N. Effect of age on alveolar bone turnover adjacent to maxillary molar roots in male rats: A histomorphometric study. Arch Oral Biol. 2007;52:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Schubert A, Jäger F, Maltha JC, Bartzela TN. Age effect on orthodontic tooth movement rate and the composition of gingival crevicular fluid: A literature review. J Orofac Orthop. 2020;81:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21:9-19. [PubMed] |
| 5. | Wilcko MT, Wilcko WM, Bissada, NF. An Evidence-Based Analysis of Periodontally Accelerated Orthodontic and Osteogenic Techniques: A Synthesis of Scientific Perspectives. Semin Orthod. 2008;14:305-316. [RCA] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Alves R, Grimalt R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018;4:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 7. | Agrawal AA. Evolution, current status and advances in application of platelet concentrate in periodontics and implantology. World J Clin Cases. 2017;5:159-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (4)] |
| 8. | Del Corso M, Vervelle A, Simonpieri A, Jimbo R, Inchingolo F, Sammartino G, Dohan Ehrenfest DM. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: Periodontal and dentoalveolar surgery. Curr Pharm Biotechnol. 2012;13:1207-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 9. | Simonpieri A, Del Corso M, Vervelle A, Jimbo R, Inchingolo F, Sammartino G, Dohan Ehrenfest DM. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol. 2012;13:1231-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Fan Y, Perez K, Dym H. Clinical Uses of Platelet-Rich Fibrin in Oral and Maxillofacial Surgery. Dent Clin North Am. 2020;64:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Murphy KG, Wilcko MT, Wilcko WM, Ferguson DJ. Periodontal accelerated osteogenic orthodontics: a description of the surgical technique. J Oral Maxillofac Surg. 2009;67:2160-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Sanivarapu S, Addanki PK, Palaparty R, Adurty C. Periodontally accelerated osteogenic orthodontics: Novel perio-ortho interrelationship. J Indian Soc Periodontol. 2018;22:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Vannala V, Katta A, Reddy MS, Shetty SR, Shetty RM, Khazi SS. Periodontal Accelerated Osteogenic Orthodontics Technique for Rapid Orthodontic Tooth Movement: A Systematic Review. J Pharm Bioallied Sci. 2019;11:S97-S106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Wilcko W, Wilcko MT. Accelerating tooth movement: the case for corticotomy-induced orthodontics. Am J Orthod Dentofacial Orthop. 2013;144:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Soltani L, Loomer PM, Chaar EE. A Novel Approach in Periodontally Accelerated Osteogenic Orthodontics (PAOO): A Case Report. Clin Adv Periodontics. 2019;9:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | AlHammadi HA, Wilcko MT, Ferguson DJ. Severe Mandibular Crowding Treated with Nonextraction Periodontally Accelerated Osteogenic Orthodontics. Int J Periodontics Restorative Dent. 2019;39:e188-e194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Coscia G, Coscia V, Peluso V, Addabbo F. Augmented corticotomy combined with accelerated orthodontic forces in class III orthognathic patients: morphologic aspects of the mandibular anterior ridge with cone-beam computed tomography. J Oral Maxillofac Surg 2013; 71: 1760.e1-1760. e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Yu H, Jiao F, Wang B, Shen SG. Piezoelectric decortication applied in periodontally accelerated osteogenic orthodontics. J Craniofac Surg. 2013;24:1750-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Ahn HW, Seo DH, Kim SH, Park YG, Chung KR, Nelson G. Morphologic evaluation of dentoalveolar structures of mandibular anterior teeth during augmented corticotomy-assisted decompensation. Am J Orthod Dentofacial Orthop. 2016;150:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Sebaoun JD, Kantarci A, Turner JW, Carvalho RS, Van Dyke TE, Ferguson DJ. Modeling of trabecular bone and lamina dura following selective alveolar decortication in rats. J Periodontol. 2008;79:1679-1688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Baloul SS, Gerstenfeld LC, Morgan EF, Carvalho RS, Van Dyke TE, Kantarci A. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am J Orthod Dentofacial Orthop. 2011;139:S83-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Kim YS, Kim SJ, Yoon HJ, Lee PJ, Moon W, Park YG. Effect of piezopuncture on tooth movement and bone remodeling in dogs. Am J Orthod Dentofacial Orthop. 2013;144:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Chin M. Establishing and Maintaining Osseointegration Within the Functional Matrix. Int J Periodontics Restorative Dent. 2016;36:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Nowzari H, Yorita FK, Chang HC. Periodontally accelerated osteogenic orthodontics combined with autogenous bone grafting. Compend Contin Educ Dent. 2008;29:200-6; quiz 207, 218. [PubMed] |
| 25. | Addanki P, Gooty JR, Palaparthy R. Clinical and Radiographic Comparative Evaluation of Buccal and Palatal Corticotomy with Buccal Corticotomy in Periodontally Accelerated Osteogenic Orthodontics with Surgical Bur. Contemp Clin Dent. 2017;8:321-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Singh S, Jayan B. Comparative Evaluation of Periodontally Accelerated Osteogenic Orthodontics (PAOO) Versus Conventional Orthodontic Tooth Movement in Adult Patients with Bimaxillary Dentoalveolar Protrusion. Int J Periodontics Restorative Dent. 2019;39:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Frost HM. The regional acceleratory phenomenon: a review. Henry Ford Hosp Med J. 1983;31:3-9. [PubMed] |
| 28. | Yaffe A, Fine N, Binderman I. Regional accelerated phenomenon in the mandible following mucoperiosteal flap surgery. J Periodontol. 1994;65:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 179] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 29. | Frost HM. The biology of fracture healing. An overview for clinicians. Part I. Clin Orthop Relat Res. 1989: 283-293. [PubMed] |
| 30. | K S, M S, D R, Gowd P. Wilckodontics - a novel synergy in time to save time. J Clin Diagn Res. 2014;8:322-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Fau V, Diep D, Bader G, Brézulier D, Sorel O. [Effectiveness of selective alveolar decortication in accelerating orthodontic treatment: a systematic review]. Orthod Fr. 2017;88:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Shih MS, Norrdin RW. Regional acceleration of remodeling during healing of bone defects in beagles of various ages. Bone. 1985;6:377-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 33. | Verna C, Dalstra M, Melsen B. The rate and the type of orthodontic tooth movement is influenced by bone turnover in a rat model. Eur J Orthod. 2000;22:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Midgett RJ, Shaye R, Fruge JF Jr. The effect of altered bone metabolism on orthodontic tooth movement. Am J Orthod. 1981;80:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 75] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Bell WH, Finn RA, Buschang PH. Accelerated orthognathic surgery and increased orthodontic efficiency: a paradigm shift. J Oral Maxillofac Surg. 2009;67:2043-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Brugnami F, Caiazzo A, Mehra P. Can corticotomy (with or without bone grafting) expand the limits of safe orthodontic therapy? J Oral Biol Craniofac Res. 2018;8:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Williams MO, Murphy NC. Beyond the Ligament: A Whole-Bone Periodontal View of Dentofacial Orthopedics and Falsification of Universal Alveolar Immutability. Semin Orthod. 2008;14:246-259. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Miron RJ, Shuang Y, Bosshardt DD, Caballé-Serrano J, Chandad F, Zhang Y. Osteogenic gene array of osteoblasts cultured on a novel osteoinductive biphasic calcium phosphate bone grafting material. Clin Oral Investig. 2017;21:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Baldwin P, Li DJ, Auston DA, Mir HS, Yoon RS, Koval KJ. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J Orthop Trauma. 2019;33:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 407] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 40. | Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006;3:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 385] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 41. | Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 689] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 42. | Munoz F, Jiménez C, Espinoza D, Vervelle A, Beugnet J, Haidar Z. Use of leukocyte and platelet-rich fibrin (L-PRF) in periodontally accelerated osteogenic orthodontics (PAOO): Clinical effects on edema and pain. J Clin Exp Dent. 2016;8:e119-e124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Canellas JVDS, Medeiros PJD, Figueredo CMDS, Fischer RG, Ritto FG. Platelet-rich fibrin in oral surgical procedures: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2019;48:395-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 44. | Albilia J DMD, MSc, Herrera-Vizcaíno C DDS, Weisleder H BSc, Choukroun J MD, Ghanaati S MD, DMD, PhD. Liquid platelet-rich fibrin injections as a treatment adjunct for painful temporomandibular joints: preliminary results. Cranio. 2020;38:292-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Anitua E, Tejero R, Zalduendo MM, Orive G. Plasma rich in growth factors promotes bone tissue regeneration by stimulating proliferation, migration, and autocrine secretion in primary human osteoblasts. J Periodontol. 2013;84:1180-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 692] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 47. | Miron RJ, Choukroun J. Platelet rich fibrin in regenerative dentistry: biological background and clinical indications. Hoboken (NJ): John Wiley and Sons, 2017. |
| 48. | Gassling V, Hedderich J, Açil Y, Purcz N, Wiltfang J, Douglas T. Comparison of platelet rich fibrin and collagen as osteoblast-seeded scaffolds for bone tissue engineering applications. Clin Oral Implants Res. 2013;24:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Shawky H, Seifeldin SA. Does Platelet-Rich Fibrin Enhance Bone Quality and Quantity of Alveolar Cleft Reconstruction? Cleft Palate Craniofac J. 2016;53:597-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Wilcko MT, Ferguson DJ, Makki L, Wilcko WM. Keratinized Gingiva Height Increases After Alveolar Corticotomy and Augmentation Bone Grafting. J Periodontol. 2015;86:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Jing WD, Jiao J, Xu L, Hou JX, Li XT, Wang XX, Xu X, Mao MX. Periodontal soft- and hard-tissue changes after augmented corticotomy in Chinese adult patients with skeletal Angle Class III malocclusion: A non-randomized controlled trial. J Periodontol. 2020;91:1419-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |