Published online Feb 26, 2021. doi: 10.12998/wjcc.v9.i6.1293
Peer-review started: November 10, 2020
First decision: December 8, 2020
Revised: December 17, 2020
Accepted: December 27, 2020
Article in press: December 27, 2020
Published online: February 26, 2021
Processing time: 87 Days and 20.7 Hours
The ideal depth of general anesthesia should achieve the required levels of hypnosis, analgesia, and muscle relaxation while minimizing physiologic responses to awareness. The choice of anesthetic strategy in patients with coronary heart disease (CHD) undergoing major noncardiac surgery is becoming an increasingly important issue as the population ages. This is because general anesthesia is associated with a risk of perioperative cardiac complications and death, and this risk is much higher in people with CHD.
To compare hemodynamic function and cardiovascular event rate between etomidate- and propofol-based anesthesia in patients with CHD.
This prospective study enrolled consecutive patients (American Society of Anesthesiologists grade II/III) with stable CHD (New York Heart Association class I/II) undergoing major noncardiac surgery. The patients were randomly allocated to receive either etomidate/remifentanil-based or propofol/remifentanil-based general anesthesia. Randomization was performed using a computer-generated random number table and sequentially numbered, opaque, sealed envelopes. Concealment was maintained until the patient had arrived in the operating theater, at which point the consulting anesthetist opened the envelope. All patients, data collectors, and data analyzers were blinded to the type of anesthesia used. The primary endpoints were the occurrence of cardiovascular events (bradycardia, tachycardia, hypotension, ST-T segment changes, and ventricular premature beats) during anesthesia and cardiac troponin I level at 24 h. The secondary endpoints were hemodynamic parameters, bispectral index, and use of vasopressors during anesthesia.
The final analysis included 40 patients in each of the propofol and etomidate groups. The incidences of bradycardia, hypotension, ST-T segment changes, and ventricular premature beats during anesthesia were significantly higher in the propofol group than in the etomidate group (P < 0.05 for all). The incidence of tachycardia was similar between the two groups. Cardiac troponin I levels were comparable between the two groups both before the induction of anesthesia and at 24 h after surgery. When compared with the etomidate group, the propofol group had significantly lower heart rates at 3 min after the anesthetic was injected (T1) and immediately after tracheal intubation (T2), lower systolic blood pressure at T1, and lower diastolic blood pressure and mean arterial pressure at T1, T2, 3 min after tracheal intubation, and 5 min after tracheal intubation (P < 0.05 for all). Vasopressor use was significantly more in the propofol group than in the etomidate group during the induction and maintenance periods (P < 0.001).
In patients with CHD undergoing noncardiac major surgery, etomidate-based anesthesia is associated with fewer cardiovascular events and smaller hemodynamic changes than propofol-based anesthesia.
Core Tip: The results show that in patients with coronary heart disease undergoing noncardiac major surgery, etomidate-based anesthesia was associated with fewer cardiovascular events and smaller hemodynamic changes than propofol-based anesthesia.
- Citation: Dai ZL, Cai XT, Gao WL, Lin M, Lin J, Jiang YX, Jiang X. Etomidate vs propofol in coronary heart disease patients undergoing major noncardiac surgery: A randomized clinical trial. World J Clin Cases 2021; 9(6): 1293-1303
- URL: https://www.wjgnet.com/2307-8960/full/v9/i6/1293.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i6.1293
The ideal depth of general anesthesia should achieve the required levels of hypnosis, analgesia and muscle relaxation while minimizing physiologic responses to awareness such as tachycardia, hypertension, sweating, lacrimation, increased skeletal muscle tone, and spontaneous movement[1,2]. The choice of anesthetic strategy in patients with coronary heart disease (CHD) undergoing major noncardiac surgery is becoming an increasingly important issue as the population ages. This is because general anesthesia is associated with a risk of perioperative cardiac complications and death, and this risk is much higher in people with CHD. Perioperative cardiac complications including myocardial infarction (MI) have been reported in around 15% of patients with CHD who undergo noncardiac surgery[3,4], although even higher rates have been described in some studies[5]. Furthermore, preoperative CHD is associated with an approximately 6-fold increased odds of reintubation following surgery under general anesthesia[6]. It is thus essential to utilize strategies that maintain hemodynamic stability, adequate oxygenation and a good analgesic effect while minimizing the risk of perioperative ischemia, and this requires a multidisciplinary approach[7-9]. Recommendations have been made regarding the perioperative medical management of CHD in patients undergoing noncardiac surgery[10]. The type of anesthesia used may also affect perioperative cardiac outcomes in patients[11].
Propofol is an intravenous anesthetic agent that is widely used for the induction and maintenance of general anesthesia. However, propofol has various effects on the cardiovascular system including a decrease in blood pressure[12-14], impaired cardiac contraction[15], and (rarely) the induction of arrhythmia[16]. Propofol has also been reported to compromise cardiovascular function in patients with CHD[17]. A study of 12 patients with CHD found that propofol anesthesia was associated with an increase in heart rate, reductions in arterial blood pressure, myocardial blood flow, and myocardial oxygen consumption, and in one patient an elevation in myocardial lactate production[18]. This latter finding and the observation of ischemic electrocardiogram (ECG) changes during propofol anesthesia[19] suggest that propofol can induce myocardial ischemia in at-risk patients.
Etomidate is an alternative intravenous anesthetic agent that has a rapid onset and a short duration of action. There is published evidence that etomidate may have smaller effects on the cardiovascular system than propofol. For example, when compared with propofol, etomidate has been reported to cause smaller reductions in blood pressure, heart rate, arterial elastance, and systemic vascular resistance during the induction of anesthesia[19,20]. However, some studies have concluded that propofol may have advantages over etomidate with regard to minimizing the stress response and alleviating ischemic ECG changes[19,21]. Thus, further research is needed to establish which of these agents is preferable in patients with CHD.
The aim of the present study was to compare hemodynamic function and cardiovascular event rate between etomidate- and propofol-based anesthesia in patients with CHD undergoing major noncardiac surgery.
This prospective, randomized clinical trial enrolled consecutive patients with stable CHD undergoing major noncardiac surgery at the Department of Anesthesiology, Shenzhen People’s Hospital, Guangdong Province, China between July 2014 and December 2015. The inclusion criteria were: (1) Age 52-88 years; (2) American Society of Anesthesiologists grade II or III; (3) A clinical history of stable angina pectoris or myocardial infarction; (4) Angiographically-confirmed stenosis of > 50% in at least one coronary artery; (5) Cardiac function graded as New York Heart Association class I or II; (6) Scheduled for major gastrointestinal, hepatobiliary, or thyroid surgery; and (7) Expected duration of hospitalization ≥ 24 h. The exclusion criteria were: (1) Scheduled for emergency surgery or cardiac surgery; (2) Congenital heart disease, rheumatic heart disease, cardiomyopathy, valvular disease, or significant left ventricular hypertrophy; (3) Myocardial infarction, percutaneous coronary intervention, or other serious cardiopulmonary dysfunction in the previous 3 mo; (4) Dysfunction of the liver, kidney, or adrenal cortex; (5) History of abnormal bleeding; (6) Adverse reactions to propofol or etomidate; (7) Implanted cardiac pacemaker; (8) Hypertension of grade 3 or above; (9) Hypotension or shock; (10) Body mass index > 30 kg/m2; and (11) Participation in another study that might interfere with the endpoints of the current trial.
The study was approved by the Ethics Committee of Shenzhen People’s Hospital (LL-KT-2014211) and conducted according to the Declaration of Helsinki. All patients provided written informed consent after having been provided with detailed information about the study aims, procedures, and risks. ChiCTR1900025174. The trial was registered in Chinese Clinical Trial Registry on August 15, 2019 (ChiCTR1900025174) and is available at http://www.chictr.org.cn/showproj.aspx?proj=42063.
The patients were randomly allocated to receive either etomidate/remifentanil-based or propofol/remifentanil-based general anesthesia. Randomization was performed using a computer-generated random number table and sequentially numbered, opaque, sealed envelopes. Concealment was maintained until the patient had arrived in the operating theater, at which point the consulting anesthetist opened the envelope. All patients, data collectors, and data analyzers were blinded to the type of anesthesia used.
For this superiority trial, a 10% difference in the rate of myocardial ischemia between groups was regarded as clinically relevant based on previous studies[9,13,14]. Assuming an alpha of 5% and a test power of 0.8, power analysis indicated that a difference of 10% would be detected with a total sample size of 160 (or 80 per group). Assuming a loss to follow-up of 20%, each group would need to include 100 patients at enrolment.
The same main anesthetist (Dr Li) was present at all the operations, and all procedures were performed by the same surgical team. The patients were admitted the day before surgery and fasted for at least 8 h before the operation. Some patients continued to receive an antihypertensive agent (captopril) until the morning of surgery. On arrival in the operating theater, an intravenous catheter was inserted into a large forearm vein, and standard monitoring was initiated including non-invasive arterial blood pressure (Datex-Ohmeda, Helsinki, Finland) and bispectral index (BIS; Aspect Medical Systems, Newton, MA, United States) monitoring. Vital parameters such as heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) were assessed at regular intervals.
Patients in both groups received oral midazolam (0.04 mg/kg; Enhua Pharmaceutical Co. Ltd., Xuzhou, China) at least 1 h before induction of anesthesia. An infusion of remifentanil (4 μg/kg; Renfu Pharmaceutical Group Co. Ltd., Yichang, China) was started during pre-oxygenation via a face mask, and anesthesia was induced 3 min later by the administration of cisatracurium (0.2 mg/kg; Jiangsu Hengrui Pharmaceutical Co. Ltd., Lianyungang, China) with either etomidate (0.3 mg/kg; Enhua Pharmaceutical Co. Ltd.; etomidate group) or propofol (2.0 mg/kg; Fresenius Kabi, Uppsala, Sweden; propofol group). The trachea was intubated, and mechanical ventilation was started with a tidal volume of 8 mL/kg, a ventilatory frequency that maintained end-tidal Pco2 between 35 and 45 mmHg, and a fraction of inspired oxygen (FIO2) of 1.0 (PhysioFlex closed-circuit anesthesia machine, Dräger, Lübeck, Germany).
Baseline hemodynamic data were recorded after at least 5 min without further changes in HR or arterial pressure. The BIS was maintained at a value of 45-60 to achieve a suitable depth of anesthesia with very low awareness possibility. The remifentanil infusion was continued and increased in increments of 0.05 mg/kg/min if one of the following occurred: Sudden increase in HR or arterial pressure by > 20%, sweating, or spontaneous movements.
For maintenance of anesthesia in the propofol group, 1% propofol (1.5-3.0 μg/mL) was administered by target-controlled infusion (Alaris Pk syringe pump, Cardinal Health, Rolle, Switzerland) according to a modified Marsh pharmacokinetic model (ke0 of 1.21 min-1; plasma mode using real weight) starting at an initial target concentration of 1.0 μg/mL and increasing at a rate of 0.5 μg/mL each minute until a plasma concentration (Cp) of 2.5 μg/mL was attained. In the etomidate group, anesthesia was maintained by the continuous infusion of etomidate emulsion at a constant rate (10-20 μg/kg/min). Intermittent bolus injections of cisatracurium were used to maintain full muscle relaxation. No other anesthetic agents were administered until 30 min before the operation finished.
Thirty minutes before the end of surgery, 0.1 mg fentanyl and 50 mg flurbiprofen were administrated intravenously, and the cisatracurium infusion was stopped. Five minutes before the end of the operation, 3 mg granisetron was administrated by intravenous injection. The infusion of remifentanil and etomidate/propofol was stopped at the end of surgery. Neostigmine was used to reverse residual muscle relaxation after spontaneous respiration had recovered. Awakening was considered as the moment when the patient opened their eyes after being called by their name. Extubation was performed when the following conditions were met: Recovery of the cough and swallowing reflexes; recovery of consciousness and awakening; tidal volume > 6 mL/kg; respiratory rate 12-30 times/min; head elevation for > 2 s; and normal BP, HR, and ECG findings.
SBP, DBP, HR, ST-T interval, and BIS were recorded immediately before the induction of anesthesia (T0), 3 min after the anesthetic was injected (T1), immediately after tracheal intubation (T2), 3 min after tracheal intubation (T3), and 5 min after tracheal intubation (T4). The levels of cardiac troponin I (cTnI) were measured before the induction of anesthesia and 24 h after surgery to determine the extent of any myocardial injury.
The patients were followed for 24 h. The primary endpoints were the occurrence of cardiovascular events (bradycardia, tachycardia, hypotension, ST-T segment changes, and ventricular premature beats) during anesthesia and cTnI level at 24 h. The secondary endpoints were hemodynamic parameters (SBP, DBP, MAP, and HR), BIS and use of vasopressors during anesthesia.
SPSS 15.0 (SPSS Inc., Chicago, IL, United States) was used for data analyses, and Microsoft Excel and Word (Microsoft Corp., Redmond, WA, United States) were used to generate graphs and tables. Measurement data were tested for normality using the Kolmogorov-Smirnov test. Quantitative data are expressed as the mean ± SD and were compared between groups using Student’s t-test with Bonferroni correction. Categorical data are presented as n (%) and were compared between groups using the chi-squared test or Fisher’s exact test. P < 0.05 was taken to indicate statistical significance.
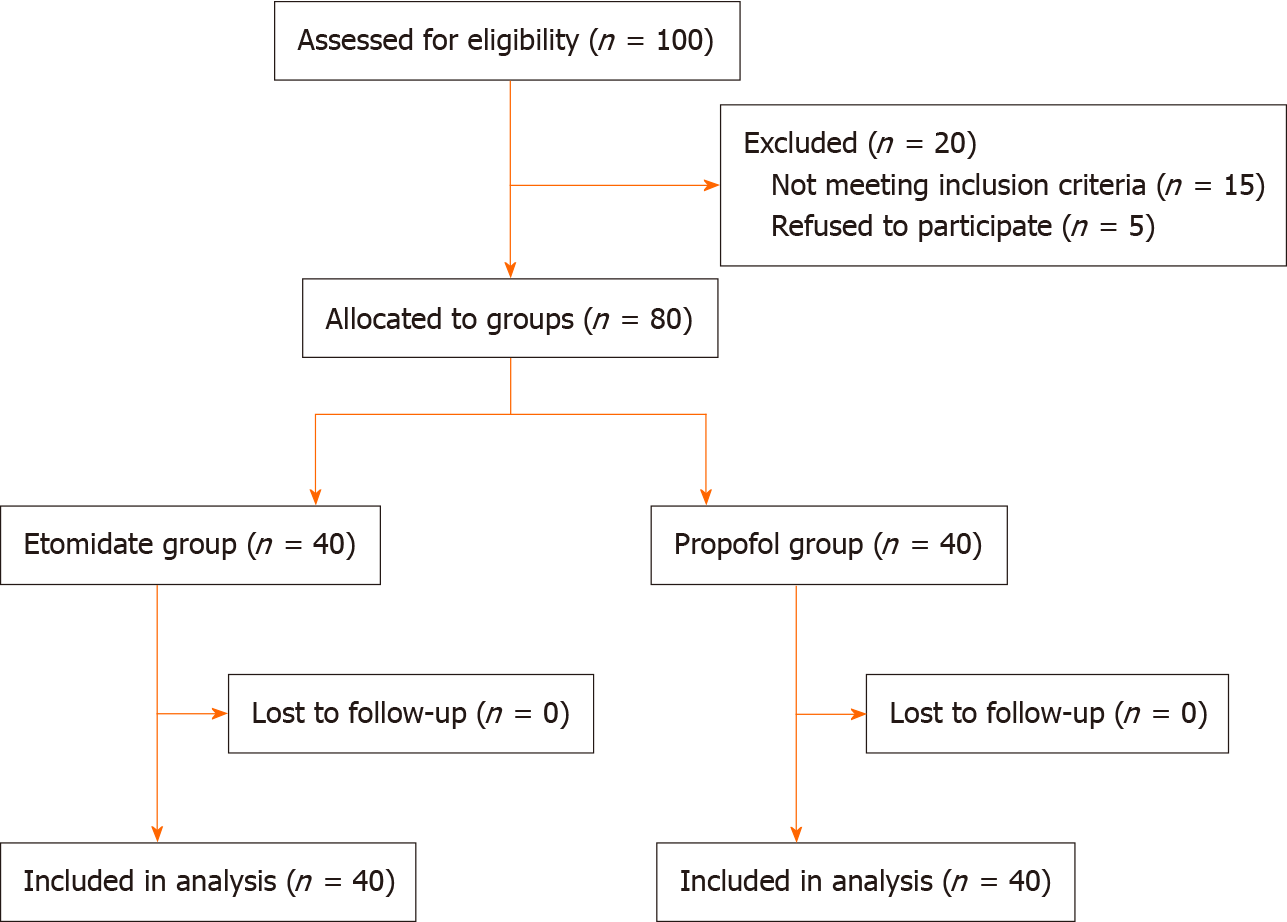
Among 100 patients screened for eligibility, 15 were excluded because they did not meet the inclusion criteria, and 5 were excluded because they refused to participate (Figure 1). Therefore, a total of 80 patients were included in the final analysis, with 40 patients in each group. There were no significant differences between groups in sex, age, height, weight, or type of surgery (Table 1).
| Propofol group (n = 40) | Etomidate group (n = 40) | P value | |
| Male sex, n (%) | 21 (52.5%) | 23 (57.5%) | 0.651 |
| Age (years), mean ± SD | 56.7 ± 6.6 | 53.6 ± 6.1 | 0.452 |
| Weight (kg), mean ± SD | 64.6 ± 12.5 | 66.4 ± 16.3 | 0.084 |
| Height (cm), mean ± SD | 165.7 ± 11.9 | 163.4 ± 13.3 | 0.732 |
| Type of surgery, n (%) | |||
| Gastrointestinal | 26 (65.0%) | 23 (57.5%) | 0.946 |
| Hepatobiliary | 8 (20.0%) | 10 (25.0%) | 0.783 |
| Thyroid | 6 (15.0%) | 7 (17.5%) | 0.562 |
The incidences of bradycardia, hypotension, ST-T segment changes, and ventricular premature beats during anesthesia were significantly higher in the propofol group than in the etomidate group (P < 0.05 for all; Table 2). The incidence of tachycardia did not differ significantly between groups (Table 2). cTnI levels in the propofol and etomidate groups were comparable both before the induction of anesthesia (0.009 ± 0.003 ng/mL and 0.010 ± 0.001 ng/mL, respectively) and 24 h after surgery (0.012 ± 0.005 ng/mL and 0.011 ± 0.002 ng/mL, respectively).
| Propofol group (n = 40) | Etomidate group (n = 40) | P value | |
| Bradycardia, n (%) | 11 (27.5%) | 3 (7.5%) | 0.037 |
| Tachycardia, n (%) | 1 (2.5%) | 3 (7.5%) | 0.615 |
| Hypotension, n (%) | 17 (42.5%) | 4 (10.0%) | 0.027 |
| ST-T segment changes, n (%) | 8 (20.0%) | 2 (5.0%) | 0.029 |
| Ventricular premature beats, n (%) | 11 (27.5%) | 3 (7.5%) | 0.041 |
There were no significant differences between the propofol and etomidate groups in HR, SBP, DBP, or MAP immediately before the induction of anesthesia (Table 3). Both groups exhibited reductions in HR, SBP, DBP, and MAP following the induction of anesthesia, but these changes were more prominent in the propofol group (Table 3). When compared with the etomidate group, the propofol group had significantly lower HR at 3 min after anesthetic injection and immediately after tracheal intubation, lower SBP at 3 min after anesthetic injection, and lower DBP and MAP at all four time points after the induction of anesthesia (P < 0.05 for all; Table 3).
| Before anesthesia induction | 3 min after anesthetic injection | Just after tracheal intubation | 3 min after tracheal intubation | 5 min after tracheal intubation | |
| Heart rate (beats/min) | |||||
| Propofol group | 82.6 ± 9.1 | 58.9 ± 7.1 | 66.5 ± 9.7 | 63.2 ± 6.7 | 68.9 ± 8.8 |
| Etomidate group | 79.0 ± 7.3 | 71.3 ± 9.3 | 76.5 ± 6.9 | 71.3 ± 6.4 | 76.8 ± 5.2 |
| P value | 0.223 | 0.012 | 0.044 | 0.253 | 0.072 |
| SBP (mmHg) | |||||
| Propofol group | 132.7 ± 12.4 | 89.4 ± 16.3 | 100.2 ± 13.1 | 93.1 ± 17.4 | 103.8 ± 13.2 |
| Etomidate group | 129.6 ± 17.2 | 100.3 ± 19.3 | 117.5 ± 12.9 | 112.3 ± 16.4 | 121.5 ± 15.3 |
| P value | 0.813 | 0.041 | 0.112 | 0.214 | 0.712 |
| DBP (mmHg) | |||||
| Propofol group | 76.7 ± 18.7 | 62.9 ± 16.3 | 72.1 ± 16.7 | 68.6 ± 17.2 | 70.9 ± 18.4 |
| Etomidate group | 79.0 ± 16.1 | 73.0 ± 16.4 | 90.8 ± 20.2 | 91.2 ± 16.3 | 82.2 ± 15.4 |
| P value | 0.60 | 0.009 | 0.008 | 0.02 | 0.01 |
| MAP (mmHg) | |||||
| Propofol group | 92.6 ± 18.7 | 68.9 ± 16.1 | 76.5 ± 16.5 | 86.2 ± 18.2 | 80.8 ± 18.2 |
| Etomidate group | 95.1 ± 17.2 | 80.2 ± 16.7 | 96.3 ± 22.5 | 93.5 ± 16.4 | 89.8 ± 15.5 |
| P value | 0.69 | 0.007 | 0.043 | 0.012 | 0.018 |
None of the observed changes in HR required intervention. All cases of hypotension (a decrease in MAP to ≤ 70% of the baseline value) were successfully treated with single injections of phenylephrine or ephedrine. Notably, vasopressor use was significantly higher in the propofol group than in the etomidate group during the induction and maintenance periods (P < 0.001 for both ephedrine and phenylephrine use; Table 4).
| Ephedrine use (mg) | Phenylephrine use (μg) | |||
| Induction | Maintenance | Induction | Maintenance | |
| Propofol group | 7.0 ± 1.6 | 9.8 ± 3.2 | 17.5 ± 3.8 | 15.1 ± 1.6 |
| Etomidate group | 3.1 ± 2.0 | 2.6 ± 1.1 | 6.2 ± 1.3 | 7.5 ± 2.1 |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
The BIS values were not significantly different between the propofol and etomidate groups at all time points (Table 5).
| Before anesthesia induction | 3 min after anesthetic injection | Just after tracheal intubation | 3 min after tracheal intubation | 5 min after tracheal intubation | |
| Propofol group | 97.0 ± 1.7 | 46.9 ± 2.1 | 47.5 ± 1.5 | 46.2 ± 1.2 | 46.8 ± 1.8 |
| Etomidate group | 97.4 ± 1.20 | 47.2 ± 1.7 | 46.5 ± 1.5 | 44.3 ± 1.4 | 45.0 ± 1.3 |
| P value | 0.91 | 0.29 | 0.38 | 0.32 | 0.43 |
An important finding of the present study was that the incidences of bradycardia, hypotension, ST-T segment changes, and ventricular premature beats in patients with CHD undergoing noncardiac major surgery were significantly higher for propofol-based general anesthesia than for etomidate-based anesthesia. Furthermore, in comparison with the etomidate group, the propofol group had significantly lower HR, SBP, DBP, and MAP at various time points after the induction of anesthesia. Additionally, vasopressor use was significantly more in the propofol group than in the etomidate group during the induction and maintenance periods. The above data suggest that etomidate-based anesthesia may be preferable to propofol-based anesthesia in patients with CHD undergoing noncardiac major surgery.
Monitored anesthesia care is frequently considered a means to increase safety when anesthetists are confronted with a patient who has complex cardiac physiology. This is understandable because general anesthesia is known to be associated with a risk of perioperative cardiac complications, and this risk is particularly high in patients with CHD[3-5]. In part, the risks of general anesthesia in patients with CHD may be related to their hemodynamic effects. In the present study, the reductions in HR, SBP, DBP, and MAP during the induction of anesthesia were significantly greater for propofol (supplemented by remifentanil) than for etomidate (supplemented by remifentanil). Our findings are in good agreement with previous research indicating that propofol decreases blood pressure[12-14] and impairs cardiovascular function in patients with CHD[17,18] whereas etomidate is associated with smaller inhibitory effects on hemodynamics in patients with CHD[19,20]. Notably, etomidate has also been reported to cause less hemodynamic instability than propofol in the setting of congenital heart disease and impaired cardiac function[22].
Adult patients with CHD are now surviving longer than ever before, and it is becoming increasingly apparent that even the simplest coronary lesions can be associated with long-term complications. A previous study concluded that propofol anesthesia was associated with decreases in arterial blood pressure, myocardial blood flow, and myocardial oxygen consumption and (in one case) an increase in myocardial lactate production[18], while another described ischemic ECG changes during propofol anesthesia[19]. These previous findings suggest that propofol-based general anesthesia might induce myocardial ischemia in certain patients with CHD. In the present analysis, propofol-based anesthesia was associated with significantly higher incidences of bradycardia, hypotension, ST-T segment abnormalities, and ventricular premature beats than etomidate-based anesthesia, implying that etomidate may be associated with a lower risk of these cardiovascular events than propofol, perhaps because etomidate is associated with better hemodynamic stability than propofol[23,24]. Although there is some evidence that propofol may have advantages over etomidate with regard to reducing the stress response and minimizing ischemic ECG changes[19,21], our results suggest that ST-T segment abnormalities and other cardiovascular events occur less frequently for etomidate than for propofol during the induction of anesthesia.
Etomidate, an imidazole-derived ultrashort-acting nonbarbiturate hypnotic, is frequently used to induce anesthesia in critically ill patients because of its hemodynamic safety, rapid onset, and short duration of action[25]. However, although etomidate offers the advantage of minimizing hypotension that can cause coronary hypoperfusion, dysrhythmia, and cardiac arrest, a previous clinical study reported that induction of anesthesia with etomidate rather than propofol was associated with increased 30 d mortality and cardiovascular morbidity after noncardiac surgery as well as a prolonged duration of hospitalization[26]. It is possible that some of the effects reported in this earlier study resulted from a suppression of adrenocortical function by etomidate[27]. However, this prior study was retrospective and the patients were not randomized to etomidate- or propofol-based anesthesia, so the results may have been influenced by confounding factors. Indeed, other investigations did not find a longer duration of hospital stay or increased mortality for etomidate-based anesthesia than for propofol-based anesthesia[28,29].
The present study did not detect any notable increase in cTnI level at 24 h after surgery in either the propofol or etomidate group. Nevertheless, we would recommend postoperative troponin monitoring in all patients with known cardiovascular risk who are undergoing major surgery, as suggested by others[30-33]. Without early postoperative monitoring of troponin, physicians would be likely to miss 2/3 of the MIs that occur, and asymptomatic MIs carry the same high risk of 30 d mortality as symptomatic MIs[32].
Our study has several limitations. First, the sample size was small, so the study may have been underpowered to detect some real differences between groups. Second, this was a single-center study, so the generalizability of the findings is not known. Third, the follow-up period was only 24 h, so longer-term outcomes such as duration of hospital stay, cardiovascular morbidity, and 30 d mortality were not evaluated. Fourth, as with any observational study, unknown confounding factors may have influenced the results.
For patients with CHD undergoing noncardiac major surgery, etomidate-based anesthesia may be preferable to propofol-based anesthesia due to a lower incidence of cardiovascular events and smaller hemodynamic changes.
The choice of anesthetic strategy in patients with coronary heart disease (CHD) undergoing major noncardiac surgery is becoming an increasingly important issue as the population ages.
Perioperative cardiac complications including myocardial infarction have been reported in around 15% of patients with CHD who undergo noncardiac surgery. It is thus essential to utilize strategies that maintain hemodynamic stability, adequate oxygenation, and a good analgesic effect while minimizing the risk of perioperative ischemia, and this requires a multidisciplinary approach.
To compare hemodynamic function and cardiovascular event rate between etomidate- and propofol-based anesthesia in patients with CHD.
This prospective, randomized clinical trial enrolled consecutive patients with stable CHD undergoing major noncardiac surgery. The patients were randomly allocated to receive either etomidate/remifentanil-based or propofol/remifentanil-based general anesthesia.
The final analysis included 40 patients in each group. The incidences of bradycardia, hypotension, ST-T segment changes, and ventricular premature beats during anesthesia were significantly higher in the propofol group than in the etomidate group (P < 0.05 for all).
In patients with CHD undergoing noncardiac major surgery, etomidate-based anesthesia was associated with fewer cardiovascular events and smaller hemodynamic changes than propofol-based anesthesia.
In the future, a large prospective randomized study will definitively address the effect of etomidate on postoperative outcomes in patients with coronary heart disease undergoing major noncardiac surgery.
We thank Hai-Bo Wang PhD from Peking University for statistical data preparation. We also thank Professor Zheng-Yuan Xia from Hong Kong University for assistance in manuscript preparation. We are grateful to Zhong-Jun Zhang, Xue-Ping Zhang, and Ya-Li Li for their crucial role in patient recruitment and data collection.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Găman MA S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Brown EN, Pavone KJ, Naranjo M. Multimodal General Anesthesia: Theory and Practice. Anesth Analg. 2018;127:1246-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 2. | Rani DD, Harsoor S. Depth of general anaesthesia monitors. Indian J Anaesth. 2012;56:437-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Seki M, Kashimoto S, Nagata O, Yoshioka H, Ishiguro T, Nishimura K, Honda O, Sakamoto A, Omi A, Ogihara Y, Fujimoto K, Iwade M, Yamada T, Nomura M, Takeda J. Are the incidences of cardiac events during noncardiac surgery in Japan the same as in the United States and Europe? Anesth Analg. 2005;100:1236-1240, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Yamada T, Nomura M, Iwade M, Omi A, Kashimoto S, Yoshioka H, Kikuchi T, Fujimoto K, Honda O, Seki M, Ishiguro T, Takeda J. [Multicenter study of cardiac events and anesthetic management of patients with ischemic heart diseases undergoing noncardiac surgery]. Masui. 2000;49:673-679. [PubMed] |
| 5. | Varma MK, Puri GD, Chari P, Verma JS, Kohli KK. Perioperative myocardial infarction in coronary artery disease patients and 'at-risk' for coronary artery disease patients undergoing non-cardiac surgery. Natl Med J India. 1996;9:214-217. [PubMed] |
| 6. | Wang K, Yin Y. Risk Factors and Prognosis of Reintubation Following Surgeries under General Anesthesia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Fellahi JL, Godier A, Benchetrit D, Berthier F, Besch G, Bochaton T, Bonnefoy-Cudraz E, Coriat P, Gayat E, Hong A, Jenck S, Le Gall A, Longrois D, Martin AC, Pili-Floury S, Piriou V, Provenchère S, Rozec B, Samain E, Schweizer R, Billard V. Perioperative management of patients with coronary artery disease undergoing non-cardiac surgery: Summary from the French Society of Anaesthesia and Intensive Care Medicine 2017 convention. Anaesth Crit Care Pain Med. 2018;37:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Sellevold OF, Stenseth R. [Non-cardiac surgery in patients with cardiac disease]. Tidsskr Nor Laegeforen. 2010;130:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Sear JW, Higham H. Issues in the perioperative management of the elderly patient with cardiovascular disease. Drugs Aging. 2002;19:429-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Feringa HH, Bax JJ, Poldermans D. Perioperative medical management of ischemic heart disease in patients undergoing noncardiac surgery. Curr Opin Anaesthesiol. 2007;20:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Gal J, Bogar L, Acsady G, Kertai MD. Cardiac risk reduction in non-cardiac surgery: the role of anaesthesia and monitoring techniques. Eur J Anaesthesiol. 2006;23:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Farhan M, Hoda MQ, Ullah H. Prevention of hypotension associated with the induction dose of propofol: A randomized controlled trial comparing equipotent doses of phenylephrine and ephedrine. J Anaesthesiol Clin Pharmacol. 2015;31:526-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Pensado A, Molins N, Alvarez J. [Cardiovascular effects of a single dose of propofol in coronary patients with good ventricular function]. Rev Esp Anestesiol Reanim. 1994;41:147-151. [PubMed] |
| 14. | Galletly DC, Short TG. Total intravenous anaesthesia using propofol infusion--50 consecutive cases. Anaesth Intensive Care. 1988;16:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Yang HS, Song BG, Kim JY, Kim SN, Kim TY. Impact of propofol anesthesia induction on cardiac function in low-risk patients as measured by intraoperative Doppler tissue imaging. J Am Soc Echocardiogr. 2013;26:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Wutzler A, De Asmundis C, Matsuda H, Bannehr M, Loehr L, Voelk K, Jungmann J, Huemer M, Attanasio P, Parwani A, Boldt LH, Brugada P, Haverkamp W. Effects of propofol on ventricular repolarization and incidence of malignant arrhythmias in adults. J Electrocardiol. 2018;51:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Baumert JH, Hein M, Hecker KE, Satlow S, Neef P, Rossaint R. Xenon or propofol anaesthesia for patients at cardiovascular risk in non-cardiac surgery. Br J Anaesth. 2008;100:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Stephan H, Sonntag H, Schenk HD, Kettler D, Khambatta HJ. Effects of propofol on cardiovascular dynamics, myocardial blood flow and myocardial metabolism in patients with coronary artery disease. Br J Anaesth. 1986;58:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 107] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Lischke V, Probst S, Behne M, Kessler P. [ST segment changes in the ECG. Anesthesia induction with propofol, etomidate or midazolam in patients with coronary heart disease]. Anaesthesist. 1993;42:435-440. [PubMed] |
| 20. | Haessler R, Madler C, Klasing S, Schwender D, Peter K. Propofol/fentanyl versus etomidate/fentanyl for the induction of anesthesia in patients with aortic insufficiency and coronary artery disease. J Cardiothorac Vasc Anesth. 1992;6:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Singh R, Choudhury M, Kapoor PM, Kiran U. A randomized trial of anesthetic induction agents in patients with coronary artery disease and left ventricular dysfunction. Ann Card Anaesth. 2010;13:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Andropoulos DB, Stayer SA, Skjonsby BS, East DL, McKenzie ED, Fraser CD. Anesthetic and perioperative outcome of teenagers and adults with congenital heart disease. J Cardiothorac Vasc Anesth. 2002;16:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Aggarwal S, Goyal VK, Chaturvedi SK, Mathur V, Baj B, Kumar A. A comparative study between propofol and etomidate in patients under general anesthesia. Braz J Anesthesiol. 2016;66:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Kaushal RP, Vatal A, Pathak R. Effect of etomidate and propofol induction on hemodynamic and endocrine response in patients undergoing coronary artery bypass grafting/mitral valve and aortic valve replacement surgery on cardiopulmonary bypass. Ann Card Anaesth. 2015;18:172-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Cherfan AJ, Arabi YM, Al-Dorzi HM, Kenny LP. Advantages and disadvantages of etomidate use for intubation of patients with sepsis. Pharmacotherapy. 2012;32:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Komatsu R, You J, Mascha EJ, Sessler DI, Kasuya Y, Turan A. Anesthetic induction with etomidate, rather than propofol, is associated with increased 30-day mortality and cardiovascular morbidity after noncardiac surgery. Anesth Analg. 2013;117:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Albert SG, Ariyan S, Rather A. The effect of etomidate on adrenal function in critical illness: a systematic review. Intensive Care Med. 2011;37:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Basciani RM, Rindlisbacher A, Begert E, Brander L, Jakob SM, Etter R, Carrel T, Eberle B. Anaesthetic induction with etomidate in cardiac surgery: A randomised controlled trial. Eur J Anaesthesiol. 2016;33:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Romito B, Stone J, Ning N, Yin C, Llano EM, Liu J, Somanath K, Lee CT, Matchett G. How Drug Shortages Affect Clinical Care: The Case of the Surgical Anesthetic Propofol. Hosp Pharm. 2015;50:798-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Biccard BM, Devereaux PJ, Rodseth RN. Cardiac biomarkers in the prediction of risk in the non-cardiac surgery setting. Anaesthesia. 2014;69:484-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators, Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, Sigamani A, Srinathan S, Biccard BM, Chow CK, Abraham V, Tiboni M, Pettit S, Szczeklik W, Lurati Buse G, Botto F, Guyatt G, Heels-Ansdell D, Sessler DI, Thorlund K, Garg AX, Mrkobrada M, Thomas S, Rodseth RN, Pearse RM, Thabane L, McQueen MJ, VanHelder T, Bhandari M, Bosch J, Kurz A, Polanczyk C, Malaga G, Nagele P, Le Manach Y, Leuwer M, Yusuf S. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 745] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 32. | Devereaux PJ, Xavier D, Pogue J, Guyatt G, Sigamani A, Garutti I, Leslie K, Rao-Melacini P, Chrolavicius S, Yang H, Macdonald C, Avezum A, Lanthier L, Hu W, Yusuf S; POISE (PeriOperative ISchemic Evaluation) Investigators. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 461] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 33. | Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons; Thygesen K; Alpert JS; White HD; Biomarker Subcommittee; Jaffe AS; Katus HA; Apple FS; Lindahl B; Morrow DA; ECG Subcommittee; Chaitman BR; Clemmensen PM; Johanson P; Hod H; Imaging Subcommittee; Underwood R; Bax JJ; Bonow JJ; Pinto F; Gibbons RJ; Classification Subcommittee; Fox KA; Atar D; Newby LK; Galvani M; Hamm CW; Intervention Subcommittee; Uretsky BF; Steg PG; Wijns W; Bassand JP; Menasche P; Ravkilde J; Trials & Registries Subcommittee; Ohman EM; Antman EM; Wallentin LC; Armstrong PW; Simoons ML; Trials & Registries Subcommittee; Januzzi JL; Nieminen MS; Gheorghiade M; Filippatos G; Trials & Registries Subcommittee; Luepker RV; Fortmann SP; Rosamond WD; Levy D; Wood D; Trials & Registries Subcommittee; Smith SC; Hu D; Lopez-Sendon JL; Robertson RM; Weaver D; Tendera M; Bove AA; Parkhomenko AN; Vasilieva EJ; Mendis S; ESC Committee for Practice Guidelines (CPG); Bax JJ; Baumgartner H; Ceconi C; Dean V; Deaton C; Fagard R; Funck-Brentano C; Hasdai D; Hoes A; Kirchhof P; Knuuti J; Kolh P; McDonagh T; Moulin C; Popescu BA; Reiner Z; Sechtem U; Sirnes PA; Tendera M; Torbicki A; Vahanian A; Windecker S; Document Reviewers; Morais J; Aguiar C; Almahmeed W; Arnar DO; Barili F; Bloch KD; Bolger AF; Botker HE; Bozkurt B; Bugiardini R; Cannon C; de Lemos J; Eberli FR; Escobar E; Hlatky M; James S; Kern KB; Moliterno DJ; Mueller C; Neskovic AN; Pieske BM; Schulman SP; Storey RF; Taubert KA; Vranckx P; Wagner DR. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2313] [Article Influence: 177.9] [Reference Citation Analysis (0)] |