Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1175
Peer-review started: October 30, 2020
First decision: November 20, 2020
Revised: December 4, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: February 16, 2021
Processing time: 92 Days and 6.9 Hours
Conventional therapies for primary plasma cell leukemia (pPCL) are usually ineffective, with a short remission time with the use of multiple myeloma medications, showing aggressiveness of pPCL. B-cell lymphoma-2 inhibitor venetoclax is usually used for relapsed/refractory multiple myeloma (RRMM) with t(11;14). There are very few studies published on the use of venetoclax in pPCL without t(11;14). Similarly, histone deacetylase inhibitors are considered effective for the treatment of RRMM, but there are no reports on their use in pPCL.
A 57-year-old woman with severe anemia, thrombocytopenia, multiple bone destruction, impaired renal function, and 42.7% of peripheral plasma cells is reported. After multiple chemotherapy regimens and chimeric antigen receptor T-cell treatment, the disease progressed again. The patient had very good partial response and was maintained for a long time on venetoclax in combination with chidamide and dexamethasone therapy.
The success of venetoclax-chidamide-dexamethasone combination therapy in achieving a very good partial response suggested that it can be used for refractory/relapsed pPCL patients who have been exhausted with the use of various drug combinations and had poor survival outcomes.
Core Tip: Primary plasma cell leukemia is a rare and high mortality disease. We herein described a case report of relapsed/refractory primary plasma cell leukemia without t(11;14) who achieved a very good partial response from venetoclax therapy in combination with chidamide and dexamethasone.
- Citation: Yang Y, Fu LJ, Chen CM, Hu MW. Venetoclax in combination with chidamide and dexamethasone in relapsed/refractory primary plasma cell leukemia without t(11;14): A case report. World J Clin Cases 2021; 9(5): 1175-1183
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1175.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1175
Plasma cell leukemia (PCL) is a rare and highly aggressive form of plasma cell dyscrasia and is characterized by the presence of more than 20% and/or more than 2.0 × 109/L of circulating plasma cells (PCs) in the peripheral blood. PCL is classified as primary (p)PCL when the leukemic phase is present at the time of diagnosis and as secondary (s)PCL when it is previously diagnosed with multiple myeloma (MM). At present, pPCL accounts for approximately 50% of PCL cases[1]. Patients with pPCL are generally younger than those with sPCL (median age 55 years vs 66 years). The characteristic pattern of PCL revealed PCL and MM as different diseases, not only clinically but also genetically[2]. Immunoglobulin H translocations in t(11;14) were observed in pPCL (33%-63%), where it predicts its sensibility to B-cell lymphoma-2 (BCL-2) inhibitor[3]. The prognosis of pPCL remains usually very poor, and the median overall survival is reported to be less than 1 year[4]. Both BCL-2 inhibitor and histone deacetylase inhibitors (HDACIs) have curative effects on relapsed/refractory multiple myeloma but are limited when used in pPCL. We herein reported a combination regimen of venetoclax-daratumumab-chidamide that led to an unexpected rapid and very deep hematologic remission in a relapsed pPCL patient. To our knowledge, this is the first case report in a pPCL patient without translocation t(11;14).
Pain in the lumbar spine and fatigue.
A 57-year-old woman with a 1 mo history of waist pain and fatigue visited the Orthopedics Department. Examination of peripheral blood revealed white blood cell count of 22.6 × 109/µL with 49% PCs, hemoglobin of 3.2 mg/dL, and a platelet count of 37000/µL. She was then transferred to our department.
The patient had rib fracture caused by trauma and anemia before 7 mo.
No data were available.
Physical examination revealed severe anemia and tenderness of the middle and lower sternum. Local tenderness at 2-3 lumbar vertebrae, and leg elevation test showed negative for both lower limbs.
The patient at the time of joining the hospital had renal function damage (serum creatinine 188.9 µmol/L) and hypercalcemia (serum calcium 2.81 mmol/L). Serum and urine immunofixation electrophoresis revealed only lambda light chain monoclonal antibodies, and the serum lambda light chain levels were found to be significantly increased (2.80 g/L; normal range, 0.90-2.10 g/L). β-2 microglobulin levels were also found to be increased (12.84 mg/L; normal range, 1.00-3.00 mg/L).
A computed tomography scan showed multiple destruction of bones (thoracic spine, lumbar spine, ribs, etc.).
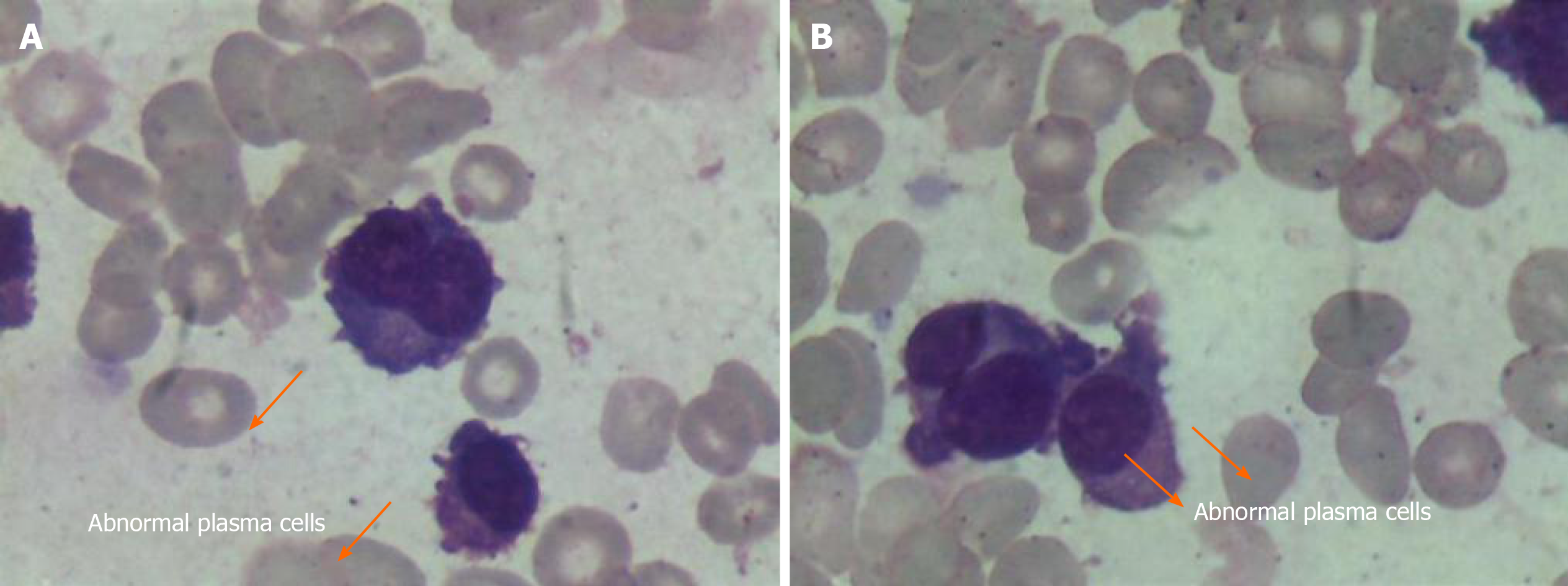
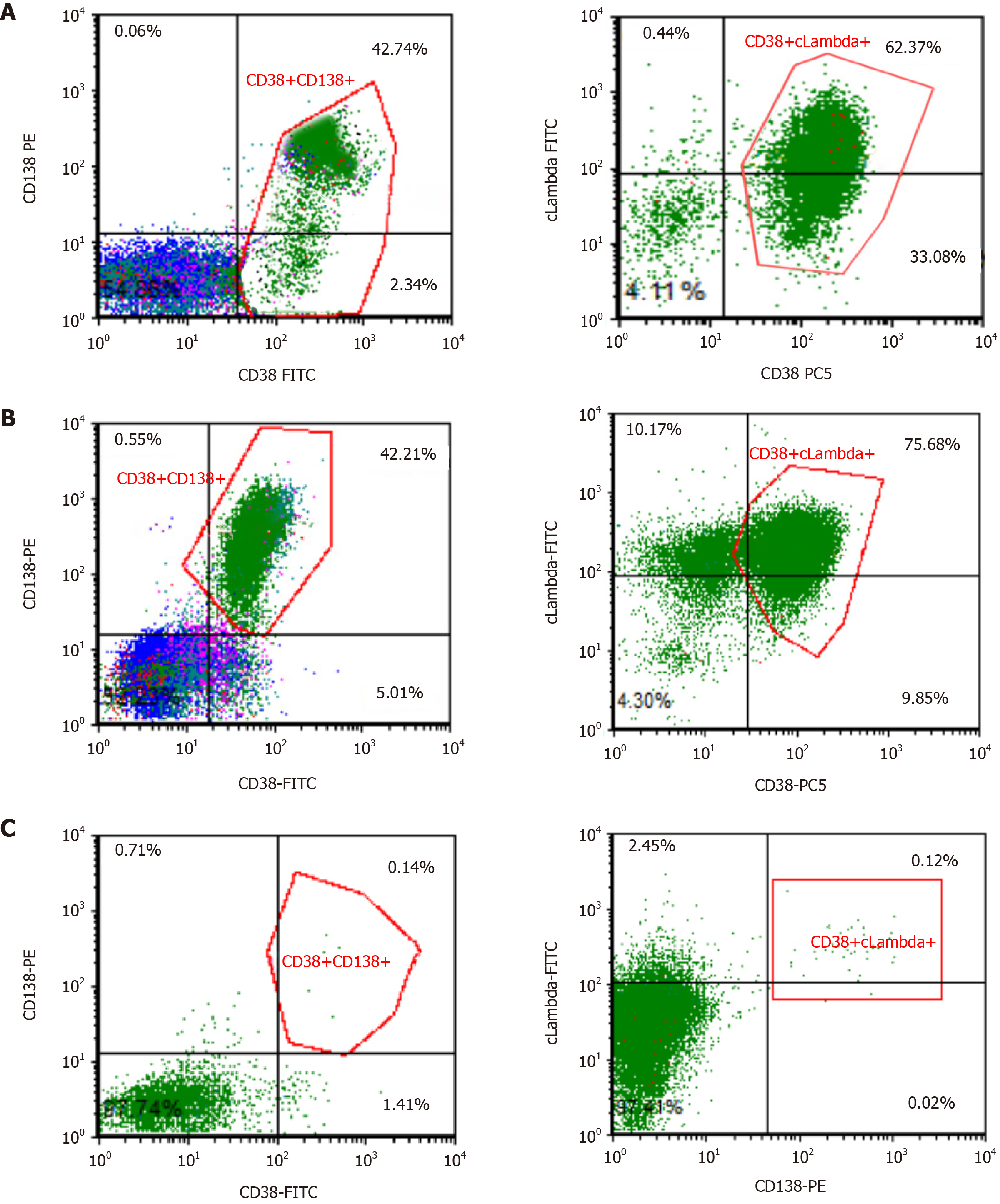
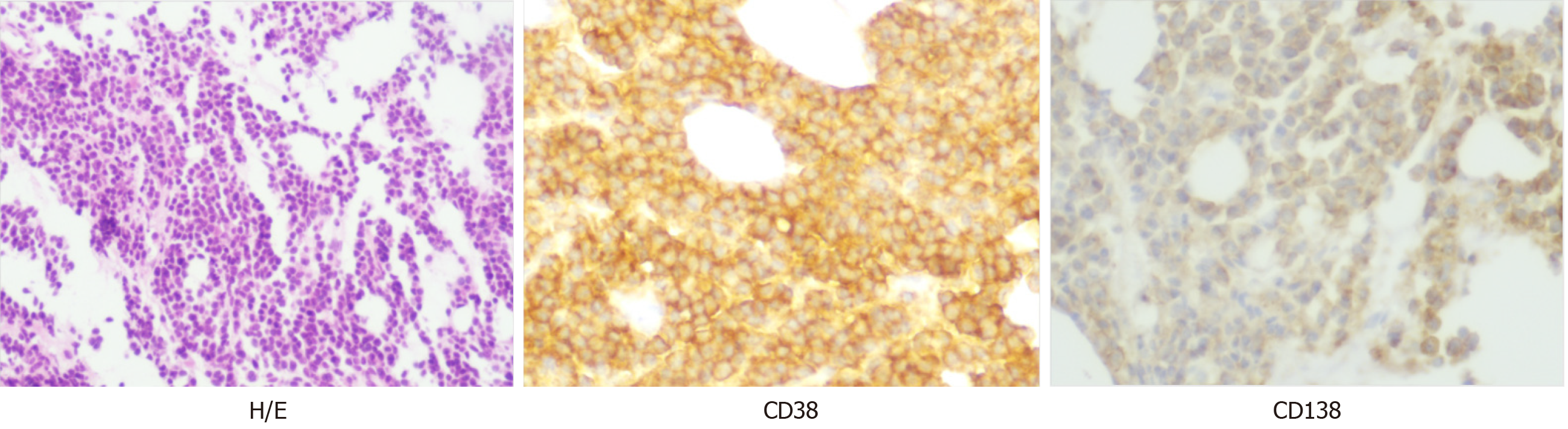
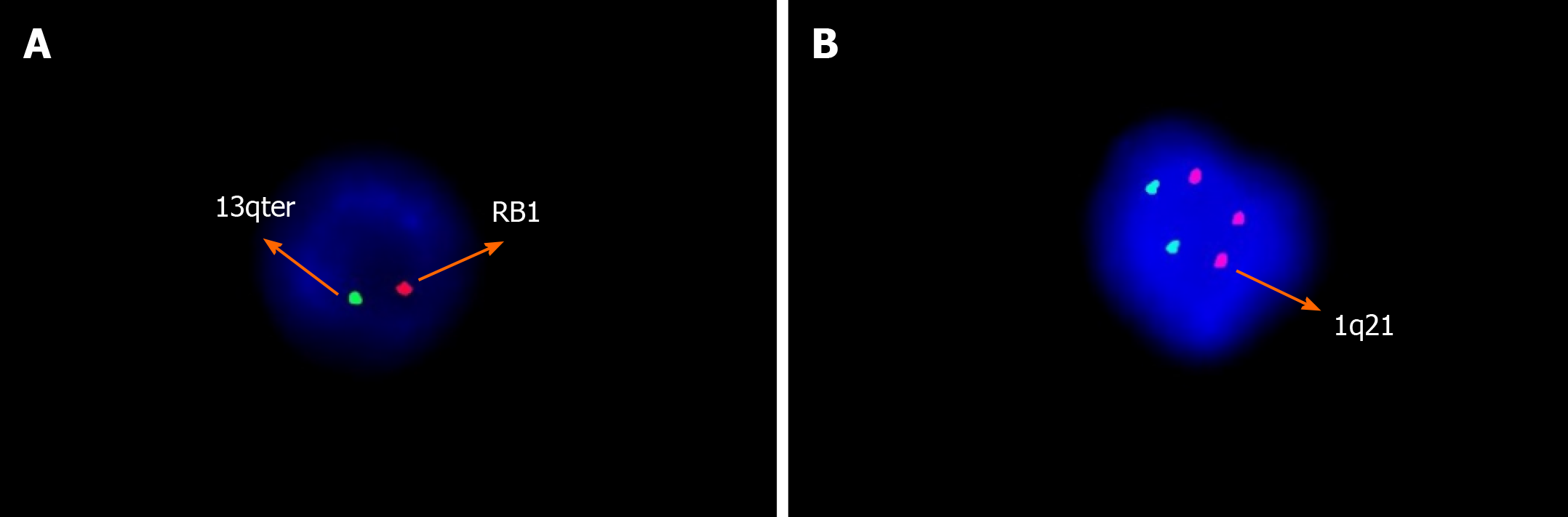
Peripheral smear showed 49% PCs in June 2015 (Figure 1). Flow cytometry (FCM) analysis confirmed that 42.7% of PCs in the peripheral blood were positive for CD38, CD138, CD56, and cytoplasmic immunoglobulin λ (Figure 2A). Bone marrow analysis also revealed that the immature-like PCs in the smear were increased to 70.5%, which was consistent with that in the peripheral blood through FCM analysis. Pathological analysis of bone marrow biopsy revealed that the morphology of abnormal PCs was similar to that of bone marrow images, and immunohistochemistry results revealed that CD38 and CD138 were positive (Figure 3). No analyzable cleavage phase was seen in conventional chromosomes. Fluorescence in situ hybridization (FISH) analysis revealed deletion of 13q14 and amplification of 1q21, but the frequency of p17 deletion and t(11;14) were within the normal range (Figure 4A and B). Therefore, the patient was diagnosed with pPCL (λ light chain type).
Primary plasma cell leukemia.
The patient was initially treated with bortezomib, thalidomide, cyclophosphamide, and dexamethasone for two cycles, and then thalidomide was changed to lenalidomide after two cycles. After treatment, the patient achieved very good partial response (VGPR) by normalizing thrombocytopenia and anemia, and achieving near resolution of her bone marrow plasmacytosis (0.8% of monoclonal PCs) and persistent positive serum immunofixation after four cycles. Autologous hemopoietic stem cell transplantation was performed, but the collection of stem cells failed. The patient refused to undergo allogeneic hematopoietic stem cell transplantation. Bortezomib, lenalidomide, cyclophosphamide, and dexamethasone (VRCD) was continued, and her condition was evaluated as complete response with negative serum immunofixation after six cycles of chemotherapy, but her FISH test was shown to be negative after eight cycles of chemotherapy. The patient’s treatment-related side effects were incomplete intestinal obstruction and mild diarrhea. A total of 10 cycles of chemotherapy were completed, and the regimens of bortezomib, lenalidomide, dexamethasone/lenalidomide, and dexamethasone (VRD/RD) were given as alternate maintenance treatment. Considering the side effects of bortezomib, VRD was used every 3 mo.
After maintenance treatment for nearly 7 mo, she experienced a biochemical relapse, wherein the bone marrow was infiltrated by 2.84% of PCs. She underwent salvage therapy with four cycles of VRCD again, and her curative effect was evaluated as stable disease. In July 2017, her treatment was switched to isazomib, lenalidomide, cyclophosphamide, and dexamethasone (IRCD), achieving a complete response with serological and urine of negative immunofixation and less than 0.01% of monoclonal PCs in the bone marrow. IRCD was given as the main treatment program for 10 cycles, but the disease progressed again. She was then immediately given B cell maturation antigen and CD269 chimeric antigen receptor T-cell immunotherapy (CAR-T) in July 2018, and the remission lasted for more than 1 year after treatment. After more than 1 year of CAR-T treatment, minor residual disease showed progressive increase.
In October 2019, she started using daratumumab, lenalidomide, and dexamethasone chemotherapy. After 6 wk, her platelet count was shown to be rapidly declined, the residual disease level as assessed by FCM was 48.29%, and her FISH test remained the same as that when the disease was first diagnosed, indicating that the disease was still in the progressive stage. Selinexor [exportin 1 (XPO1) inhibitor]/dexamethasone regimen was then given, and the regimen was rechecked after two cycles, but the treatment regimen failed to achieve control, with approximately 42.2% monoclonal PCs in her peripheral blood immunophenotyping (Figure 2B). So, the regimen was considered invalid. Because there was limited access to carfilzomi or pomalimidone in China, salvage immunotherapy including venetoclax in combination with chidaniline and dexamethasone (chidaniline 20 mg twice a week, venetoclax 300 mg/d, dexamethasone 20 mg once a week) was given for 28 d (i.e. for one cycle). Reassessment after two cycles revealed progressive decline in her peripheral PCs, the bone marrow had 0.5% of PCs, and bone marrow FCM identified 0.1% of clonal PCs (Figure 2C). A summary report of clinical and treatment assessments are presented in Table 1.
| Regimen | Duration of therapy (mo range) | Best response | Reason for stopping |
| Bortezomib/thalidomide/cyclophosphamide/dexamethasone (VTCD) | 2 (Jun-Aug 2015) | PR | PR |
| Bortezomib/lenalidomide/cyclophosphamide/dexamethasone (VRCD) | 9 (Aug 2015-May 2016) | CR | Started maintenance |
| Lenalidomide/dexamethasone (RD) Bortezomib/lenalidomide/dexamethasone (VRD) alternate maintenance | 10 (May 2016-Mar 2017) | CR | PD |
| Bortezomib/lenalidomide/cyclophosphamide/dexamethasone (VRCD) | 4 (Mar 2017-Jul 2017) | SD | SD |
| Isazomib/lenalidomide/cyclophosphamide/dexamethasone (IRCD) | 11 (Jul 2017-Jun 2018) | CR | PD |
| BCMA CAR-T | 15 (Jul 2018-Oct 2019) | CR | PD |
| Daratumumab/lenalidomide/dexamethasone (DRD) | 2 (Oct 2019-Jan 2020) | PD | PD |
| Selinexor/dexamethasone | 2 (Jan 2020-Mar 2020) | SD | SD |
| Chidaniline/venetoclax/dexamethasone | 7 (Mar 2020-present) | VGPR | VGPR |
The patient experienced the deepest response of VGPR after four cycles. Currently, the patient is still receiving triplet therapy with chidaniline twice a week and dexamethasone once a week and venetoclax daily for 7 mo.
Primary PCL is one of the most aggressive leukemias. When compared to MM patients, more cases of pPCL have deletion of 1p, 6q, 13q, 16q, and 17p and a significant gain of chromosome 1q than MM, showing poor prognosis[5]. FISH analysis in our patient showed deletion of 13q14 and amplification of 1q21, suggesting a poor prognosis. First-line induction treatment for pPCL combines immuno-regulatory drugs[6] (thalidomide and lenalidomide) and proteasome inhibitors (bortezomib and carfilzomib)[7] as well as anthracyclines or alkylating agents. Allogeneic stem cell transplantation[8] or autologous stem cell transplantation[9] further improved patient survival. However, several studies have reported that many pPCL patients die within a few months after diagnosis. Due to the exceptionally poor prognosis of patients with pPCL, novel combination therapies are urgently needed.
The frequency of t(11;14) in pPCL is higher than that of newly diagnosed MM. The translocation of t(11;14) is related to high expression of BCL-2[10]. Venetoclax (ABT199) inhibited antiapoptotic BCL-2 protein, resulting in tumor cell apoptosis[11]. Venetoclax achieved successful treatment of relapsed/refractory MM in phase I-III clinical trials[12-14]. Thus, a venetoclax based regimen could be a standard approach for treating pPCL patients with translocation t(11;14) abnormality[15,16]. Although venetoclax is particularly effective in patients with t(11;14), the drug was also shown to be effective in patients without such genetic changes[12]. Venetoclax treatment has never been tested or reported in pPCL without t(11;14). Our patient had more than two prior therapy failures and acquired resistance to proteasome inhibitors and immunomodu-lators. Although CAR-T has a short remission, treatment with dacetuzumab and XPO1 inhibitors (Selinexor) was shown to be ineffective, and triple therapy of venetoclax-chidamide-dexamethasone was started as independent therapy with t(11;14). According to recent discontinuation of BELLINI phase 3 trial (M14-031), venetoclax showed a higher proportion of deaths due to infection when compared with the control group[14]. Therefore, the Food and Drug Administration has temporarily suspended the evaluation of venetoclax in clinical trials for MM research treatment. This is because our patient had pancytopenia before treatment, and venetoclax at a low dose (300 mg/d) was taken continuously.
HDACIs are one of the most promising therapeutic drugs used for the treatment of many types of cancers. In the pathogenesis of MM, the imbalance of histone acetylation plays a vital role[17]. The overall remission rate of HDACI-based program in clinical trials of MM is 42%-61%[18], and a promising targeted therapy for MM treatment was shown to be HDACIs. Panobinostat is a non-selective HDACIs approved by the Food and Drug Administration in 2015. It can be used in combination with bortezomib and dexamethasone for the treatment of refractory/relapsed MM[19]. Panobinostat’s anti-myeloma activity alters the gene expression through epigenetic modification and inhibition of protein metabolism. In the MM cell line, panobinostat and venetoclax are used in combination to enhance anti-myeloma activity. This synergistic effect might be attributed to the activation of intrinsic apoptosis and the inhibition of mammalian target of rapamycin signaling pathway[20]. However, panobinostat is shown to cause many adverse events, especially diarrhea, nausea, fatigue, and hematological toxicity. To reduce the adverse events associated with pan-HDACI, selective HDACIs with higher efficacy and lower toxicity might act as promising drugs for the treatment of MM[21]. Chidamide is a new type of benzamide HDACI that can selectively inhibit the activity of class I HDACIs. In 2014, chidamide has been approved for the treatment of relapsed/refractory peripheral T-cell lymphoma by the China Food and Drug Administration. Many studies have shown that chidamide has anti-tumor effects in a variety of hematological malignancies (such as lymphoma, myeloma, and leukemia)[22]. Many studies have confirmed the anti-myeloma effect of chidamide, and it mainly promotes the G0/G1 arrest and apoptosis of G0/G1 in a caspase-dependent manner in myeloma cells[23]. We herein reported the results of a highly successful treatment for the diagnosis of pPCL 5 years ago. The patient received six prior lines of therapy, including all available treatment drugs (such as thalidomide, lenalidomide, ixazomib, daratumumab, XPO1 inhibitor, and CAR-T). We described a 62-year-old female patient who took oral chidamide 30 mg twice weekly in combination with venetoclax 300 mg daily and dexamethasone 20 mg weekly for the treatment of relapsed/refractory pPCL. The patient achieved VGPR in four courses of treatment. At present, the original maintenance treatment is still continued for 7 mo. For the first time in this field, the efficacy of chidamide in combination with venetoclax for the treatment of pPCL has been explored and tried to provide more options for treatment of this disease. We believe that the combination of venetoclax-chidamide-dexamethasone has the ability to salvage high risk, multi-refractory in patients without t(11;14).
The combination treatment of venetoclax-chidamide-dexamethasone has achieved successful results for refractory/relapsed pPCL. This is also the first case report that described the use of BCL-2 inhibitors in combination with HDACIs in a patient with refractory/relapsed sPCL. This case report showed that the triplet was well tolerated, and even lower doses of venetoclax might lead to a deeper response (VGPR) for several months, as shown by bone marrow cytology, FCM, and immunosolid phase electrophoresis. Based on the successful results obtained, it is necessary to conduct further clinical studies to explore the combination of venetoclax and chidamide therapy for the treatment of pPCL.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Singer J S-Editor: Chen XF L-Editor: Filipodia P-Editor: Li JH
| 1. | Fernández de Larrea C, Kyle RA, Durie BG, Ludwig H, Usmani S, Vesole DH, Hajek R, San Miguel JF, Sezer O, Sonneveld P, Kumar SK, Mahindra A, Comenzo R, Palumbo A, Mazumber A, Anderson KC, Richardson PG, Badros AZ, Caers J, Cavo M, LeLeu X, Dimopoulos MA, Chim CS, Schots R, Noeul A, Fantl D, Mellqvist UH, Landgren O, Chanan-Khan A, Moreau P, Fonseca R, Merlini G, Lahuerta JJ, Bladé J, Orlowski RZ, Shah JJ; International Myeloma Working Group. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27:780-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 259] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 2. | Musto P, Statuto T, Valvano L, Grieco V, Nozza F, Vona G, Bochicchio GB, La Rocca F, D'Auria F. An update on biology, diagnosis and treatment of primary plasma cell leukemia. Expert Rev Hematol. 2019;12:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Mihalyova J, Jelinek T, Growkova K, Hrdinka M, Simicek M, Hajek R. Venetoclax: A new wave in hematooncology. Exp Hematol. 2018;61:10-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Gonsalves WI, Rajkumar SV, Go RS, Dispenzieri A, Gupta V, Singh PP, Buadi FK, Lacy MQ, Kapoor P, Dingli D, Lust JA, Zeldenrust SR, Hayman SR, Kyle RA, Gertz MA, Kumar SK. Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood. 2014;124:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Plunkett GA, West VC. Systemic joint laxity and mandibular range of movement. Cranio. 1988;6:320-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Musto P, Simeon V, Martorelli MC, Petrucci MT, Cascavilla N, Di Raimondo F, Caravita T, Morabito F, Offidani M, Olivieri A, Benevolo G, Mina R, Guariglia R, D'Arena G, Mansueto G, Filardi N, Nobile F, Levi A, Falcone A, Cavalli M, Pietrantuono G, Villani O, Bringhen S, Omedè P, Lerose R, Agnelli L, Todoerti K, Neri A, Boccadoro M, Palumbo A. Lenalidomide and low-dose dexamethasone for newly diagnosed primary plasma cell leukemia. Leukemia. 2014;28:222-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Katodritou E, Terpos E, Delimpasi S, Kotsopoulou M, Michalis E, Vadikolia C, Kyrtsonis MC, Symeonidis A, Giannakoulas N, Vadikolia C, Michael M, Kalpadakis C, Gougopoulou T, Prokopiou C, Kaiafa G, Christoulas D, Gavriatopoulou M, Giannopoulou E, Labropoulou V, Verrou E, Kastritis E, Konstantinidou P, Anagnostopoulos A, Dimopoulos MA. Real-world data on prognosis and outcome of primary plasma cell leukemia in the era of novel agents: a multicenter national study by the Greek Myeloma Study Group. Blood Cancer J. 2018;8:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Royer B, Minvielle S, Diouf M, Roussel M, Karlin L, Hulin C, Arnulf B, Macro M, Cailleres S, Brion A, Brechignac S, Belhadj K, Chretien ML, Wetterwald M, Chaleteix C, Tiab M, Leleu X, Frenzel L, Garderet L, Choquet S, Fuzibet JG, Dauriac C, Forneker LM, Benboubker L, Facon T, Moreau P, Avet-Loiseau H, Marolleau JP. Bortezomib, Doxorubicin, Cyclophosphamide, Dexamethasone Induction Followed by Stem Cell Transplantation for Primary Plasma Cell Leukemia: A Prospective Phase II Study of the Intergroupe Francophone du Myélome. J Clin Oncol. 2016;34:2125-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Jurczyszyn A, Radocha J, Davila J, Fiala MA, Gozzetti A, Grząśko N, Robak P, Hus I, Waszczuk-Gajda A, Guzicka-Kazimierczak R, Atilla E, Mele G, Sawicki W, Jayabalan DS, Charliński G, Szabo AG, Hajek R, Delforge M, Kopacz A, Fantl D, Waage A, Avivi I, Rodzaj M, Leleu X, Richez V, Knopińska-Posłuszny W, Masternak A, Yee AJ, Barchnicka A, Druzd-Sitek A, Guerrero-Garcia T, Liu J, Vesole DH, Castillo JJ. Prognostic indicators in primary plasma cell leukaemia: a multicentre retrospective study of 117 patients. Br J Haematol. 2018;180:831-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Kumar S, Kaufman JL, Gasparetto C, Mikhael J, Vij R, Pegourie B, Benboubker L, Facon T, Amiot M, Moreau P, Punnoose EA, Alzate S, Dunbar M, Xu T, Agarwal SK, Enschede SH, Leverson JD, Ross JA, Maciag PC, Verdugo M, Touzeau C. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130:2401-2409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 379] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 11. | Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25:27-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 401] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 12. | Moreau P, Chanan-Khan A, Roberts AW, Agarwal AB, Facon T, Kumar S, Touzeau C, Punnoose EA, Cordero J, Munasinghe W, Jia J, Salem AH, Freise KJ, Leverson JD, Enschede SH, Ross JA, Maciag PC, Verdugo M, Harrison SJ. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130:2392-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 215] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 13. | Costa LJ, Stadtmauer EA, Morgan G, Monohan G, Kumar SK. Phase 2 study of venetoclax plus carflizomib and dexamethasone in patients with relapsed/refractory multiple myeloma. Blood. 2018;132:303. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Kumar S, Harrison SJ, Cavo M, Rubia JD, Popat R, Gasparetto CJ, Hungria V, Salwender HJ, Suzuki K, Kim I, Punnoose E, Hong WJ, Freise KJ, Sood A, Jalaluddin M, Ross J, Ward JE, Maciag P, Moreau P. A phase 3 study of venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma. Clin Lymphoma, Myeloma Leuk. 2019;19:e31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Jelinek T, Mihalyova J, Kascak M, Duras J, Popkova T, Benkova K, Richterova P, Plonkova H, Zuchnicka J, Broskevicova L, Huvarova L, Cerna L, Growkova K, Simicek M, Havel M, Gumulec J, Navratil M, Koristek Z, Paiva B, Hajek R. Single-agent venetoclax induces MRD-negative response in relapsed primary plasma cell leukemia with t(11;14). Am J Hematol. 2019;94:E35-E37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Gonsalves WI, Buadi FK, Kumar SK. Combination therapy incorporating Bcl-2 inhibition with Venetoclax for the treatment of refractory primary plasma cell leukemia with t (11;14). Eur J Haematol. 2018;100:215-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Mithraprabhu S, Kalff A, Chow A, Khong T, Spencer A. Dysregulated Class I histone deacetylases are indicators of poor prognosis in multiple myeloma. Epigenetics. 2014;9:1511-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Raje NS, Bensinger W, Cole CE, Lonial S, Jagannath S, Arce-Lara CE, Valent J, Rosko AE, Harb WA, Sandhu I, Bahlis NJ, Reece D, Terpos E, Supko J, Tamang D, Jones SS, Wheeler C, Markelewicz, Jr. RJ, Richardson PG. Ricolinostat (ACY-1215), the first selective HDAC6 inhibitor, combines safely with pomalidomide and dexamethasone and shows promosing early results in relapsed- and- refractory myeloma (ACE-MM-102 Study). Blood. 2015;126:4228. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A, Jedrzejczak WW, Günther A, Nakorn TN, Siritanaratkul N, Corradini P, Chuncharunee S, Lee JJ, Schlossman RL, Shelekhova T, Yong K, Tan D, Numbenjapon T, Cavenagh JD, Hou J, LeBlanc R, Nahi H, Qiu L, Salwender H, Pulini S, Moreau P, Warzocha K, White D, Bladé J, Chen W, de la Rubia J, Gimsing P, Lonial S, Kaufman JL, Ocio EM, Veskovski L, Sohn SK, Wang MC, Lee JH, Einsele H, Sopala M, Corrado C, Bengoudifa BR, Binlich F, Richardson PG. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 607] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 20. | Valdez BC, Li Y, Murray D, Liu Y, Nieto Y, Bashir Q, Qazilbash MH, Andersson BS. Panobinostat and venetoclax enhance the cytotoxicity of gemcitabine, busulfan, and melphalan in multiple myeloma cells. Exp Hematol. 2020;81:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Redic KA, Hough SM, Price EM. Clinical developments in the treatment of relapsed or relapsed and refractory multiple myeloma: impact of panobinostat, the first-in-class histone deacetylase inhibitor. Onco Targets Ther. 2016;9:2783-2793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Xu L, Tang HL, Gong X, Xin XL, Dong Y, Gao GX, Shu MM, Chen XQ. [Inducing effect of chidamide on apoptosis of multiple myeloma cells and its relerance to DNA damage response]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015;23:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Yuan XG, Huang YR, Yu T, Jiang HW, Xu Y, Zhao XY. Chidamide, a histone deacetylase inhibitor, induces growth arrest and apoptosis in multiple myeloma cells in a caspase-dependent manner. Oncol Lett. 2019;18:411-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |