Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1119
Peer-review started: September 10, 2020
First decision: November 23, 2020
Revised: December 3, 2020
Accepted: January 7, 2021
Article in press: January 7, 2021
Published online: February 16, 2021
Processing time: 142 Days and 3.8 Hours
Adrenal incidentaloma (AI) has been frequently encountered in the clinical setting. It has been shown that primary aldosteronism (PA) or subclinical Cushing’s syndrome (SCS) are the representative causative diseases of AI. However, the coexistence of PA and SCS has been reportedly observed. Recently, we encountered a case of AI, in which PA and SCS coexisted, confirmed by histopathological examinations after a laparoscopic adrenalectomy. We believe that there were some clinical implications in the diagnosis of the present case.
A 58-year-old man presented with lower right abdominal pain with a blood pressure of 170/100 mmHg. A subsequent computed tomography scan revealed right ureterolithiasis, which was the cause of right abdominal pain, and right AI measuring 22 mm × 25 mm. After the disappearance of right abdominal pain, subsequent endocrinological examinations were performed. Aldosterone-related evaluations, including adrenal venous sampling, revealed the presence of bilateral PA. In addition, several cortisol-related evaluations showed the presence of SCS on the right adrenal adenoma. A laparoscopic right adrenalectomy was then performed. The histopathological examination of the resected right adrenal revealed the presence of a cortisol-producing adenoma, while CYP11B2 immunoreactivity was absent in this adenoma. However, in the adjacent non-neoplastic adrenal, multiple CYP11B2-positive adrenocortical micronodules were detected, showing the presence of aldosterone-producing adrenocortical micronodules.
Careful clinical and pathological examination should be performed when a patient harboring AI presents with concomitant SCS and PA.
Core Tip: Adrenal incidentaloma (AI) has been frequently encountered in the clinical setting. It has been shown that primary aldosteronism (PA) or subclinical Cushing’s syndrome (SCS) are the representative causative diseases of AI. However, the coexistence of PA and SCS has been reportedly observed. In the present case report, we describe a case of AI, in which PA and SCS coexisted after endocrinologic examinations, which was ultimately confirmed by histopathological examinations after a laparoscopic adrenalectomy. Careful clinical examinations should be performed when patients harboring AI present with concomitant SCS and PA.
- Citation: Teragawa H, Oshita C, Orita Y, Hashimoto K, Nakayama H, Yamazaki Y, Sasano H. Primary aldosteronism due to bilateral micronodular hyperplasia and concomitant subclinical Cushing’s syndrome: A case report. World J Clin Cases 2021; 9(5): 1119-1126
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1119.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1119
Adrenal incidentaloma (AI) has been frequently encountered in the daily clinical practice. The concomitant clinical presentation of subclinical Cushing’s syndrome (SCS) and/or primary aldosteronism (PA) in patients with AI is not necessarily rare. However, the rate of those patients among AI cases also depends on the lesion’s laterality and size, the presence of hypertension, and gender[1-4]. Conversely, among hypertensive patients, the prevalence of PA was recently reported to be relatively higher, in the range of 5%–10%[5,6]. Among those patients described above, the coexistence of SCS and PA has also been reported to range from 11.3% to 53.8%[7-10], and careful clinical evaluation is required to identify those patients harboring SCS and PA. We recently encountered a case with concomitant manifestation of bilateral PA and SCS in the right adrenal gland confirmed by detailed histopathological examination. In view of these findings, this case should provide clinically important information regarding the approach to AI patients harboring these two different endocrine symptoms.
A 58-year-old man visited our outpatient clinic in September 2018 because of a sudden development of lower right abdominal pain, which occurred one hour previously.
He reported lower right abdominal pain that had continued for one hour at the same degree and in the same portion.
He had no prior contributory medical history, although no health checkups had been performed.
He had no obvious family history of the disease.
The results of his physical examination were as follows: height, 1.75 m; weight, 79.4 kg; body mass index, 25.9 kg/m2; blood pressure, 170/100 mmHg (systolic/diastolic); and pulse rate, 76 beats/min. No cardiac murmur nor abnormal respiratory sounds were noted, and he complained of knocking pain in his right back. There was no tenderness on his abdomen, with normal body shape and no particular dermal findings.
Routine laboratory examination revealed a reduced estimated glomerular filtration rate (45.8 mL/min/1.73 m2) with normal potassium levels (4.5 mEq/L). The patient’s fasting blood glucose level was 87 mg/dL, with hemoglobin A1C of 6.0%.
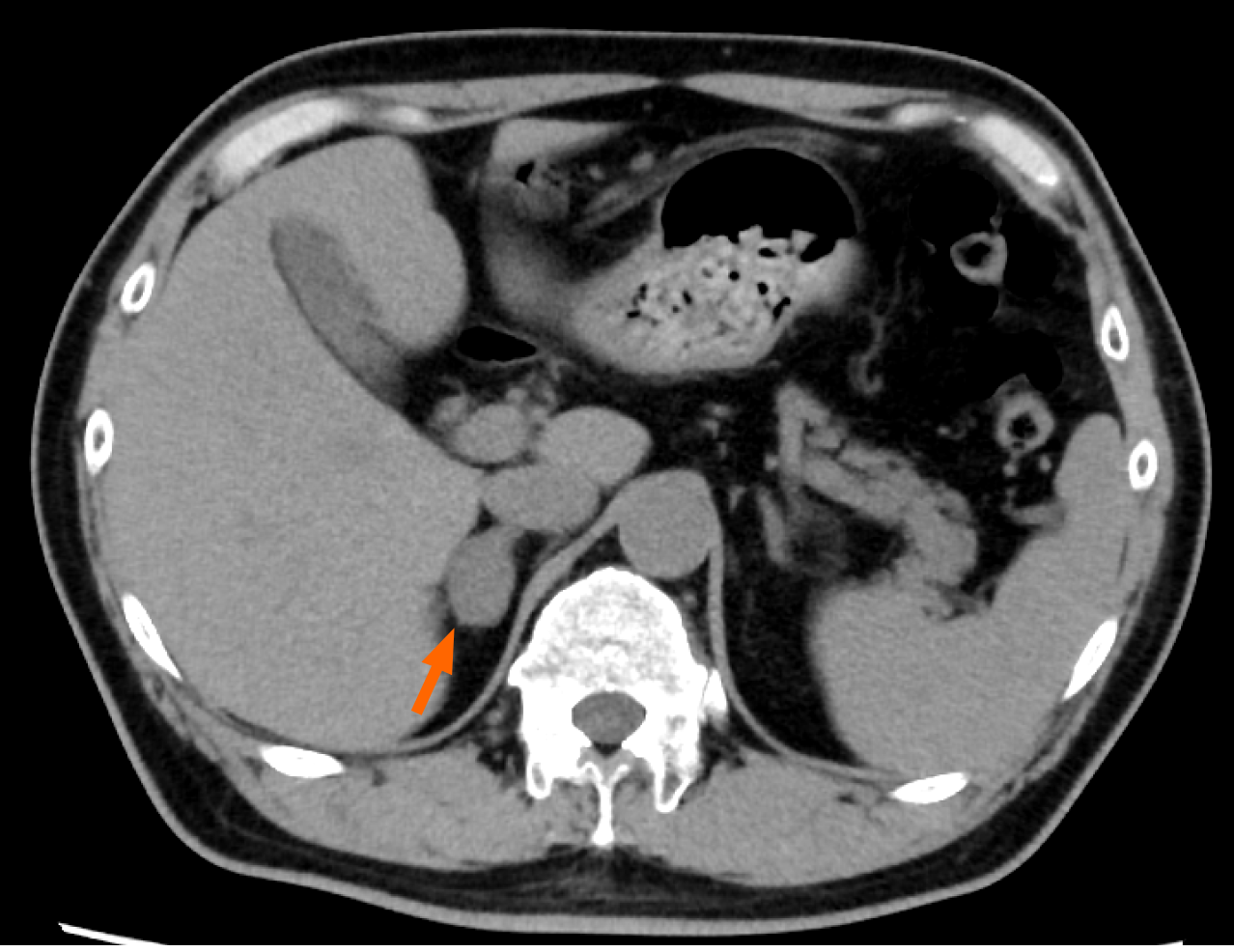
An electrocardiogram demonstrated regular sinus rhythm without any evidence of left ventricular hypertrophy (LVH) and an echocardiography showed normal left ventricular wall motion and no indications for LVH. Subsequent computed tomography (CT) performed for exploration of his abdominal pain revealed the presence of a right ureterolithiasis, which could account for his symptoms, and a right adrenal adenoma measuring 22 mm × 25 mm (Figure 1).
The patient was subsequently treated with analgesic drugs to control his pain due to the right ureterolithiasis, and the stones were excreted after a few weeks.
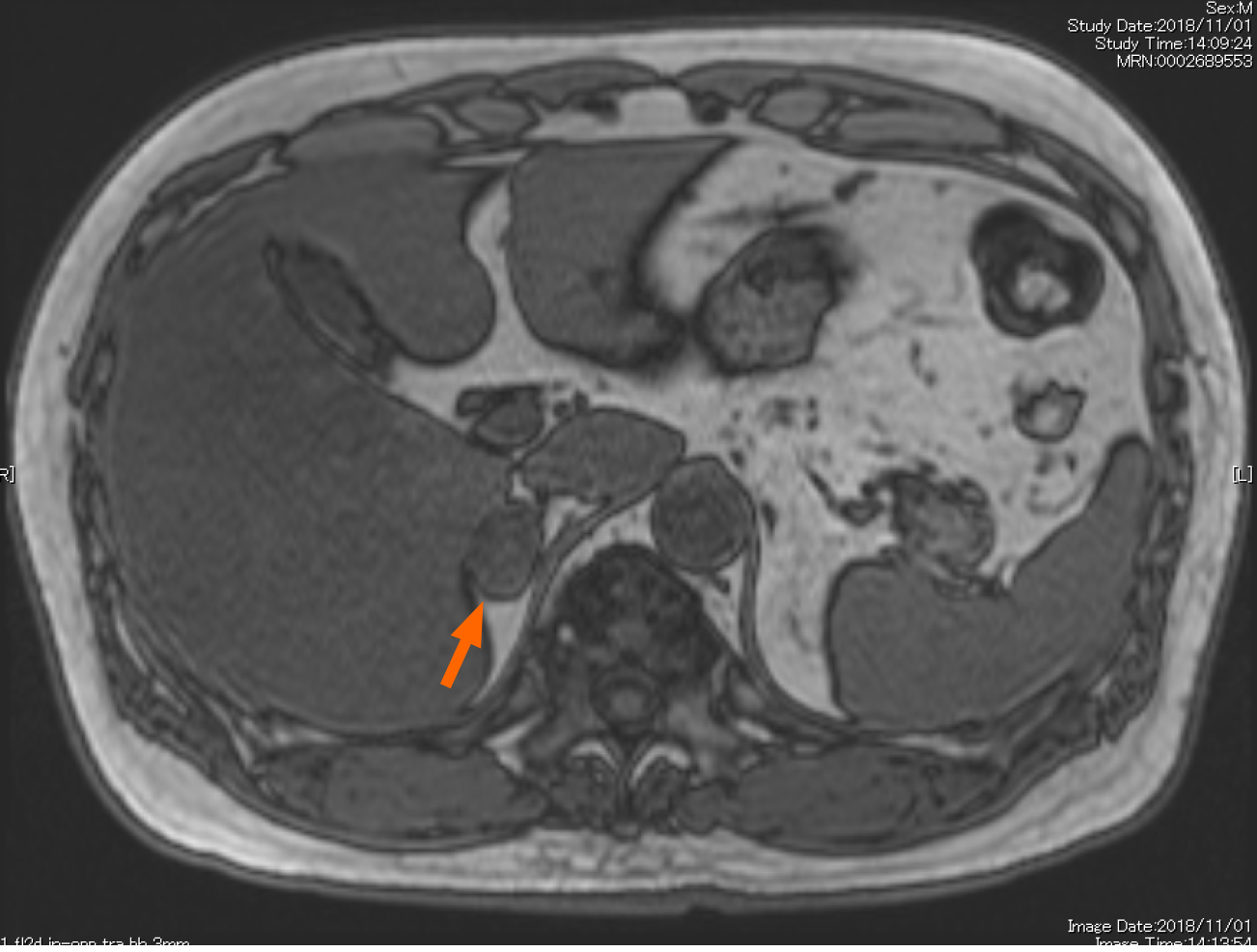
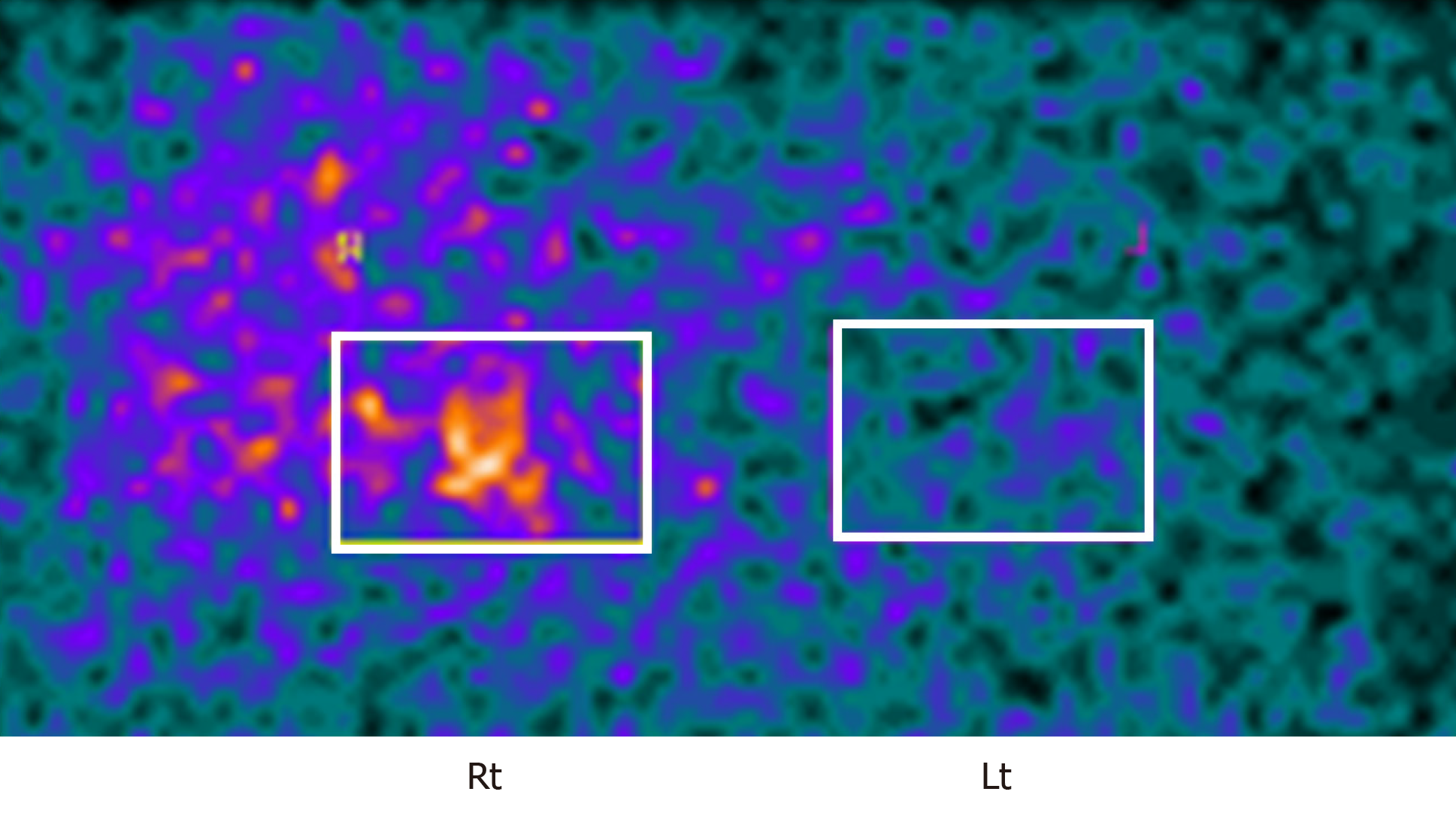
While his hypertension had been treated with a calcium channel blocker with amlodipine (10 mg), detailed examination on his right AI was also performed for the possible presence of pheochromocytoma, PA, and SCS. The urinary metanephrines were within the normal range. Magnetic resonance imaging revealed the presence of a right adrenal tumor (Figure 2), as detected in CT. The salt loading test was positive, with a plasma aldosterone concentration (PAC) of 83 pg/mL, consistent with PA. However, the captopril challenge test was negative, and the aldosterone/renin ratio (ARR) after the challenge was in the range of 192.5 (< 200). The subsequent adrenal venous sampling (AVS) a revealed bilateral increase of plasma aldosterone levels following intravenous infusion of adrenocorticotrophic hormone (ACTH) (aldosterone: 15251 pg/mL in the right adrenal vein, 14.271 pg/mL in the left adrenal vein; cortisol: 618.1 µg/dL in the right adrenal vein, 530.5 µg/dL in the left adrenal vein, and a lateralized ratio 1.09), with a higher increase in the aldosterone level in the left adrenal gland before ACTH infusion (Table 1). Based on these findings, we ultimately diagnosed this patient as bilateral PA. In addition, dexamethasone administrations (1 or 8 mg) did not suppress the production of cortisol (cortisol: 4.5 µg/dL in 1 mg dexamethasone; 3.3 µg/dL in 8 mg dexamethasone). ACTH and cortisol concentrations in the early morning were 2.4 pg/mL and 5.9 µg/dL, respectively. Conversely, the concentration of cortisol at midnight was 4.6 µg/dL. These findings all indicated the presence of diurnal variation in cortisol patterns. In addition, an increased ACTH concentration was noted in the corticotropin-releasing hormone (CRH) challenge test. Adrenal scintigraphy revealed an increased uptake in the right adrenal gland with its decreased uptake in the left adrenal gland (Figure 3).
| At baseline | Right adrenal vein | Left adrenal vein | Inferior vena cava |
| Aldosterone (pg/mL) | 971 | 1562 | 99 |
| Cortisol (µg/dL) | 75.9 | 35.6 | 6.3 |
| Aldosterone/Cortisol | 1.27 | 4.39 | |
| Laterized ratio (L/R) | 3.43 | ||
| After ACTH infusion | Right adrenal vein | Left adrenal vein | Inferior vena cava |
| Aldosterone (pg/mL) | 15251 | 14271 | 166 |
| Cortisol (µg/dL) | 618.1 | 530.5 | 16.1 |
| Aldosterone/Cortisol | 2.47 | 2.69 | |
| Laterized ratio (L/R) | 1.09 | ||
These findings above all indicated the presence of SCS in this patient. He was therefore diagnosed with concomitant bilateral PA and right SCS.
His treatment included the administration of the combination of three antihypertensive drugs, including amlodipine (10 mg), eplerenone (50 mg), and bisoprolol (5 mg). A laparoscopic right adrenalectomy was subsequently performed in February 2019 to remove the source of cortisol excess.
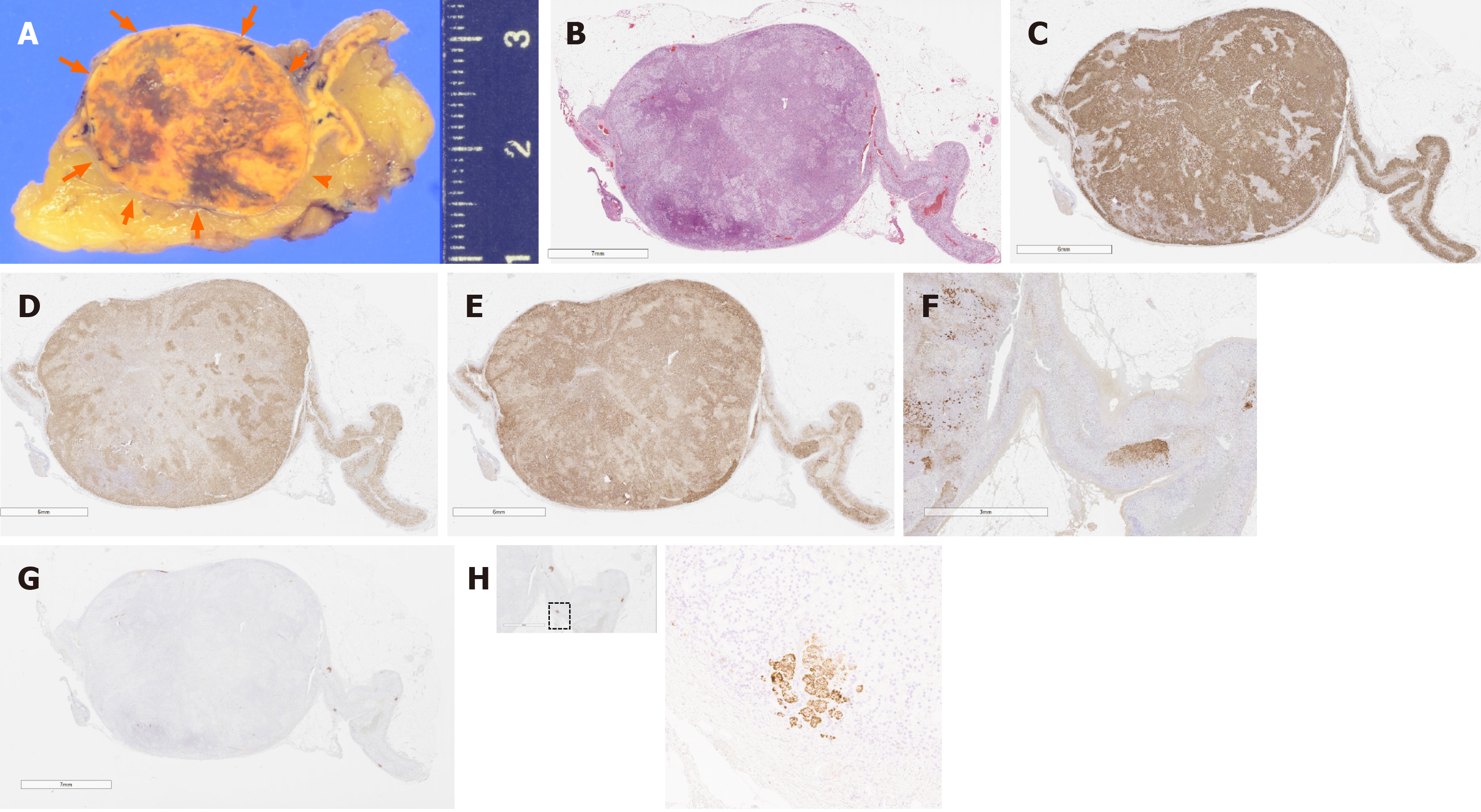
An adrenal tumor measuring 2.5 cm × 2.3 cm × 2.0 cm (Figure 4A) was detected in the resected right adrenal gland. The cut surface of the tumor appeared heterogenous, admixed with yellow and brown areas. The tumor was histologically diagnosed as adrenocortical adenoma according to the criteria of Weiss[11] (Figure 4B). Subsequent immunohistochemical examination revealed that the tumor cells were positive for HSD3B2, CYP17A, and CYP11B1 (Figure 4C-E), consistent with the finding of cortisol-producing adrenocortical adenoma. The zona reticularis of the adjacent adrenal cortex was atrophic, with decreased DHEA-ST immunoreactivity (Figure 4F). CYP11B2 immunoreactivity was completely absent in this adenoma (Figure 4G), but in the adjacent non-neoplastic adrenal tissue, multiple CYP11B2-positive adrenocortical micronodules or cell clusters were detected at the subcapcular area, consistent with the histological finding of “Multiple adrenocortical micronodules” (Figure 4H). Therefore, these aldosterone-producing adrenocortical micronodules or cell clusters were histopathologically diagnosed as the responsible lesion of PA in this patient.
The clinical control of his hypertension was achieved with two antihypertensive drugs, including amlodipine (5 mg) and eplerenone (50 mg). The hormonal examinations indicated the following: PAC, 374 pg/mL; renin activity, 3.5; ARR, 107; and cortisol, 12.4 µg/dL.
We report a case with concomitant bilateral PA and cortisol-producing right adrenocortical adenoma resulting in SCS. The inferences drawn from the present case are as follows: (1) PA and SCS can coexist in AI, and it is important to determine whether those two endocrinopathies could be due to a tumor; and (2) The detailed pathological evaluation revealed different sources of aldosterone and cortisol excess: the former in micronodules or clusters and the latter in an adenoma, which should highlight the importance of careful examination of the resected adrenal gland in this particular case.
The frequency of the concomitant presence of SCS and PA has been reported to be in the range of 11.3%-53.8%[7-10], which may depend upon various factors pertaining to the studied population[12], degree of hypertension[13], or institutions at which the evaluation was conducted[7,14]. Some clinical patient characteristics have been reported in the cases harboring SCS and PA concomitantly[7,10,15], whereas the presence of diabetes mellitus (DM) may be more prevalent in PA patients with SCS compared with those without SCS. Cortisol rather than aldosterone has been considered to influence the onset or development of DM, although the presence of DM was not noted in our case. In addition, Furuta et al[10] reported that the average size was larger in PA patients with SCS (3.0 cm) than in PA patients without SCS (1.5 cm), and the maximum size of the adenoma was 2.5 cm in our case. The presence of DM and/or the larger adenoma sizes quantified with imaging could provide important information regarding the coexistence of PA and SCS, but endocrinological confirmation should be required. The degree and/or duration of increased production of cortisol may contribute to the presence of the clinical characteristics of SCS.
The clinical diagnosis of SCS has been considered difficult in some cases because of the rather stringent clinical criteria of SCS. In this case, the results of normal cortisol concentrations in the early morning, dexamethasone suppression tests (1 mg and 8 mg), suppression of ACTH in the early morning, and findings of adrenal scintigraphy with increased uptake in the diseased side and decreased uptake in the normal side met the SCS criteria. However, it is also true that the cortisol concentration at midnight was relatively low, indicating that the preservation of the diurnal variation of cortisol and the normal response of ACTH in the CRH challenge test did not meet the criteria of SCS. In addition, the supplementation of steroid therapy after adrenalectomy, which constitutes one of the SCS criteria, was not necessarily required in this case. These findings may be attributed to the degree of production of cortisol in the adenoma and the preserved cortisol-producing function in the ipsilateral non-neoplastic adrenal tissue and the adrenal gland on the contralateral side. However, the pathological findings in this case did provide important information regarding the inconsistent clinical findings above, including the ability of the adenoma to produce cortisol and the status of the adjacent non-neoplastic adrenal tissue.
In patients with SCS and PA, careful interpretation of the results of AVS is generally required because the aldosterone/cortisol ratio is reduced on the diseased side and elevated on the normal side. In our present case, the lateralized ratio was increased in the left adrenal gland before the ACTH infusion, although the difference was not marked after the ACTH infusion. These findings are also considered to be attributed to the increased ability of the adenoma to produce cortisol and to the preserved function of the adrenal gland. The localized diagnosis of PA should be carefully interpreted in patients with a coexistence of PA and SCS.
Clinical evaluations regarding the PA in the present case include the following: negative captopril challenge test, positive saline loading test, and bilateral PA on AVS. These findings indicated the potential presence of idiopathic hyperaldosteronism (IHA), which is frequent among cases of PA[16]. However, detailed pathological examinations did reveal that the micronodular hyperplasia detected suggested the bilateral micronodular hyperplasia as a responsive lesion of PA in this case. These detailed pathological examinations, especially the immunohistochemical analysis of steroidogenic enzymes involved in cortisol and aldosterone biosynthesis in the resected adrenal tissue, did provide pivotal information regarding the definitive diagnosis of the adrenocortical lesions producing excessive amounts of two different adrenocortical hormones.
We reported a case of hypertension and right AI with concomitant SCS and bilateral PA based on clinical evaluations. Detailed pathological examination of the resected adrenal tissue did reveal that the patient had a cortisol-producing adrenocortical adenoma and micronodular hyperplasia in the nonneoplastic adrenal tissue or that cortisol and aldosterone were produced in the different adrenocortical lesions. It is important to assess causative diseases carefully in those harboring concomitant PA and SCS in patients with AI.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sperti C S-Editor: Liu M L-Editor: A P-Editor: Xing YX
| 1. | Zeiger MA, Siegelman SS, Hamrahian AH. Medical and surgical evaluation and treatment of adrenal incidentalomas. J Clin Endocrinol Metab. 2011;96:2004-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Patrova J, Jarocka I, Wahrenberg H, Falhammar H. Clinical outcomes in adrenal incidentaloma: experience from one center. Endocr Pract. 2015;21:870-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Cho YY, Suh S, Joung JY, Jeong H, Je D, Yoo H, Park TK, Min YK, Kim KW, Kim JH. Clinical characteristics and follow-up of Korean patients with adrenal incidentalomas. Korean J Intern Med. 2013;28:557-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Tabuchi Y, Otsuki M, Kasayama S, Kosugi K, Hashimoto K, Yamamoto T, Tsugawa M, Mineo I, Yamada Y, Kurebayashi S, Ohashi M, Umayahara Y, Kouhara H, Nakamura T, Taki H, Matsuoka TA, Imagawa A, Funahashi T, Shimomura I. Clinical and endocrinological characteristics of adrenal incidentaloma in Osaka region, Japan. Endocr J. 2016;63:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Käyser SC, Dekkers T, Groenewoud HJ, van der Wilt GJ, Carel Bakx J, van der Wel MC, Hermus AR, Lenders JW, Deinum J. Study Heterogeneity and Estimation of Prevalence of Primary Aldosteronism: A Systematic Review and Meta-Regression Analysis. J Clin Endocrinol Metab. 2016;101:2826-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 6. | Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:1889-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1842] [Article Influence: 204.7] [Reference Citation Analysis (0)] |
| 7. | Adachi J, Hirai Y, Terui K, Nakano T, Fukuda Y, Suda T, Sasano H. A report of 7 cases of adrenal tumors secreting both cortisol and aldosterone. Intern Med. 2003;42:714-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Hiraishi K, Yoshimoto T, Tsuchiya K, Minami I, Doi M, Izumiyama H, Sasano H, Hirata Y. Clinicopathological features of primary aldosteronism associated with subclinical Cushing's syndrome. Endocr J. 2011;58:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Fujimoto K, Honjo S, Tatsuoka H, Hamamoto Y, Kawasaki Y, Matsuoka A, Ikeda H, Wada Y, Sasano H, Koshiyama H. Primary aldosteronism associated with subclinical Cushing syndrome. J Endocrinol Invest. 2013;36:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 10. | Furuta N, Naruoka T, Igarashi T, Bando S, Yamada H, Kimura T, Egawa S. [Clinical Evaluation of Primary Aldosteronism with Subclinical Cushing's Syndrome]. Hinyokika Kiyo. 2015;61:185-190. [PubMed] |
| 11. | Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 573] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, Kurihara I, Umakoshi H, Ichijo T, Katabami T, Wada N, Ogawa Y, Yoshimoto T, Kawashima J, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Miyauchi S, Kamemura K, Fukuoka T, Yamamoto K, Otsuki M, Suzuki T, Naruse M; JPAS Study Group. Obesity as a Key Factor Underlying Idiopathic Hyperaldosteronism. J Clin Endocrinol Metab. 2018;103:4456-4464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Nagasawa M, Yamamoto K, Rakugi H, Takeda M, Akasaka H, Umakoshi H, Tsuiki M, Takeda Y, Kurihara I, Itoh H, Ichijo T, Katabami T, Wada N, Shibayama Y, Yoshimoto T, Ogawa Y, Kawashima J, Sone M, Inagaki N, Takahashi K, Fujita M, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Kamemura K, Otsuki M, Fujii Y, Ogo A, Okamura S, Miyauchi S, Yanase T, Suzuki T, Kawamura T, Naruse M; JPAS Study Group. Influence of antihypertensive drugs in the subtype diagnosis of primary aldosteronism by adrenal venous sampling. J Hypertens. 2019;37:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Fujii Y, Takeda Y, Kurihara I, Itoh H, Katabami T, Ichijo T, Wada N, Shibayama Y, Yoshimoto T, Ogawa Y, Kawashima J, Sone M, Inagaki N, Takahashi K, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Kamemura K, Otsuki M, Yamamto K, Ogo A, Yanase T, Okamura S, Miyauchi S, Fujita M, Suzuki T, Umakoshi H, Ogasawara T, Tsuiki M, Naruse M; JPAS Study Group. Historical changes and between-facility differences in adrenal venous sampling for primary aldosteronism in Japan. J Hum Hypertens. 2020;34:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Akehi Y, Yanase T, Motonaga R, Umakoshi H, Tsuiki M, Takeda Y, Yoneda T, Kurihara I, Itoh H, Katabami T, Ichijo T, Wada N, Shibayama Y, Yoshimoto T, Ashida K, Ogawa Y, Kawashima J, Sone M, Inagaki N, Takahashi K, Fujita M, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Kamemura K, Otsuki M, Fujii Y, Yamamoto K, Ogo A, Okamura S, Miyauchi S, Fukuoka T, Izawa S, Hashimoto S, Yamada M, Yoshikawa Y, Kai T, Suzuki T, Kawamura T, Naruse M; Japan Primary Aldosteronism Study Group. High Prevalence of Diabetes in Patients With Primary Aldosteronism (PA) Associated With Subclinical Hypercortisolism and Prediabetes More Prevalent in Bilateral Than Unilateral PA: A Large, Multicenter Cohort Study in Japan. Diabetes Care. 2019;42:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66:607-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 433] [Article Influence: 24.1] [Reference Citation Analysis (0)] |