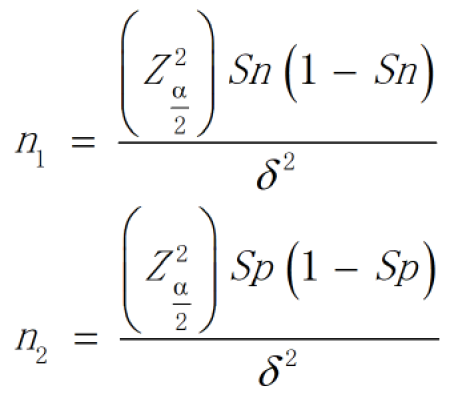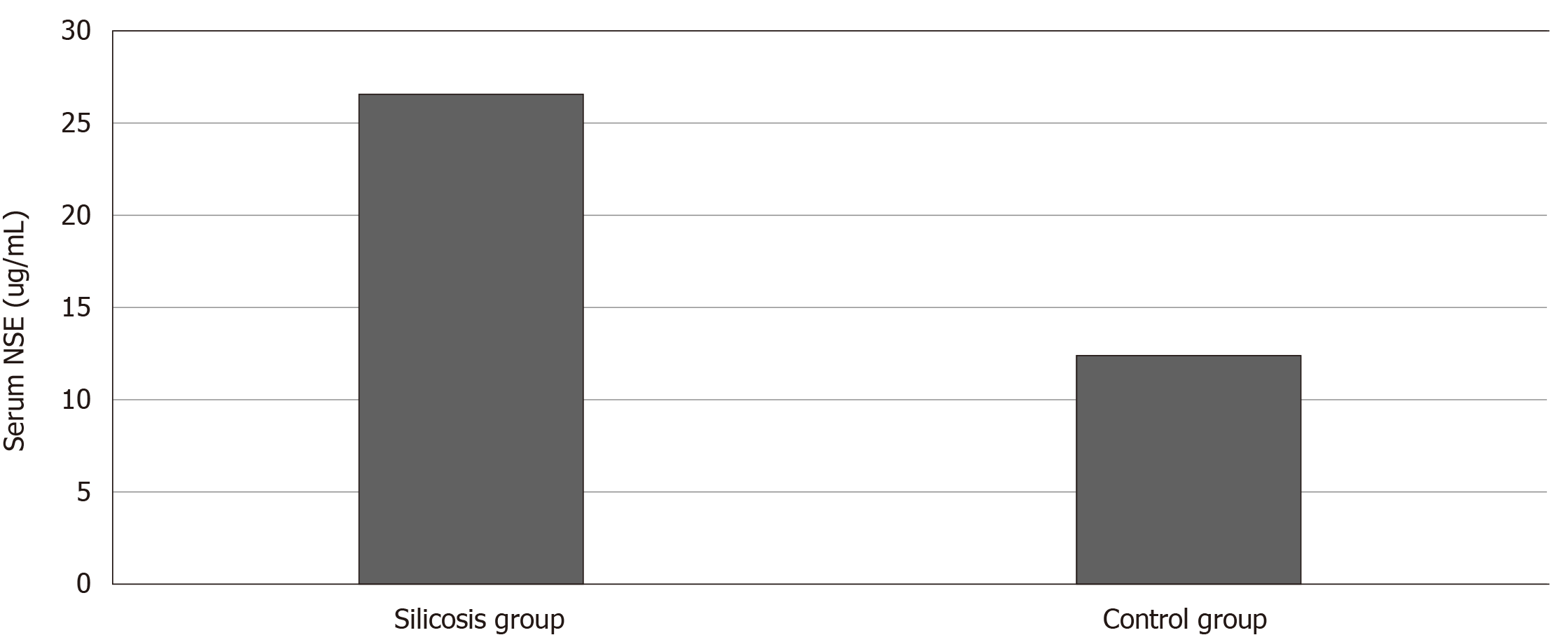Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1016
Peer-review started: November 10, 2020
First decision: November 29, 2020
Revised: December 12, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: February 16, 2021
Processing time: 80 Days and 22.6 Hours
Silicosis is a type of chronic pulmonary fibrosis caused by long-term inhalation of silica dust particles. There has been no ideal biomarker for the diagnosis and differential diagnosis of silicosis until now. Studies have found that elevated neuron-specific enolase (NSE) concentration in the serum of silicosis patients is helpful for diagnosis and severity assessment of the disease. However, the number of cases in these studies was not enough to arouse attention.
To investigate the clinical significance of serum NSE in the diagnosis and staging of silicosis.
From January 2017 to June 2019, 326 cases of silicosis confirmed in Quanzhou First Hospital Affiliated to Fujian Medical University were included in the silicosis group. A total of 328 healthy individuals or medical patients without silicosis were included in the control group. Serum NSE concentrations of all subjects were determined by electrochemical luminescence.
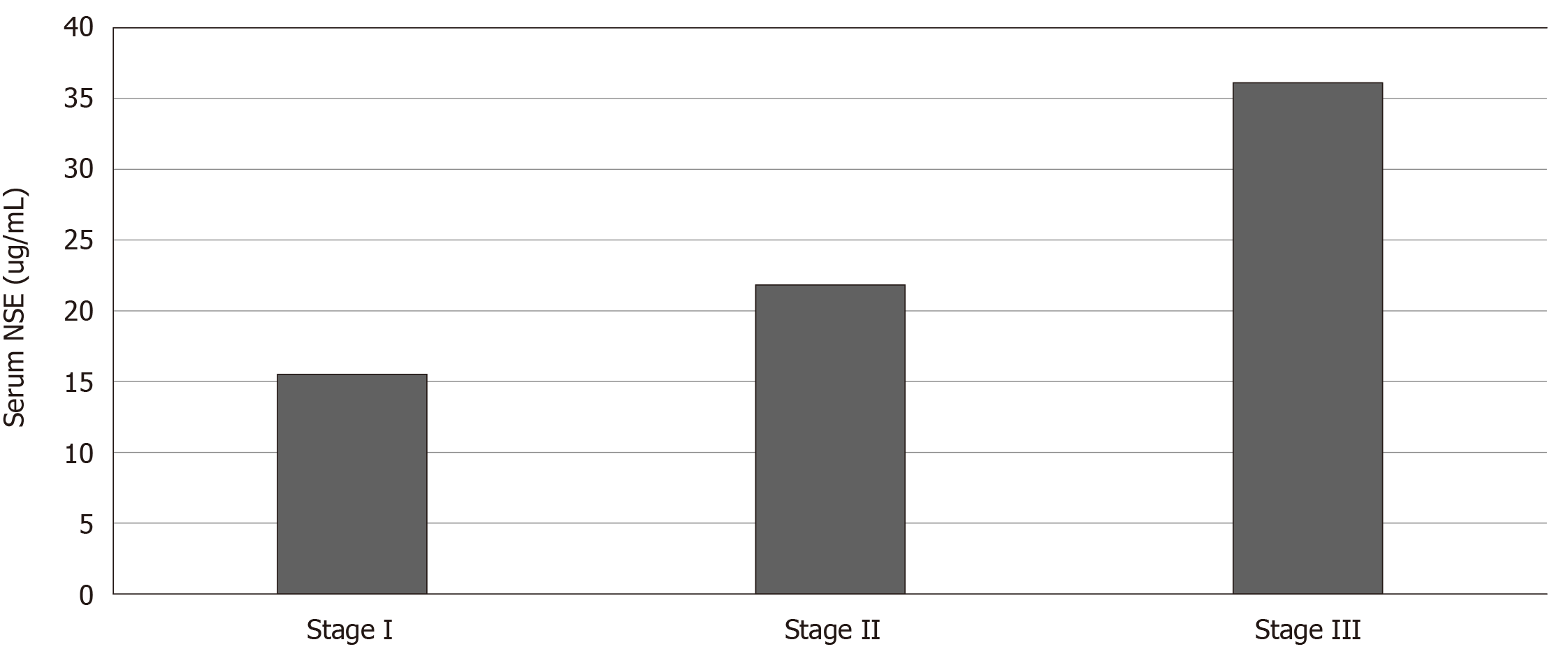
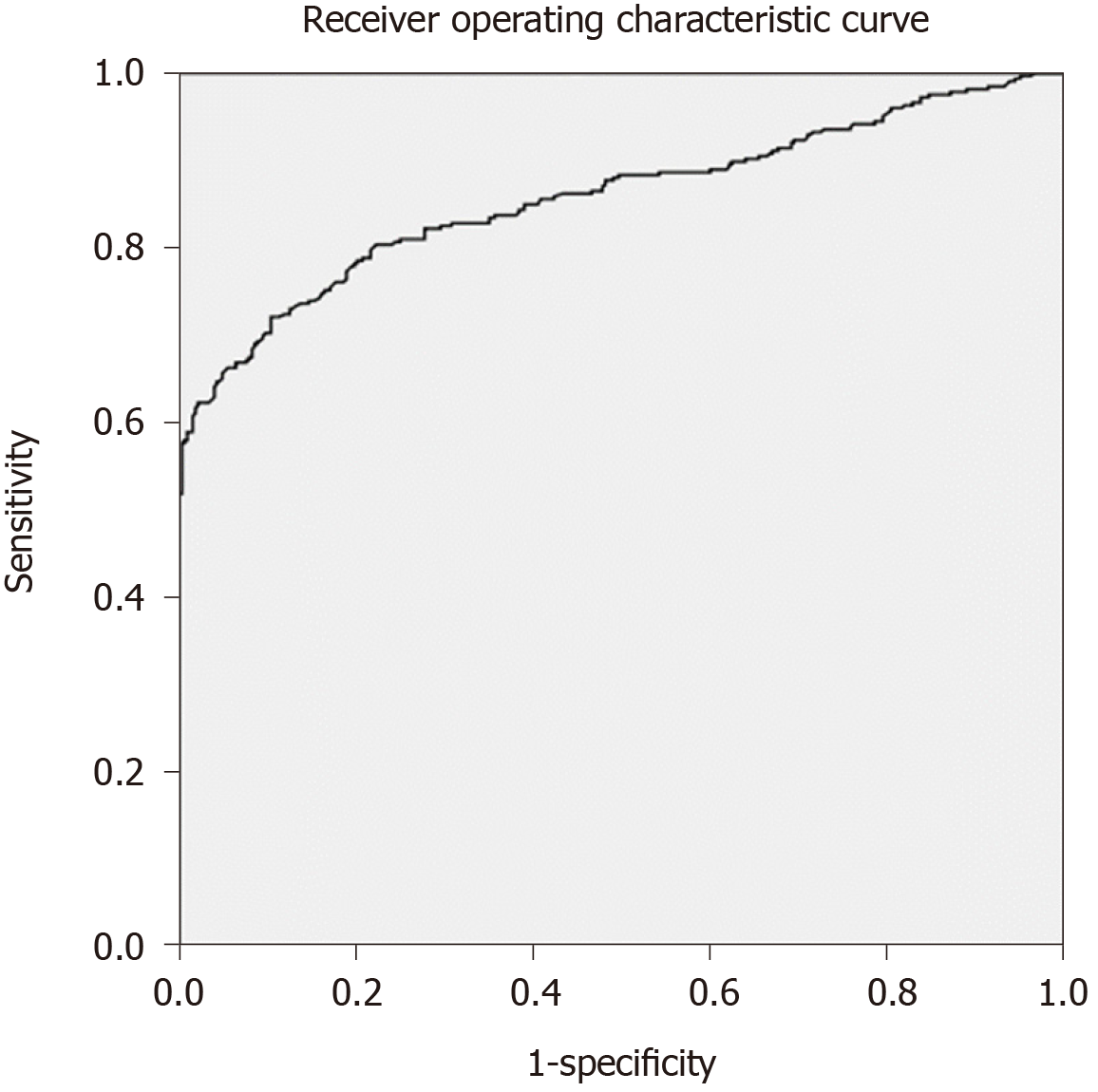
There were no significant differences in sex, age, smoking index and complications between the silicosis and control groups. The mean serum NSE concentration was 26.57 ± 20.95 ng/mL in the silicosis group and 12.42 ± 2.68 ng/mL in the control group. The difference between the two groups was significant (U = 15187, P = 0.000). Among the 326 patients with silicosis, 103 had stage I silicosis, and the mean serum NSE concentration was 15.55 ± 6.23 ng/mL. The mean serum NSE concentration was 21.85 ± 12.05 ng/mL in 70 patients with stage II silicosis. The mean serum NSE concentration was 36.14 ± 25.72 ng/mL in 153 patients with stage III silicosis. Kruskal–Wallis H test suggested that the difference in serum NSE concentration in silicosis patients in the three groups was significant (H = 130.196, P = 0.000). Receiver operating characteristic curve analysis indicated that the area under the curve was 0.858 (95% confidence interval: 0.828-0.888; P = 0.000). When the NSE concentration was 15.82 ng/mL, the Jorden index was the largest, the sensitivity was 72%, and the specificity was 90%.
Serum NSE concentration may be a promising biomarker for the diagnosis and assessment of severity of silicosis.
Core Tip: The purpose of this study was to investigate the clinical significance of serum neuron-specific enolase (NSE) in the diagnosis and staging of silicosis. The results showed that the serum NSE concentration of silicosis patients was significantly higher than that of non-silicosis patients. With the progression of silicosis, NSE concentration increased gradually. The receiver operating characteristic curve suggested that when the diagnostic threshold of NSE was 15.82 ng/mL, the area under the curve was 0.858 (P = 0.000), with sensitivity of 72% and specificity of 90%. To the best of our knowledge, this study is the largest study on silicosis biomarkers to date.
- Citation: Huang HB, Huang JL, Xu XT, Huang KB, Lin YJ, Lin JB, Zhuang XB. Serum neuron-specific enolase: A promising biomarker of silicosis. World J Clin Cases 2021; 9(5): 1016-1025
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1016.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1016
Silicosis is a type of chronic pulmonary fibrosis caused by long-term inhalation of silica dust particles. The diagnosis of silicosis[1] is mainly based on occupational history, symptoms and imaging, among which imaging is the current gold standard. However, in practice, in some cases (unsuitable for chest X-ray during pregnancy, atypical chest X-ray appearance in early stage of disease, and difficulties in differential diagnosis after chest X-ray examination), chest X-ray examination cannot fully meet the clinical needs. Therefore, laboratory tests are needed to assist in differential diagnosis. Unfortunately, there has been no ideal biomarker for the diagnosis and differential diagnosis of silicosis until now.
Neuron-specific enolase (NSE) is one of the isoenzymes of enolase[2], which exists specifically in neurons, neuroendocrine cells and oligoglial cells and is usually not secreted[3]. However, excessive release of NSE into the blood under pathological conditions can lead to elevated serum NSE concentrations. Therefore, serum NSE can be used to evaluate neuronal and neuroendocrine cell damage and provide diagnostic information for neuroendocrine neoplasms[4-8].
Studies have found that elevated NSE concentration in serum of silicosis patients was helpful for diagnosis and severity assessment of the disease[9-11]. However, the number of cases in these studies was not enough to arouse attention. In this study, the sample size was increased and the design was improved to further explore the role of serum NSE concentration in the diagnosis and staging of silicosis.
The silicosis group comprised patients with silicosis confirmed in Quanzhou First Hospital Affiliated to Fujian Medical University (Fujian, China) (outpatients or inpatients) between January 2017 and June 2019. Healthy individuals or internal medicine patients without silicosis (outpatients or inpatients) formed the control group. The study was approved by the Ethics Committee of Quanzhou First Hospital Affiliated to Fujian Medical University, and all participants gave signed informed consent.
Inclusion criteria for the silicosis group were: Definite history of silica dust exposure for 6 mo, silicosis confirmed by chest X-ray[1], and continued follow-up for 6 mo to exclude the possibility of complicated malignant tumor. The exclusion criteria were: Lung malignancies (including non-small-cell lung cancer and small-cell lung cancer), neuroblastoma, previous or present history of other systemic malignancies, acute cerebrovascular disease (including cerebral hemorrhage and cerebral infarction), epilepsy, acute brain trauma; Creutzfeldt–Jakob disease, Alzheimer’s disease and vascular dementia, schizophrenia; Guillain–Barré syndrome, chronic obstructive pulmonary disease, or tuberculosis.
The required sample size was estimated according to the formula for diagnostic tests (Figure 1).
According to relevant literature review[9], the sensitivity was 0.7 and specificity was 0.85, a was 0.05 and d was 0.05. After calculation, n1 and n2 were 323 and 196, respectively. Considering the possibility that the participants may be lost to follow-up, the number of cases should be increased appropriately. In conclusion, the sample size of both the silicosis group and the control group was 330 cases.
All participants were followed up for 6 mo after blood sample collection (outpatient or telephone follow-up) to determine whether silicosis, malignant tumor and other diseases were diagnosed within the last 6 mo.
The silicosis group initially included 330 patients, among whom 4 were excluded due to loss of follow-up data (Table 1). A total of 326 patients were eventually included: 322 males and four females, with an average age of 47.9 ± 9.8 years, and an average silica dust exposure history of 16.9 ± 7.2 years. There were 87 patients who had never smoked, 90 who had quit smoking, and 149 who currently smoked. The median smoking index was 20 pack-years. There were 103 cases of silicosis stage I, 70 of silicosis stage II and 153 of silicosis stage III. There were 7 cases of pulmonary hypertension, 62 of pulmonary infection, 46 of organizing pneumonia eight of bronchiectasis, 17 of pneumothorax, 3 of diabetes, 8 of hypertension, 6 of arrhythmia, and 6 of coronary heart disease. The control group initially included 330 participants, of whom 2 were excluded due to loss of follow-up data. A total of 328 participants were finally included: 324 males and four females, with an average age of 46.4 ± 13.6 years. There were 80 participants who had never smoked, 93 who had quit smoking, and 155 who currently smoked. The median smoking index was 20 pack-years. There were 170 healthy individuals, and 5 participants with pulmonary hypertension, 61 with pulmonary infection, 23 with organizing pneumonia, 8 with bronchiectasis, 5 with pneumothorax, 12 with diabetes, 20 with hypertension, 11 with arrhythmia and 13 with coronary heart disease.
| Characteristics | Silicosis group, n = 326 | Control group, n = 328 |
| Age in yr | 47.9 ± 9.8 | 46.4 ± 13.6 |
| Sex | ||
| Men (%) | 322 (98.8) | 324 (98.8) |
| Women (%) | 4 (1.2) | 4 (1.2) |
| Dust-exposing time, yr | 16.9 ± 7.2 | N/A |
| Smoking status | ||
| Never smoked (%) | 87 (26.7) | 80 (24.4) |
| Former smoker (%) | 90 (27.6) | 93 (28.3) |
| Current smoker (%) | 149 (45.7) | 155 (47.3) |
| Stage of silicosis | ||
| I (%) | 103 (31.6) | N/A |
| II (%) | 70 (21.5) | N/A |
| III (%) | 153 (46.9) | N/A |
| Coexisting conditions | ||
| Pulmonary infection (%) | 7 (2.1) | 5 (1.5) |
| Pulmonary hypertension (%) | 62 (19.0) | 61 (18.6) |
| Organizing pneumonia (%) | 46 (14.1) | 23 (7.0) |
| Bronchiectasis (%) | 8 (2.5) | 8 (2.4) |
| Pneumothorax (%) | 17 (5.2) | 5 (1.5) |
| Diabetes (%) | 3 (0.9) | 12 (3.7) |
| Hypertension (%) | 8 (2.5) | 20 (6.1) |
| Arrhythmia (%) | 6 (1.8) | 11 (3.4) |
| Coronary heart disease (%) | 6 (1.8) | 13 (4.0) |
Venous blood (4 mL) was extracted from all participants in the morning. The blood samples were centrifuged at 1000 g for 6 min and the supernatant was extracted and stored at -80 °C. Serum NSE concentrations were detected by electrochemical luminescence. The detector (Cobas800 automatic immune analyzer) and the kit were purchased from Roche, Switzerland. Each blood sample was tested three times and the average value was taken as the final result. During the test, the relevant personnel were blinded.
Statistic Package for Social Science for Windows version 19.0 was used for statistical analysis. Data with normal distribution are expressed as the mean ± standard deviation, and data with non-normal distribution are expressed as the median. One-way analysis of variance was used for data with homogeneous variance, and the Mann–Whitney U test or Kruskal–Wallis H test was used for data with heterogeneous variance. Numerical data are expressed as the rate or composition ratio. The R × C table χ2 test was used for comparison of numerical data. The area under the curve (AUC) was calculated by drawing the receiver operating characteristic (ROC) curve, and the optimal threshold was selected accordingly. P < 0.05 was considered statistically significant.
The χ2 test showed that there was no significant difference in sex and complications between the silicosis and control groups (χ2 = 0.000, P = 1.000 and χ2 = 0.219, P = 0.640, respectively). The Mann–Whitney U test showed that there was no significant difference in age and smoking index between the two groups (U = 50632, P = 0.241 and U = 50688.5, P = 0.244, respectively).
Serum NSE concentration of the silicosis group was 26.57 ± 20.95 ng/mL, compared with 12.42 ± 2.68 ng/mL in the control group (Figure 2). The Mann–Whitney U test showed that serum NSE concentration in the silicosis group was significantly increased compared with that in the control group, and the difference between the two groups was significant (U = 15187, P = 0.000).
Among the 326 patients with silicosis, 103 with stage I silicosis had an average age of 48.5 ± 10.3 years, average silica dust exposure history of 15.7 ± 7.8 years, average time free from silica dust exposure of 9.5 ± 5.6 years, and average serum NSE concentration of 15.55 ± 6.23 ng/mL (Figure 3). Seventy patients with stage II silicosis had an average age of 48.1 ± 10.7 years, average silica dust exposure history of 17.1 ± 8.4 years, average time free from silica dust exposure of 5.9 ± 4.1 years, and average serum NSE concentration of 21.85 ± 12.05 ng/mL. The average age of 153 patients with stage III silicosis was 47.4 ± 9.0 years, average silica dust exposure history was 17.6 ± 6.0 years, average time free from silica dust exposure was 4.9 ± 3.2 years, and average serum NSE concentration was 36.14 ± 25.72 ng/mL. One-way analysis of variance suggested that there was no significant difference in age among the three groups (F = 0.372, P = 0.690). Kruskal–Wallis H test suggested that serum NSE concentrations of silicosis patients in the three groups were significantly different (H = 130.196, P = 0.000). Pearson correlation analysis indicated that there was no linear correlation between serum NSE concentration and silica dust exposure history (R = -0.009, P = 0.875). There was a negative correlation between serum NSE concentration and time free from silica dust exposure (R = -0.139, P = 0.012).
The AUC was 0.858 (95% confidence interval: 0.828–0.888, P = 0.000) (Figure 4). When the NSE value was 15.82 ng/mL, the Jorden index was the largest, the sensitivity was 72%, and the specificity was 90%.
The purpose of this study was to investigate the role of serum NSE concentration in the diagnosis and staging of silicosis. The results showed that the serum NSE concentration of silicosis patients was significantly higher than that of non-silicosis patients. With the progression of silicosis, NSE concentration increased gradually. The ROC curve suggested that when the diagnostic threshold of NSE was 15.82 ng/mL, the AUC was 0.858, with sensitivity of 72% and specificity of 90%. These results suggest that serum NSE can be used in the auxiliary diagnosis of silicosis, and to some extent, reflects the severity of the disease. To the best of our knowledge, this study is the largest study on silicosis biomarkers so far.
Silicosis is a type of chronic pulmonary fibrosis caused by long-term inhalation of silica dust particles. Silicosis is one of the occupational diseases with the highest number of cases reported annually in China[12]. To date, the diagnosis of silicosis is still mainly based on chest X-ray[1]. This has caused difficulty in clinical practice as: Some patients (such as pregnant women) are not suitable or unwilling to accept X-ray examination; due to the technical limitations of X-ray examination, the early recognition rate for many diseases including silicosis is low; and due to the similarity of imaging manifestations, chest X-ray is often not able to distinguish silicosis from acute pulmonary miliary tuberculosis, mediastinal lymph node tuberculosis, sarcoidosis and other diseases. In these cases, clinicians often hope to obtain further evidence through laboratory tests to assist in diagnosis and differential diagnosis. Therefore, it is particularly important to study the biomarkers related to silicosis.
NSE is one of the isoenzymes of enolase[2], which exists specifically in neurons, neuroendocrine cells and oligoglial cells and is usually not secreted. Excessive release of neuron-specific enolase into the blood under pathological conditions can lead to elevated serum neuron-specific enolase concentrations. Serum neuron-specific enolase can be used to evaluate neuronal and neuroendocrine cell damage[4,5] and provide diagnostic information for neuroendocrine neoplasms[6].
Similar to the gastrointestinal tract, the lung is an endocrine organ. Pulmonary neuroendocrine cells are scattered and distributed in other epithelial tissues[13,14]. Many neuroactive substances have been found involving in a variety of physiological and pathophysiological processes, such as lung growth and development, and respiratory damage repair[15]. During the pathophysiological development of silicosis, silica dust particles continuously stimulate lung tissue, causing local damage and activating the body’s repair mechanism. In the process of sustained lung injury and repair, pulmonary neuroendocrine cells are also activated[16]. Elizegi et al[17] found that the neuroendocrine cells in a silicosis mouse model had excessive proliferation, and their secretion of peptide substances (such as NSE) also increased. Excessive secretion of NSE directly into the blood or release into the blood after neuroendocrine cell injury can lead to increased serum NSE concentration in silicosis patients. This may be the pathophysiological mechanism of elevated NSE concentration in silicosis patients.
Previously, some researchers have explored the relationship between NSE and silicosis. Zhang et al[9] found that serum NSE concentrations in the silicosis group was significantly higher than that of the healthy group. The area under the diagnostic curve of NSE was 0.804. When the diagnostic threshold of NSE was 12.9 ng/mL, the diagnostic sensitivity and specificity of NSE were 71% and 84%, respectively. Therefore, they believed that serum NSE had good sensitivity and specificity for diagnosis of silicosis, and might become a serological auxiliary diagnostic indicator for silicosis. However, that study had some shortcomings including insufficient sample size; and only healthy people were recruited as the control group, excluding patients with similar symptoms. Fang et al[10] reported that the serum NSE concentration of the silicosis group was significantly higher than that of the healthy control group. NSE concentration in bronchoalveolar lavage fluid was also detected in 20 patients, and NSE concentration in bronchoalveolar lavage fluid was higher than serum NSE concentration. Among the 14 patients who underwent whole-lung lavage and had complete preoperative and postoperative laboratory data, serum NSE concentration decreased in 11 patients. Lung biopsy was performed in one of the patients, and immunohistochemistry suggested positive NSE. Therefore, it is suggested that serum NSE concentration provides diagnostic information for the assessment of silicosis severity. That study also had some shortcomings including insufficient sample size, no ROC curve provided; and as a retrospective study, lung function examination and specimen examination were not simultaneous, and some clinical data were missing. On this basis, Fang and colleagues[11] increased the sample size (160 cases in both the silicosis and control groups), and reported a similar study again in 2017, with results similar to those in 2014.
Other researchers have studied biomarkers of silicosis, including CA125[9-11], Club cell protein 16[18,19], Krebs von den Lungen-6[19,20], surfactant protein D[20], matrix metalloproteinase-2[20], matrix metalloproteinase-9[21], tumor necrosis factor (TNF)-α[21], heme oxygenase-1[22,23], interleukin-6[24], interleukin-10[24], soluble TNF receptor 1[24], soluble TNF receptor 2[25], neopterin[26], miR-19a[27], miR-146a[28], L-selectin[29], homeobox mobility group protein B1[30], and angiotensin-converting enzyme[31]. However, the sample size of these trials was small, and the clinical significance remains to be further determined.
The advantages of this test were as follows. The sample size was calculated strictly according to the diagnostic test sample size formula, and a sufficient number of study cases were included. The silicosis group included patients with stage I, II and III silicosis, as well as patients with or without complications. In addition to healthy volunteers, the control group included patients with common medical diseases. Patients in the silicosis group were followed up for 6 mo, during which time, no diagnosis of malignant tumor or neurological disease was reported.
At the same time, this study also had some limitations. First of all, we used disease stage, rather than lung function, to assess the severity of silicosis, so data on lung function were not collected. Second, this study only followed up for 6 mo, and no pathological biopsy was performed, so the possibility of complicated early neuroendocrine tumor could not be completely ruled out.
In summary, elevated serum NSE concentration in silicosis patients is associated with disease stage. Serum NSE concentration can be used as a biomarker for diagnosis and severity assessment of silicosis.
The diagnosis of silicosis is mainly based on occupational history, symptoms and imaging, among which imaging is the current gold standard. However, in some cases, chest X-ray examination cannot fully meet the clinical needs. There has been no ideal biomarker of silicosis until now.
Studies have found that elevated neuron-specific enolase (NSE) concentration in serum of silicosis patients was helpful for diagnosis and severity assessment of the disease. However, there were many deficiencies in these studies. Among them, the most important deficiency was insufficient sample size.
The purpose of this study was to investigate the clinical significance of serum NSE in diagnosis and staging of silicosis. We hypothesized that the serum NSE concentration of silicosis patients was higher than that of non-silicosis patients, and the serum NSE concentration of silicosis patients increased with the progression of disease stage.
The study was carried out in strict accordance with the criteria of diagnostic tests. The required sample size was estimated according to the formula for diagnostic tests. In addition, many measures had been taken to control bias.
The results agreed with our hypothesis. The results showed that the serum NSE concentration of silicosis patients was significantly higher than that of non-silicosis patients. With the progression of silicosis, NSE concentration increased gradually. The receiver operating characteristic curve suggested that when the diagnostic threshold of NSE was 15.82 ng/mL, the area under the curve was 0.858, with sensitivity of 72% and specificity of 90%.
Serum NSE concentration may be a promising biomarker for diagnosis and assessment of severity of silicosis.
Subsequently, we hope to increase the sample size to further evaluate the role of serum NSE concentrations in the diagnosis of silicosis patients and determine the optimal cut-off value. We also hope to conduct further studies on the pathophysi-ological mechanism of elevated serum NSE concentration in silicosis patients.
We thank You-Xi Wang of the Information Department of Quanzhou First Hospital Affiliated to Fujian Medical University for help with data collection.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dudek M, Mueller S S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li JH
| 1. | National Health and Family Planning Commission of the People's Republic of China. Diagnosis of occupational pneumoconiosis: GBZ70-2015. Beijing: People's Medical Publishing House, 2015. Available from: http://news.zybw.com/flfg/bz/1087.html. |
| 2. | Shimizu A, Suzuki F, Kato K. Characterization of alpha alpha, beta beta, gamma gamma and alpha gamma human enolase isozymes, and preparation of hybrid enolases (alpha gamma, beta gamma and alpha beta) from homodimeric forms. Biochim Biophys Acta. 1983;748:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Li XY, Feng DF. Diffuse axonal injury: novel insights into detection and treatment. J Clin Neurosci. 2009;16:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Cheng F, Yuan Q, Yang J, Wang W, Liu H. The prognostic value of serum neuron-specific enolase in traumatic brain injury: systematic review and meta-analysis. PLoS One. 2014;9:e106680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Sahu S, Nag DS, Swain A, Samaddar DP. Biochemical changes in the injured brain. World J Biol Chem. 2017;8:21-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Jiang ZF, Wang M, Xu JL. Thymidine kinase 1 combined with CEA, CYFRA21-1 and NSE improved its diagnostic value for lung cancer. Life Sci. 2018;194:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Xu CM, Luo YL, Li S, Li ZX, Jiang L, Zhang GX, Owusu L, Chen HL. Multifunctional neuron-specific enolase: its role in lung diseases. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Isgrò MA, Bottoni P, Scatena R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:125-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 357] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 9. | Zhang J, Zhang FL, Zhang ZA. Changes of serum CA125 and neuron-specific enolase in silicosis patients and their clinical significance. Shiyong Yaowu Yu Linchuang. 2015;18:791-794. |
| 10. | Fang SC, Zhang HT, Wang CY, Zhang YM. Serum CA125 and NSE: biomarkers of disease severity in patients with silicosis. Clin Chim Acta. 2014;433:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Fang SC, Zhang HT, Xu B, Wang C, Zhang YM. The relationship between serum tumor markers and the severity of silicosis. Zhonghua Jianyan Yixue Zazhi. 2017;40:515-519. [DOI] [Full Text] |
| 12. | Sun GX, Zhou C, Yang XB, Yang B, Liu YY, Chen XF. Analysis of current situation of occupational poisoning in China from 1993 to 2016 and prevention and Control countermeasures. Zhongguo Anquan Shengchan Kexue Jishu. 2018;14:187-192. |
| 13. | Modlin IM, Champaneria MC, Bornschein J, Kidd M. Evolution of the diffuse neuroendocrine system--clear cells and cloudy origins. Neuroendocrinology. 2006;84:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Cutz E, Yeger H, Wong V, Bienkowski E, Chan W. In vitro characteristics of pulmonary neuroendocrine cells isolated from rabbit fetal lung. I. Effects of culture media and nerve growth factor. Lab Invest. 1985;53:672-683. [PubMed] |
| 15. | Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci USA. 2007;104:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Deterding RR, Pye C, Fan LL, Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr Pulmonol. 2005;40:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Elizegi E, Pino I, Vicent S, Blanco D, Saffiotti U, Montuenga LM. Hyperplasia of alveolar neuroendocrine cells in rat lung carcinogenesis by silica with selective expression of proadrenomedullin-derived peptides and amidating enzymes. Lab Invest. 2001;81:1627-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Naha N, Muhamed JCJ, Pagdhune A, Sarkar B, Sarkar K. Club cell protein 16 as a biomarker for early detection of silicosis. Indian J Med Res. 2020;151:319-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Liu J, Zhang R, Song HY, Xia Q, Zhao TT, Pan LP, Qian XL. [The effects of long-term exposure to silica dust on serum CC16 and KL-6 Levels]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2019;37:567-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Xue C, Wu N, Li X, Qiu M, Du X, Ye Q. Serum concentrations of Krebs von den Lungen-6, surfactant protein D, and matrix metalloproteinase-2 as diagnostic biomarkers in patients with asbestosis and silicosis: a case-control study. BMC Pulm Med. 2017;17:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Jiang PR, Cao Z, Qiu ZL, Pan JW, Zhang N, Wu YF. Plasma levels of TNF-α and MMP-9 in patients with silicosis. Eur Rev Med Pharmacol Sci. 2015;19:1716-1720. [PubMed] |
| 22. | Sato T, Takeno M, Honma K, Yamauchi H, Saito Y, Sasaki T, Morikubo H, Nagashima Y, Takagi S, Yamanaka K, Kaneko T, Ishigatsubo Y. Heme oxygenase-1, a potential biomarker of chronic silicosis, attenuates silica-induced lung injury. Am J Respir Crit Care Med. 2006;174:906-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Sato T, Saito Y, Inoue S, Shimosato T, Takagi S, Kaneko T, Ishigatsubo Y. Serum heme oxygenase-1 as a marker of lung function decline in patients with chronic silicosis. J Occup Environ Med. 2012;54:1461-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Braz NF, Carneiro AP, Avelar NC, Miranda AS, Lacerda AC, Teixeira MM, Teixeira AL, Mendonça VA. Influence of Cytokines and Soluble Receptors in the Quality of Life and Functional Capacity of Workers Exposed to Silica. J Occup Environ Med. 2016;58:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Braz NF, Carneiro AP, Amorim MR, de Oliveira Ferreira F, Lacerda AC, Silva de Miranda A, Teixeira MM, Teixeira AL, Mendonça VA. Association between inflammatory biomarkers in plasma, radiological severity, and duration of exposure in patients with silicosis. J Occup Environ Med. 2014;56:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Altindag ZZ, Baydar T, Isimer A, Sahin G. Neopterin as a new biomarker for the evaluation of occupational exposure to silica. Int Arch Occup Environ Health. 2003;76:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Yang Z, Li Q, Yao S, Zhang G, Xue R, Li G, Wang Y, Wang S, Wu R, Gao H. Down-Regulation of miR-19a as a Biomarker for Early Detection of Silicosis. Anat Rec (Hoboken). 2016;299:1300-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Zhang Y, Zhou D, Wang F, Ren X, Gao X, Zhang Q, Lan Y. Bronchoalveolar Lavage Fluid microRNA-146a: A Biomarker of Disease Severity and Pulmonary Function in Patients With Silicosis. J Occup Environ Med. 2016;58:e177-e182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Nardi J, Nascimento S, Göethel G, Gauer B, Sauer E, Fão N, Cestonaro L, Peruzzi C, Souza J, Garcia SC. Inflammatory and oxidative stress parameters as potential early biomarkers for silicosis. Clin Chim Acta. 2018;484:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Ma J, Zhou Y, Li W, Xiao L, Yang M, Tan Q, Xu Y, Chen W. Association between Plasma HMGB-1 and Silicosis: A Case-Control Study. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Pandey JK, Agarwal D. Biomarkers: A potential prognostic tool for silicosis. Indian J Occup Environ Med. 2012;16:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |