Published online Feb 6, 2021. doi: 10.12998/wjcc.v9.i4.878
Peer-review started: September 1, 2020
First decision: November 8, 2020
Revised: November 20, 2020
Accepted: December 6, 2020
Article in press: December 6, 2020
Published online: February 6, 2021
Processing time: 146 Days and 2.3 Hours
Dubin-Johnson syndrome (DJS) is a benign autosomal recessive liver disease involving mutations of the ABCC2 gene. It is characterized by chronic or intermittent conjugated hyperbilirubinemia, with chronic idiopathic jaundice as the main clinical manifestation. Genetic alterations of the ABCC2 gene are commonly used for diagnosing DJS; however, the causative ABCC2 point mutation in Chinese patients remains unknown. Research on ABCC2 mutations in Chinese DJS patients is extremely rare, and the diagnosis of DJS remains limited. The routine analysis of ABCC2 mutations is helpful for the diagnosis of DJS. Here, we report the clinical characteristics and ABCC2 genotype of an adult female DJS patient. This article is to expound the discovery of more potentially pathogenic ABCC2 variants will that contribute to DJS identification.
This study investigated a woman referred for DJS and involved clinical and genetic analyses. ABCC2 mutations were identified by next-generation sequencing (NGS). The patient showed intermittent jaundice and conjugated hyper-bilirubinemia. Histopathological examinations were consistent with the typical phenotype of DJS. Genetic diagnostic analysis revealed an ABCC2 genotype exhibiting a pathogenic variant, namely c.2443C>T (p.Arg815*), which has not been reported previously in the domestic or foreign literature.
Pathogenic ABCC2 mutations play an important role in the diagnosis of DJS, especially in patients with atypical presentations. Currently, NGS is used in the routine analysis of DJS cases and such tests of further cases will better illuminate the relationship between various genotypes and phenotypes of DJS.
Core Tip: Our study reported the pathological characteristics and a new ABCC2 mutation in a patient with Dubin-Johnson syndrome (DJS). A 24-year-old female with intermittent jaundice and conjugated hyperbilirubinemia underwent laparoscopic exploration and liver biopsy, which confirmed the diagnosis of DJS. Next-generation sequencing analysis showed ABCC2 mutations. Our study reports a novel ABCC2 mutation in a DJS patient.
- Citation: Wu H, Zhao XK, Zhu JJ. Clinical characteristics and ABCC2 genotype in Dubin-Johnson syndrome: A case report and review of the literature. World J Clin Cases 2021; 9(4): 878-885
- URL: https://www.wjgnet.com/2307-8960/full/v9/i4/878.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i4.878
Dubin-Johnson syndrome (DJS) is a benign autosomal recessive liver disease that is characterized by chronic or intermittent conjugated hyperbilirubinemia[1]. During laparoscopy, DJS cases display a pathognomonic "black liver", which results from excessive deposition of a melanin-like pigment in the lysosomes of hepatocytes[2,3]. Surprisingly, few diagnostic tools are available to detect this syndrome during liver function and physical examinations[4-7]. Additionally, some DJS cases share symptoms, such as dull pain in the liver area, generalized weakness, fatigue, and loss of appetite, with other diseases, which inevitably leads to misdiagnosis[8]. The main means of effective diagnosis of DJS are laparoscopic exploration and liver biopsy[9,10]. However, experience in genetic diagnosis, precision treatment, and follow-up observation of DJS patients remains insufficient.
Mutations in ABCC2 have been observed in DJS patients since 1997[11-13], and more than 50 different mutations causing amino acid changes in the ABCC2/MRP2 protein have been described in DJS patients[12,14-16]. These mutations result in a dysfunctional MRP2 protein or accelerate mRNA degradation, thus causing diminished glycosylation and impaired sorting[2,11,17]. Moreover, acquired or hereditary dysfunction of ABCC2 contributes to liver dysfunction, which alters bilirubin metabolism and results in hyperbilirubinemia[9,18,19]. Genetic alterations of the ABCC2 gene are commonly used for diagnosing DJS; however, the causative ABCC2 point mutation in Chinese patients remains unknown. Here, we present the case of a female DJS patient. Laparoscopic exploration and liver biopsy were used for a definitive diagnosis. Subsequently, we performed next-generation sequencing (NGS) with the aimed of identifying new ABCC2 mutations.
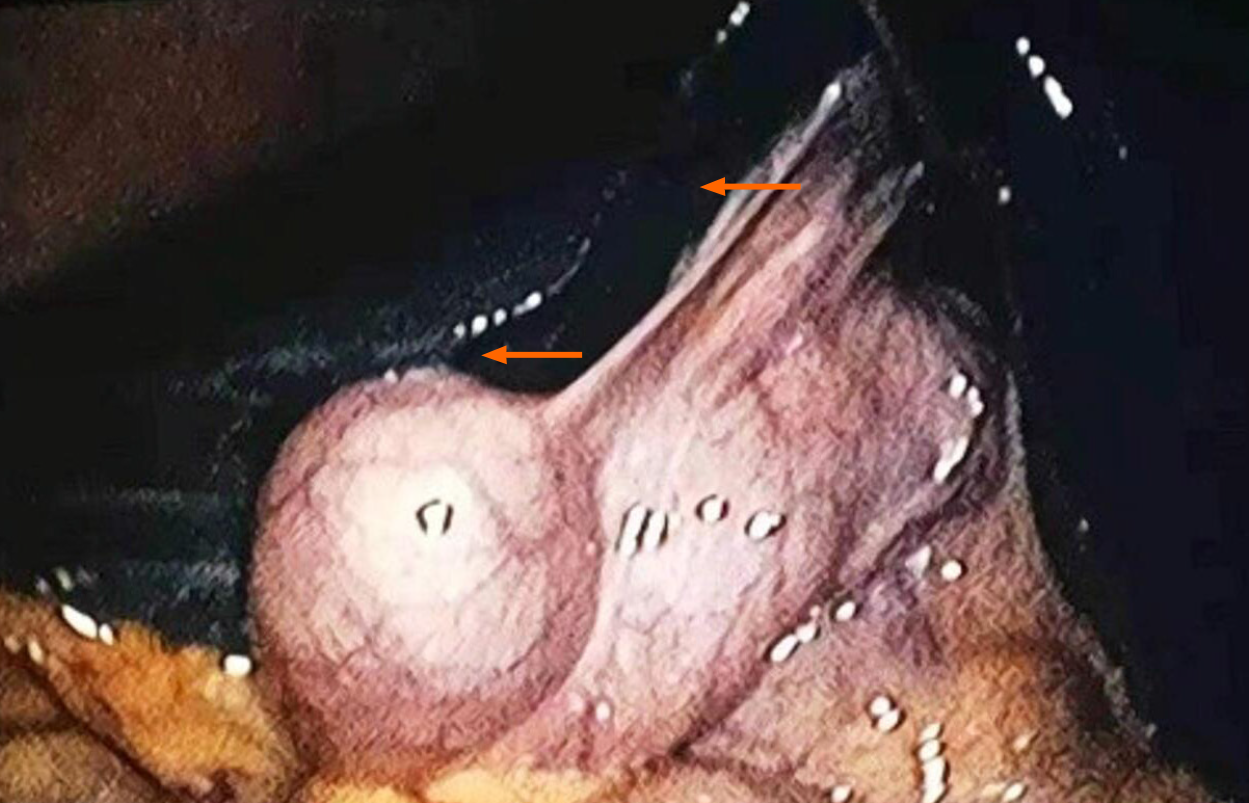
A 24-year-old female was reported as having "gallbladder stones and acute cholecystitis" by the local hospital, and laparoscopic cholecystectomy was performed. During the operation, the dark brown liver surface, slightly shorter edges, and a stone-filled gallbladder led to the termination of cholecystectomy (Figure 1). She was then transferred to our department for further diagnosis.
She is currently not ill.
She had not consumed alcohol or drugs hazardous to the liver, and reported no family history of liver disease. She had given birth three times and had episodes of jaundice during each pregnancy.
She has no family history of liver disease.
The patient was conscious and stable when she arrived at our department. She presented with visible yellow stains on her skin, mucous membranes, and sclera. Her superficial features were undiluted capillaries on the face, no spider nevus in the anterior of the chest wall and neck region, and negative liver palmar. The abdomen was soft without varicose veins or exposure. The liver and spleen were non-palpable under the ribs and percussion of the hepatic region was barely painful.
The relevant auxiliary examination involved liver function tests, routine urinalysis, immunohistochemistry tests of the blood, and a single computed tomography (CT) scan. The results of these examinations which are summarized in Table 1.
| Method | Index | Result |
| Liver function | TBIL | 69.3 μmol/L |
| DBIL | 40.8 μmol/L | |
| IBIL | 28.5 μmol/L | |
| Routine urinalysis | Urinary bilirubin | + |
| HbsAb | + | |
| HBeAb | + | |
| HBcAb | + | |
| Routine blood analysis | HBV-DNA | - |
| Non-chronic hepatitis virus markers | - | |
| ANA antibody spectrum | - | |
| Anti-neutrophil cytoplasmic antibody | - | |
| Autoimmune liver disease antibody spectrum | - | |
| Markers of ceruloplasmin and iron metabolism | - | |
| Immunohistochemistry | HbsAg | - |
| HbcAg | - | |
| Hepatocytes | + | |
| CD10 (bile capillaries) | + | |
| CD3 (hepatic lobules) | + | |
| CD3 (inflammatory cells in the hepatic duct) | + | |
| CD34 (blood vessels) | + | |
| CD68 (Kupffer cells in the hepatic sinus) | + | |
| CD68 (macrophages in the portal area) | - | |
| MUMI (inflammatory cells in the portal area) | ||
| CK7/CK19 (interlobular bile ducts) |
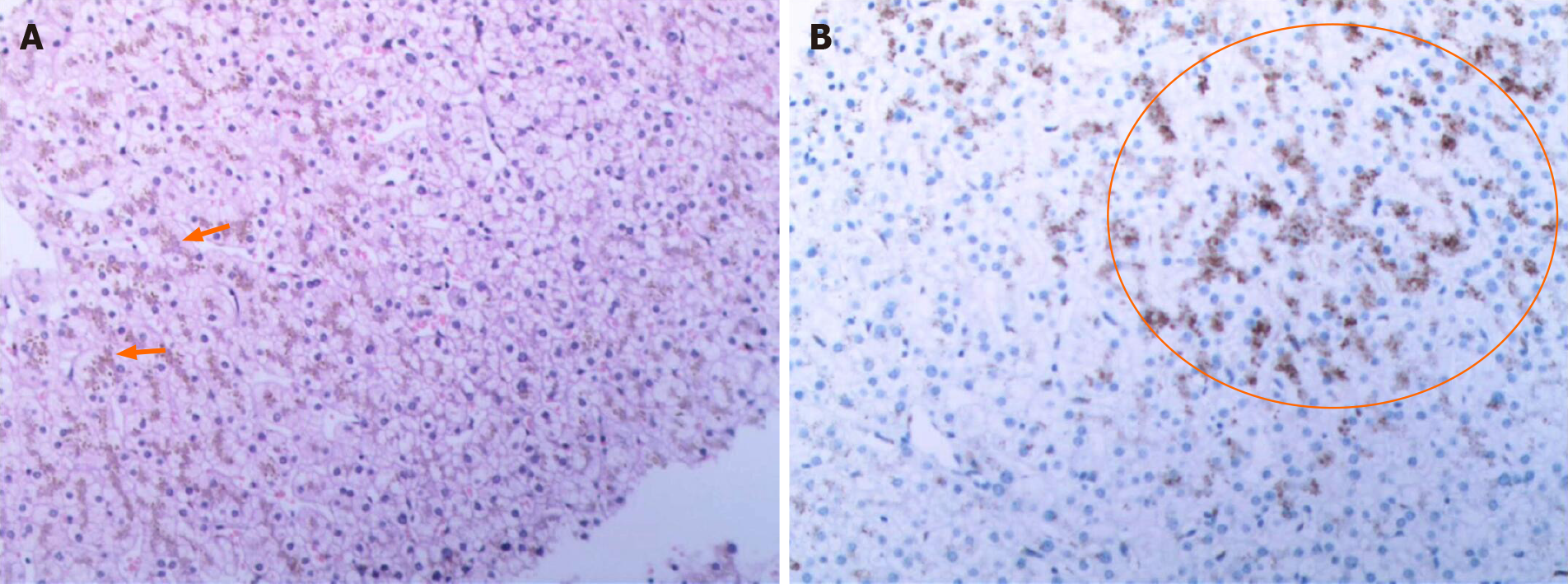
Pathological examination of the liver showed a massive deposition of pigment particles in hepatocytes, especially in the centrilobular region. These particles showed no refractive properties; therefore, they were assumed to be a biliary pigment (Figure 2A). Under the microscope, brownish-yellow pigmental granules were observed in the hepatocytes, especially in those surrounding the central vein. Furthermore, some hepatocytes showed watery and balloon-like degeneration, and nucleated inflammatory cells were present in the hepatic sinus. No obvious abnormalities were found in the portal area, fibrous tissue, bile ducts, or blood vessels between the lobules (Figure 2B).
A The whole-abdomen CT scan found multiple gallbladder stones, increased liver parenchymal density, and an enlarged spleen. After excluding liver contraindications, an ultrasound-guided liver biopsy was performed for jaundice. A melanin-like pigment deposition was observed in hepatocyte lysosomes sing laparoscopy. Additionally, periodic acid-Schiff staining was negative, and reticular fiber and collagen fiber staining (Masson) showed no obvious abnormalities. We performed NGS to identify mutations in the ABCC2 gene.
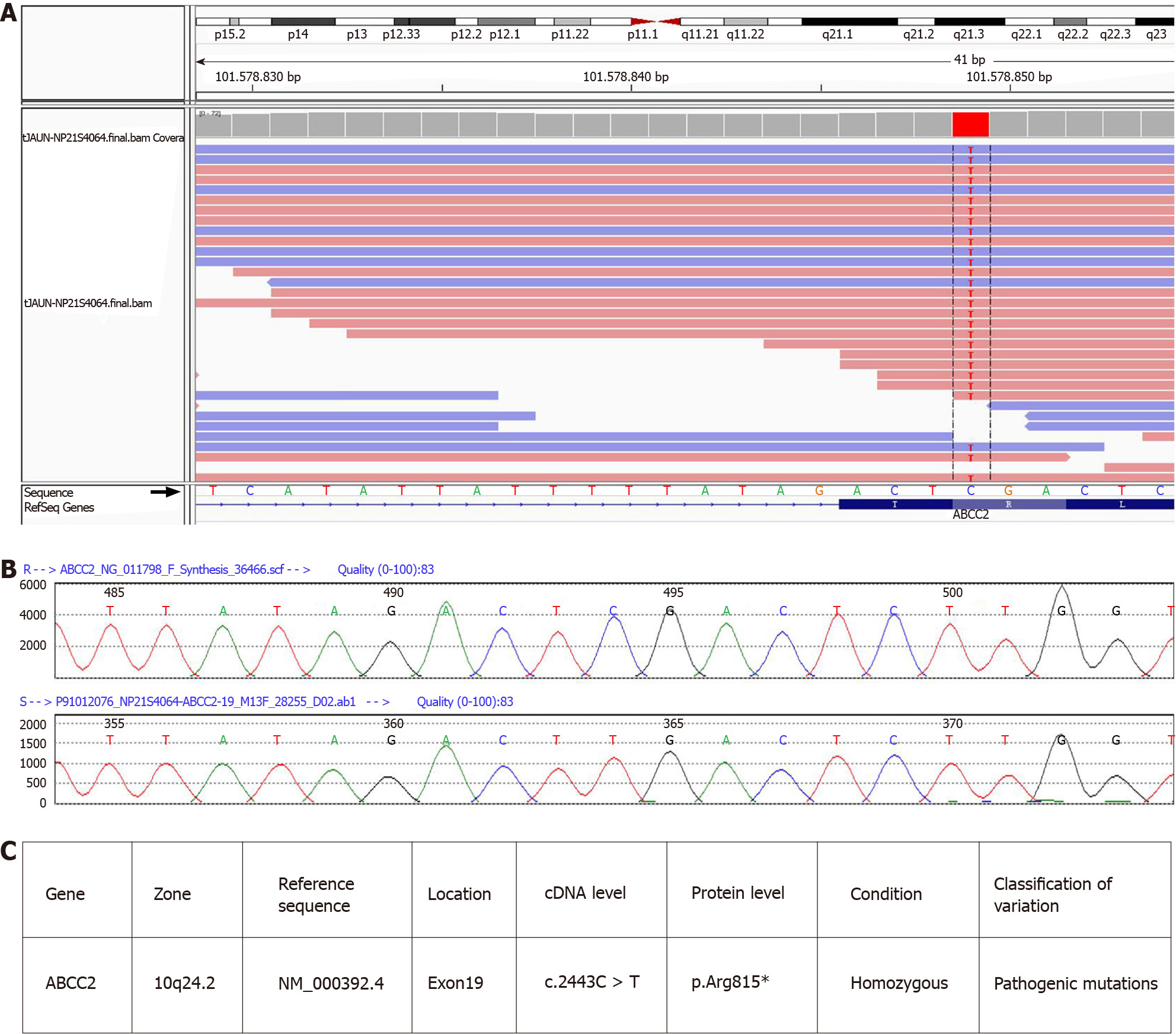
We confirmed the presence of a homozygous mutation, c.2443C>T (p.Arg815*), in ABCC2 in this patient (Figure 3). The mutation was located in exon 19 of ABCC2 and resulted in a change from cytosine to thymine. The amino acid at position 815 of the encoded protein changed from arginine to a stop codon, leading to the premature termination of the peptide chain synthesis. This mutation is novel, and does not appear in HGMD, Esp6500siv2_ALL, or 1000g2015aug_ALL databases. In the dbSNP147 database, it is recorded as rs773850184. According to a comprehensive assessment of the clinical situation, this patient carried a pathogenic mutation in ABCC2, which was the main cause of DJS.
The final diagnosis of the presented case is DJS.
After admission, the patient was administered a light digestible diet and class I nursing. Treatment medicines included Shuganning and glutathione to protect the liver and eliminating jaundice, omeprazole to stomach acid, and hepatocyte growth-promoting hormone to promote liver cell regeneration)[20-22]. Additionally, compound coenzymes, lipid soluble vitamins (II), and water-soluble vitamins were used for nutritional support.
The treatment went well. The blood biochemical indices of the patient significantly improved to the following values: Total bilirubin 34.6 μmol/L; direct bilirubin, 23.1 μmol/L; indirect bilirubin, 11.5 μmol/L; alanine aminotransferase, 11.7 U/L; aspartate aminotransferase, 12.3 U/L; gamma-glutamyl transferase, 11.0 U/L; alkaline phosphatase, 78 U/L; cholinesterase, 6454 U/L; PA, 220.4 mg/L; and total bile acid, 14.3 μmol/L. Blood coagulation function and routine blood analysis showed no abnormalities. The patient’s samples were submitted to Kingmed Diagnostics for mutation detection and the patient was discharged from the hospital. Further follow-up observation is needed for long-term prognosis.
This case of DJS had the typical features of familial conjugated hyperbilirubinemia, including elevated levels of conjugated bilirubin that developed into chronic intermittent jaundice. Pathologically, the matrix deposited with ferritin-like particles led to a black liver, with a lipofuscin like pigment deposited in the middle of the hepatic lobule. However, these symptoms are merely used as a diagnostic indicators and they lack specificity. A review of the condition showed that the patient had been pregnant three times, and that yellow staining of the systemic skin, mucosa, and sclera was present during each pregnancy. The patient was diagnosed with acute cholecystitis, and the corresponding treatment was adopted, given the pregnancy factor[23-26]. The main pathological changes observed in the liver tissue were massive pigment particle deposition in hepatocytes and significant pigment granules in the central area of the lobules. It was difficult to distinguish whether the pigment particle was iron, copper, or bile pigment using hematoxylin and eosin staining. As the serum copper-blue protein level of the patient was normal, liver injury due to copper accumulation was ruled out. The pigment granules tended to have bile pigment deposits, rather than iron, as indicated by their lack of refraction. Therefore, considering the histological features and relevant clinical examination, the patient was diagnosed with DJS. The laboratory examinations performed on the patient included a liver function test, which showed increased direct bilirubin levels; a sulfo-bromophthalein sodium test; a urine porphyrin isomer test; an oral cholecystic contrast agent test, which showed gallbladder non-development; radionuclide hepatobiliary imaging; and a liver biopsy, which is the gold standard for the diagnosis of DJS. However, these diagnostic measures are not widely available in health-care facilities in China. As DJS is a rare hereditary disease, the lack of experience with its diagnosis and treatment brings difficulties in clinical practice.
With the development of sequencing technology, genetic, and epigenetic research of complex disorders is becoming more common. Therefore, multiple mutation sites have been detected in DJS patients. The ABCC2 mutations identified in DJS patients include gene deletions, missense mutations, nonsense mutations, and splice site mutation. A previous study analyzing the clinical diagnosis of seven DJS patients in China found that all patients had at least one non-synonymous mutation in the ABCC2 gene. All identified mutations were heterozygous mutations, while only two cases had complex heterozygous mutations[2], suggesting that other clinically relevant ABCC2 variants were involved. Further, two new homozygous mutations, c.1013_1014delTG and 974C->G, in the eighth exon of the ABCC2 gene, have been identified in other countries[27-30]. However, genetic analysis of DJS cases is relatively inadequate in China. In this study, the patient’s mutation was confirmed to be c.2443C>T (p.Arg815*) by sequencing, and was identified as a nonsense mutation. Therefore, a cytosine in the coding region of ABCC2 was mutated to thymine, resulting in the amino acid at position 815 changing from arginine to a stop codon, which causes the premature termination of peptide chain synthesis, leading to a defective MRP2 protein. There was no report of this mutation in domestic and foreign literature databases, including China HowNet, Wanfang, Weipu, and PubMed, except in the dbSNP147 database. The DJS cases with the ABCC2 (p.Arg815*) nonsense mutation may contribute to potentially pathogenic ABCC2 variants of the genetic or metabolic liver diseases.
We reported the clinical manifestations and the methods of diagnosis of a Chinese DJS patient, who had a homozygous mutation, c.2443C>T (p.Arg815*), in ABCC2. Patients with unexplained jaundice should be genotyped as early as possible, for their effective clinical management.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cure E S-Editor: Huang P L-Editor: Filipodia P-Editor: Liu JH
| 1. | Dubin IN, Johnson FB. Chronic idiopathic jaundice with unidentified pigment in liver cells; a new clinicopathologic entity with a report of 12 cases. Medicine (Baltimore). 1954;33:155-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 233] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Wu L, Zhang W, Jia S, Zhao X, Zhou D, Xu A, Duan W, Wu Z, Li H, Zheng S, Nan Y, Jia J, Huang J, Ou X. Mutation analysis of the ABCC2 gene in Chinese patients with Dubin-Johnson syndrome. Exp Ther Med. 2018;16:4201-4206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Baranguán Castro ML, García Romero R, Miramar Gallart MD. Conjugated hyperbilirubinemia after surgery. A diagnosis of Dubin-Johnson syndrome confirmed by genetic testing. Rev Esp Enferm Dig. 2017;109:801-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Geng JH, Lin VC, Yu CC, Huang CY, Yin HL, Chang TY, Lu TL, Huang SP, Bao BY. Inherited Variants in Wnt Pathway Genes Influence Outcomes of Prostate Cancer Patients Receiving Androgen Deprivation Therapy. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Corpechot C, Barbu V, Chazouillères O, Broué P, Girard M, Roquelaure B, Chrétien Y, Dong C, Lascols O, Housset C, Jéru I. Genetic contribution of ABCC2 to Dubin-Johnson syndrome and inherited cholestatic disorders. Liver Int. 2020;40:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Mao X, He Z, Zhou F, Huang Y, Zhu G. Prognostic significance and molecular mechanisms of adenosine triphosphate-binding cassette subfamily C members in gastric cancer. Medicine (Baltimore). 2019;98:e18347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Lee JH, Chen HL, Chen HL, Ni YH, Hsu HY, Chang MH. Neonatal Dubin-Johnson syndrome: long-term follow-up and MRP2 mutations study. Pediatr Res. 2006;59:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Machida I, Wakusawa S, Sanae F, Hayashi H, Kusakabe A, Ninomiya H, Yano M, Yoshioka K. Mutational analysis of the MRP2 gene and long-term follow-up of Dubin-Johnson syndrome in Japan. J Gastroenterol. 2005;40:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Morii K, Yamamoto T. IMAGES IN CLINICAL MEDICINE. Dubin-Johnson Syndrome. N Engl J Med. 2016;375:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Park SW, Jun CH, Choi SK, Kim HJ, Kim GE. Hepatobiliary and Pancreatic: A black liver of Dubin-Johnson syndrome. J Gastroenterol Hepatol. 2018;33:562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Paulusma CC, Kool M, Bosma PJ, Scheffer GL, ter Borg F, Scheper RJ, Tytgat GN, Borst P, Baas F, Oude Elferink RP. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology. 1997;25:1539-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 340] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Nies AT, König J, Cui Y, Brom M, Spring H, Keppler D. Structural requirements for the apical sorting of human multidrug resistance protein 2 (ABCC2). Eur J Biochem. 2002;269:1866-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Marrone J, Tocchetti GN, Danielli M, Mottino AD, Marinelli RA. Improved hepatic MRP2/ABCC2 transport activity in LPS-induced cholestasis by aquaporin-1 gene transfer. Biochimie. 2019;165:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Meng LL, Qiu JW, Lin WX, Song YZ. [Clinical features and ABCC2 genotypic analysis of an infant with Dubin-Johnson syndrome]. Zhongguo Dangdai Erke Zazhi. 2019;21:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Uchiumi T, Tanamachi H, Kuchiwaki K, Kajita M, Matsumoto S, Yagi M, Kanki T, Kang D. Mutation and functional analysis of ABCC2/multidrug resistance protein 2 in a Japanese patient with Dubin-Johnson syndrome. Hepatol Res. 2013;43:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Erlinger S, Arias IM, Dhumeaux D. Inherited disorders of bilirubin transport and conjugation: new insights into molecular mechanisms and consequences. Gastroenterology. 2014;146:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 17. | Slachtova L, Seda O, Behunova J, Mistrik M, Martasek P. Genetic and biochemical study of dual hereditary jaundice: Dubin-Johnson and Gilbert's syndromes. Haplotyping and founder effect of deletion in ABCC2. Eur J Hum Genet. 2016;24:704-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science. 1996;271:1126-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 537] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 19. | Chen HL, Li HY, Wu JF, Wu SH, Chen HL, Yang YH, Hsu YH, Liou BY, Chang MH, Ni YH. Panel-Based Next-Generation Sequencing for the Diagnosis of Cholestatic Genetic Liver Diseases: Clinical Utility and Challenges. J Pediatr 2019; 205: 153-159. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 20. | Wei Y, Yao WX, Zhou H, Wang LY, Xie H, Zhao X. Clinical observation on effectiveness of Shuganning in treatment of patients with hepatic injury caused by chemotherapy drug. Zhongguo Ganzangbing Zazhi. 2010;2. |
| 21. | Feng X, Yang XL. Study on the economic burdens of Shuganning injection and reduced glutathione injection for the treatment of hepatobiliary diseases based on medical insurance data. Zhongguo Xinyao Zazhi. 2018;27:2962-2968. |
| 22. | Högberg J, Kristoferson A. A correlation between glutathione levels and cellular damage in isolated hepatocytes. Eur J Biochem. 1977;74:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 171] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Gupta A, Tiwari P, Sachdeva P. A Case of Dubin-Johnson Syndrome in Pregnancy. Cureus. 2019;11:e4048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Kularatnam GAM, Warawitage D, Vidanapathirana DM, Jayasena S, Jasinge E, de Silva N, Liyanarachchi KLAMS, Wickramasinghe P, Devgun MS, Barbu V, Lascols O. Dubin-Johnson syndrome and intrahepatic cholestasis of pregnancy in a Sri Lankan family: a case report. BMC Res Notes. 2017;10:487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Zhang HX, Hao R, Qin YZ. Dubin-Johnson syndrome with pregnancy: a case report. Fuchan Yu Yichuan. 2013;3:56-57. |
| 26. | Gan Y, Zhang HF, Zhu SS, Huo CH. Clinical Features of Dubin-Johnson Syndrome. Zhongguo Quanke Yixue. 2011;14:2089-2090. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Martínez-Solís M, Pinos D, Endo H, Portugal L, Sato R, Ferré J, Herrero S, Hernández-Martínez P. Role of Bacillus thuringiensis Cry1A toxins domains in the binding to the ABCC2 receptor from Spodoptera exigua. Insect Biochem Mol Biol. 2018;101:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (MRP2). Pflugers Arch. 2007;453:643-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 29. | Corpechot C, Ping C, Wendum D, Matsuda F, Barbu V, Poupon R. Identification of a novel 974C-->G nonsense mutation of the MRP2/ABCC2 gene in a patient with Dubin-Johnson syndrome and analysis of the effects of rifampicin and ursodeoxycholic acid on serum bilirubin and bile acids. Am J Gastroenterol. 2006;101:2427-2432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Huynh MT, Chrétien Y, Grison S, Delaunay JL, Lascols O, Tran CT, Goria O, Ramond MJ, Barbu V. Novel compound heterozygous ABCC2 variants in patients with Dubin-Johnson syndrome and intrahepatic cholestasis of pregnancy. Clin Genet. 2018;94:480-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |