Published online Feb 6, 2021. doi: 10.12998/wjcc.v9.i4.854
Peer-review started: August 4, 2020
First decision: November 3, 2020
Revised: December 3, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: February 6, 2021
Processing time: 173 Days and 22 Hours
Hutchinson-Gilford progeria syndrome (HGPS) is an extremely rare disease characterized by the rapid appearance of aging with an onset in childhood. Serious cardiovascular complications can be life-threatening events for affected patients and the cause of early death. Herein we report a HGPS patient with osteosarcoma hat was successfully managed and is alive 13 years after the diagnosis. This is the first report describing the detailed surgical procedure and long-term follow-up of osteosarcoma in a patient with HGPS.
The patient was diagnosed with HGPS at 5 years of age with typical features and was referred to our department with a suspected bone tumor of the left proximal tibia at the age of 18. Open biopsy of the tibial bone tumor revealed a conventional fibroblastic osteosarcoma. We have developed and performed a freezing technique using liquid nitrogen for tumor reconstruction. This technique overcame the small size of the tibia for megaprosthesis and avoided amputation and limb salvage was achieved 13 years post-operatively. Although the patient had a number of surgical site complications, such as wound dehiscence, and superficial and deep infections due to vulnerable skin in HGPS, no recurrence or metastases were detected for 13 years, and she walks assisted by crutches. Her general health was good at the latest follow-up at 31 years of age.
A HGPS patient with osteosarcoma was successfully managed and she was alive 13 years after the diagnosis.
Core Tip: We report the first case describing the detailed surgical procedure and long-term follow-up of osteosarcoma in a patient with Hutchinson-Gilford progeria syndrome (HGPS). This patient had symptoms of HGPS at birth and was diagnosed at 5 years of age. After the diagnosis of osteosarcoma in proximal tibia, a pedicle frozen autograft using liquid nitrogen was performed to cure her osteosarcoma, and limb salvage was achieved 13 years post-operatively. The patient had a number of surgical site complications, such as wound dehiscence, and superficial and deep infections due to vulnerable skin in HGPS. If the megaprosthesis was chosen, infection could not be subsided and an amputation would eventually be done. Even though amputation was selected, a prosthetic leg would not be applicable because of the poor condition of the skin. Cardiovascular disorders did not develop until 24 years of age and thyroid cancer has been successfully managed by surgery and levothyroxine for 13 years. Due to the morphologic characteristics of bone, vulnerable skin condition, and decreased subcutaneous tissue, the treatment strategy for osteosarcoma in HGPS is complicated. A biological reconstruction method is recommended to manage post-operative complications.
- Citation: Hayashi K, Yamamoto N, Takeuchi A, Miwa S, Igarashi K, Araki Y, Yonezawa H, Morinaga S, Asano Y, Tsuchiya H. Long-term survival in a patient with Hutchinson-Gilford progeria syndrome and osteosarcoma: A case report. World J Clin Cases 2021; 9(4): 854-863
- URL: https://www.wjgnet.com/2307-8960/full/v9/i4/854.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i4.854
Hutchinson-Gilford progeria syndrome (HGPS) is an extremely rare disease characterized by the rapid appearance of aging with an onset in childhood that was initially reported by Johnathan Hutchinson in 1886 and further described by Hastings Gilford in 1904[1]. The patients typically have a normal appearance at birth; however, they grow slowly and do not gain weight as expected. HGPS causes extremely short stature, low body weight, lipodystrophy, alopecia, joint abnormalities, osteolysis, and scleroderma. Patients with HGPS develop a distinctive facial appearance, including prominent eyes, a thin nose with a beaked tip, thin lips, a small chin, and protruding ears. The patients are alert and attentive with normal intelligence and emotions[2]. They tend to develop arteriosclerosis in childhood, which greatly increases the risk of ischemic heart disease at a young age. These serious cardiovascular complications can be life-threatening events for affected patients and the cause of early death between 10 and 15 years of age[3]. Based on previous studies, the prevalence of HGPS is estimated to be approximately 1 in 18 million[4]. HGPS is usually classified as classical and atypical. Atypical HGPS includes growth retardation and involves the same body systems (bones, body, fat, skin, and hair) as in classical HGPS, but the course and severity of the symptoms vary[5,6]. The majority of patients with classical HGPS have a de novo point mutation in exon 11 of the LMNA gene, which provides instructions for producing lamin A protein[7,8]. Mutations result in the production of progerin, which is a truncated version of lamin A. Progerin causes nuclear envelope instability, progressive damage to the nucleus, and premature death of cells. There are no reports of an increased cancer incidence in HGPS patients[2,9,10], but there are several case reports of osteosarcoma in HGPS patients[11,12]. Considering the prevalence of osteosarcoma (3 cases/million population/year) and total number of HGPS patients [as of 31 December 2019, 166 children were identified living with progeria in 51 countries (https://www.progeriaresearch.org/)], the incidence of malignancy in HGPS is higher than average.
The standard surgery for osteosarcoma patients consists of wide resection and reconstruction with megaprosthesis or biological methods[13]. Modular-type megaprostheses are useful for adult reconstruction and provide relatively stable results after surgery. For younger patients, custom-made prostheses, such as a growing HMRS system, can be used for immature bones. If the proper prosthesis is not available for an unusual bone condition, the patient might require amputation to achieve resection of the tumor. Biological reconstruction is one of the alternatives to prosthetic reconstruction. Biological reconstruction includes vascularized fibular grafts[14], distraction osteogenesis[15], allografts[16], and tumor-sterilized autologous tumor-bearing bone grafts[17]. Previously, we reported performing a trans-epiphyseal osteotomy and resecting a malignant bone tumor followed by reconstruction with a massive frozen tumor-bearing bone treated with liquid nitrogen[17,18]. The development of the liquid nitrogen method was based on basic studies of the hypothermic effects of liquid nitrogen. We confirmed that one cycle of −196 °C for 20 min and thawing in room temperature is sufficient to kill all tumor cells by inducing ice crystal formation and cell dehydration in tumor-bearing bone. Since then, we have clinically applied the liquid nitrogen method to musculoskeletal tumor surgery. This recycling procedure works well for patients with short stature, such as HGPS, who are not suitable candidates for a regular prosthetic replacement device or an allograft.
Herein we report a HGPS patient with osteosarcoma who had short stature with co-morbidities. She had dual cancers that were successfully managed and she was alive 13 years after the diagnosis. This is the first report describing the surgical resection and reconstruction of osteosarcoma in a patient with HGPS.
An 18-year-old female presented with left knee pain.
She presented to another hospital with a month history of left knee pain and was referred to our department with a suspected bone tumor of the left proximal tibia.
She was the product of a normal pregnancy and delivery, but incomplete extension of her knee joints was noted. She also had ichthyosis-like skin and occipital dysplasia. A chromosome abnormality was not detected and a diagnosis was not established. She was diagnosed with HGPS at 5 years of age with typical features, such as bird-like facies, lack of subcutaneous fat, aged-appearing skin, short stature, and low weight for height. She also had Perthes disease of the hip joints bilaterally and lamellar cataracts.
She had no significant personal or family history.
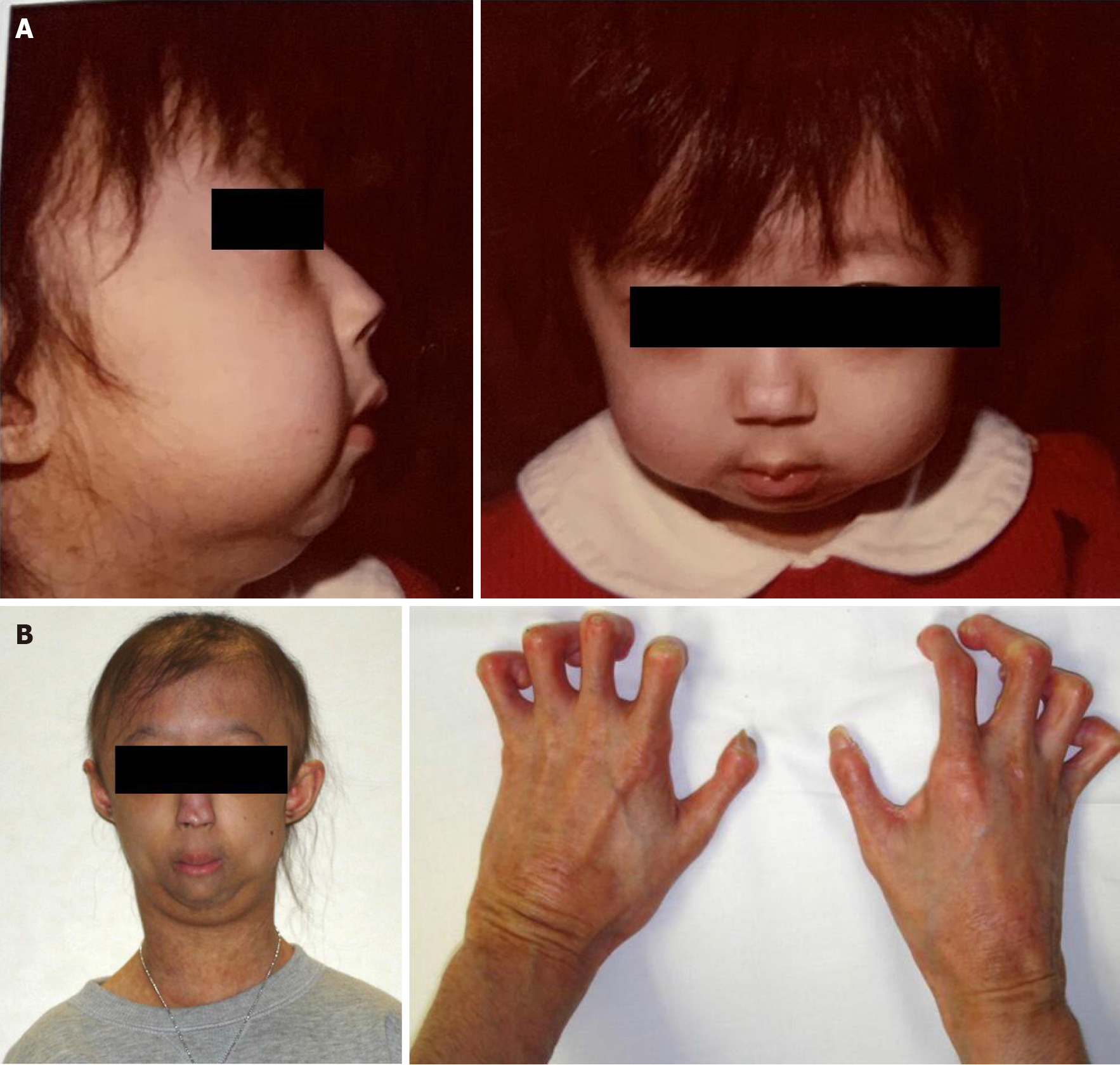
Her first physical findings at our clinic were as follows: Height, 137 cm; weight, 23 kg; and body mass index, 12.3 kg/m2. She had ichthyosis-like skin, bird-like facies, lack of subcutaneous fat, hair loss, occipital dysplasia, contractures of the extremities, marked flexion contractures of the fingers, and sloping shoulders (Figure 1). Intelligence was normal and she walked without support and she did not use any medications. A hard bony mass was palpable in the left proximal tibia with mild tenderness that was painful when she walked.
Initial laboratory examination was largely insignificant with normal hemoglobin, white blood cell count, platelets, liver function, renal function, and electrolytes. Only thyroglobulin level was elevated (138.4 ng/mL, normal level 1.4-78).
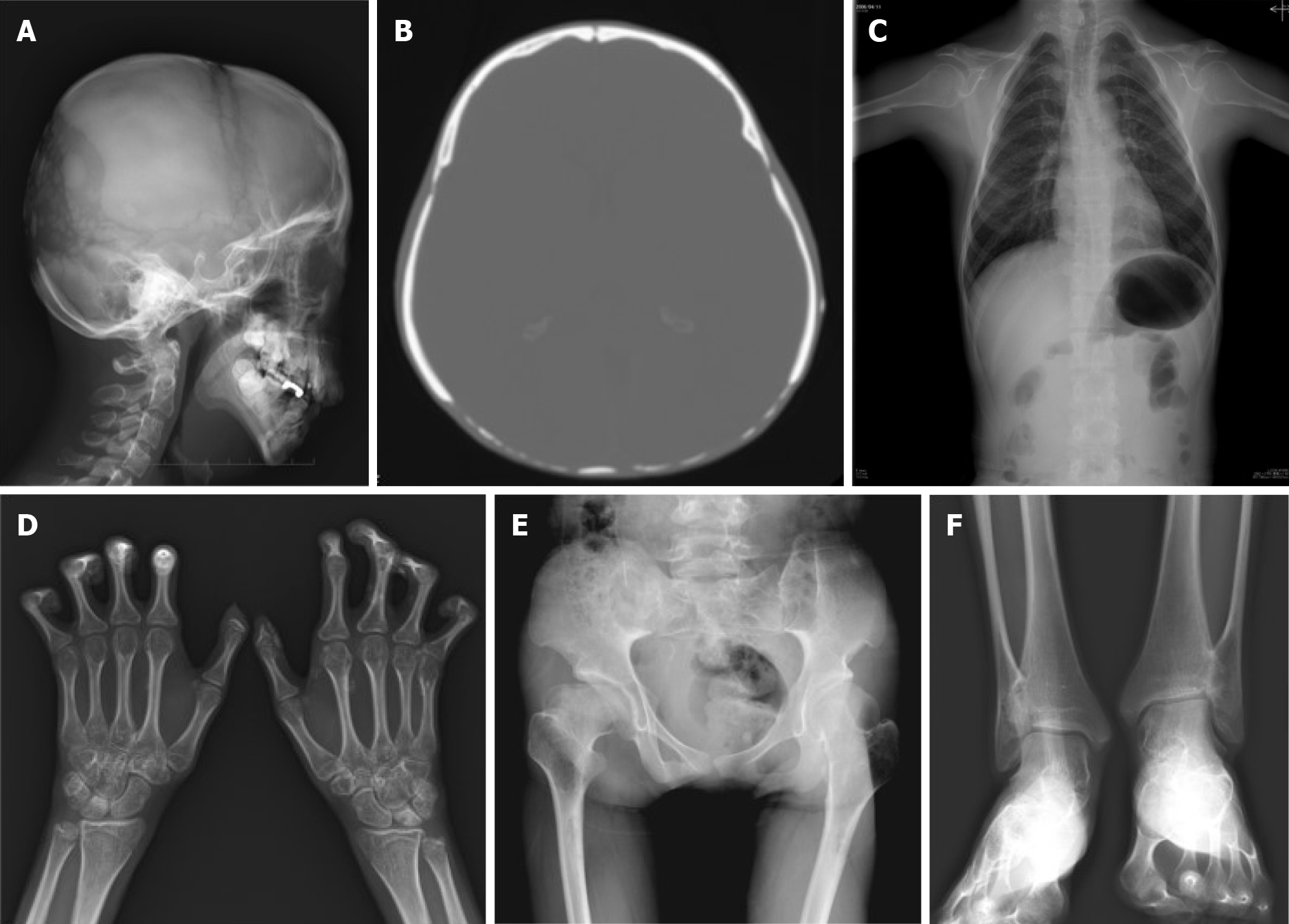
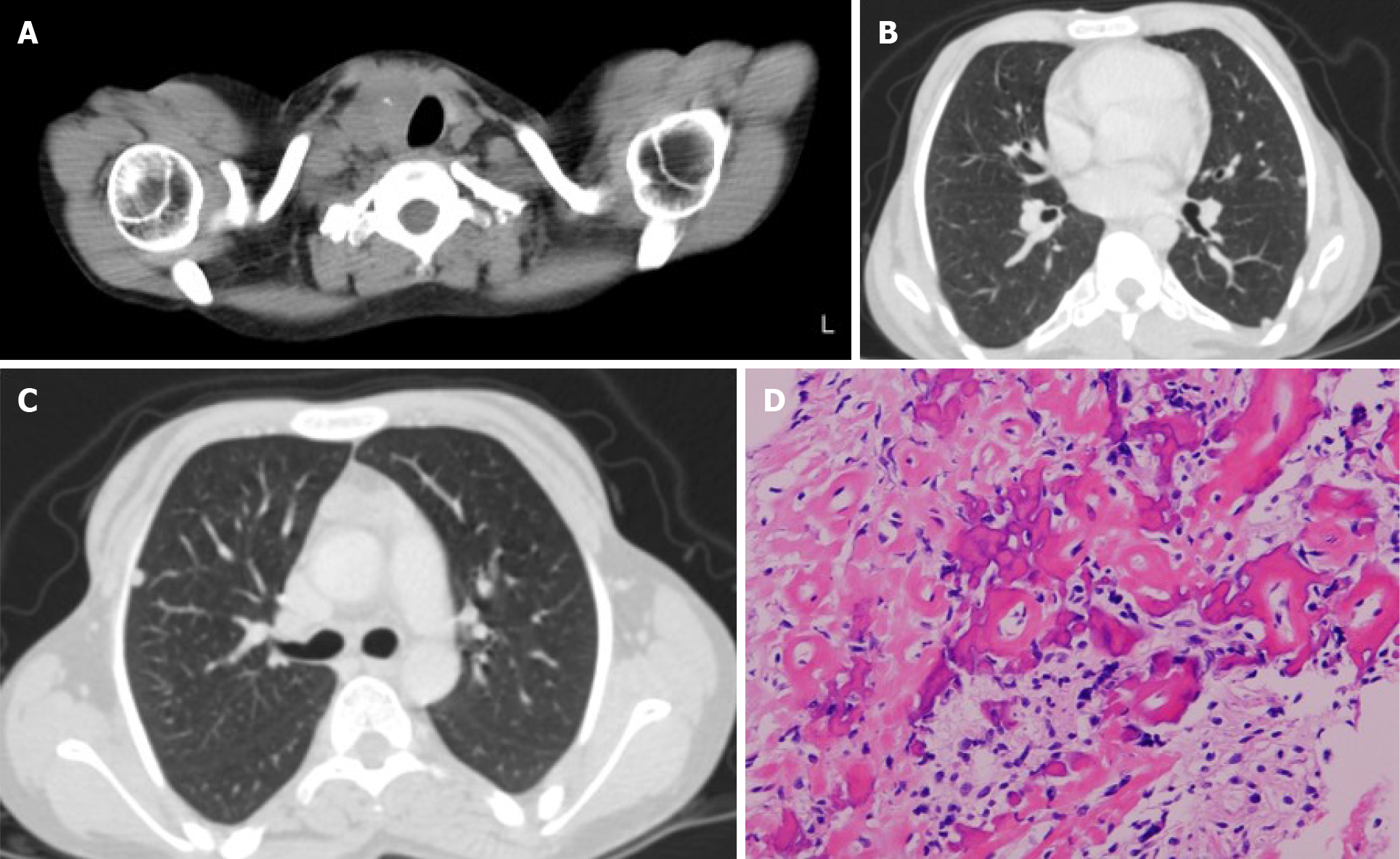
Based on systemic X-rays, the occipital bone was dysplastic, the DIP and PIP joints in the hands bilaterally had flexion contractures, and heterotopic ossifications were seen (Figure 2). The lumbar spine showed marked lordosis and vertebral deformities. The hip joints had post-Perthes disease deformities and the ankle joints had distal tibiofibular synostosis. Bilateral tibial X-rays revealed shafts that were morphologically small in diameter and narrow in the medullary cavities (Figure 3A). In the proximal part of the diaphysis, an irregular osteolytic lesion was noted on the anteroposterior view, and posterior cortical destruction and extraosseous calcified lesions were observed on the lateral view (Figure 3A and B). Magnetic resonance imaging of the tibia showed intra- and extra-osseous lesions with homogenous low intensity on T1-weighted images, low-to-high intensity on T2-weighted images, and enhanced inhomogeneity by gadolinium (Figure 3C). Bone scintigraphy showed accumulation in the proximal tibia, but no other bone lesions were detected (Figure 3D). Thallium scintigraphy showed high accumulation at the tumor site in the tibia in both early and delay phase (Figure 3E). Computed tomography showed left tracheal deviation, and a thyroid tumor and multiple small nodules in the lungs were also detected (Figure 4A-C). Metabolic disorders, hypertension, arteriosclerosis, and cerebrovascular disorders were not detected.
Open biopsy of the tibial bone tumor revealed that the tumor cells produced eosinophilic immature osteoids and varied in size with nuclear atypia. Both low- and high-grade variants were included, but the final diagnosis was a conventional fibroblastic osteosarcoma (Figure 4D). A fine needle aspiration biopsy was also obtained from the thyroid tumor that was diagnosed as papillary carcinoma.
Conventional fibroblastic osteosarcoma of proximal tibia and thyroid papillary carcinoma with HGPS.
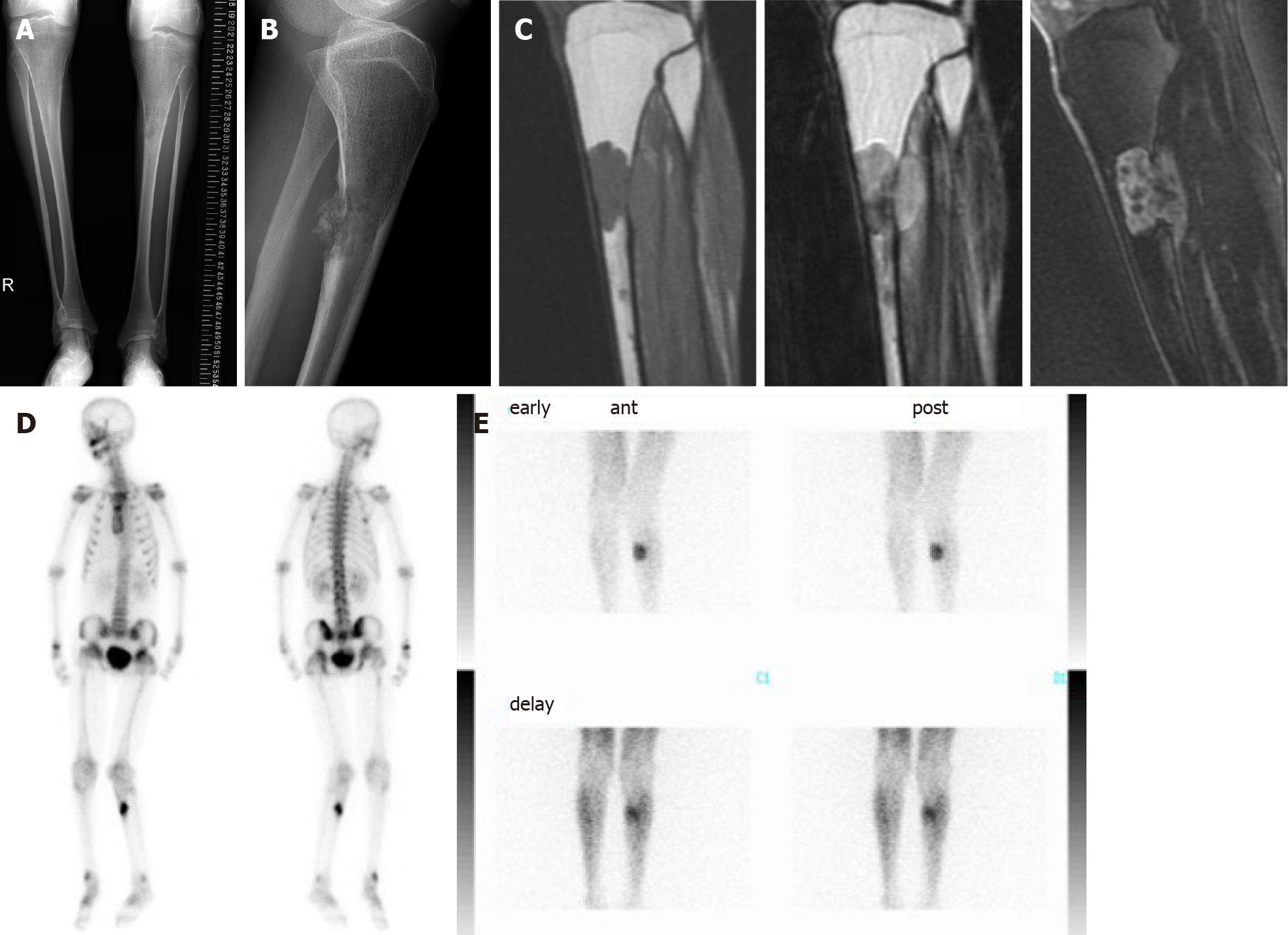
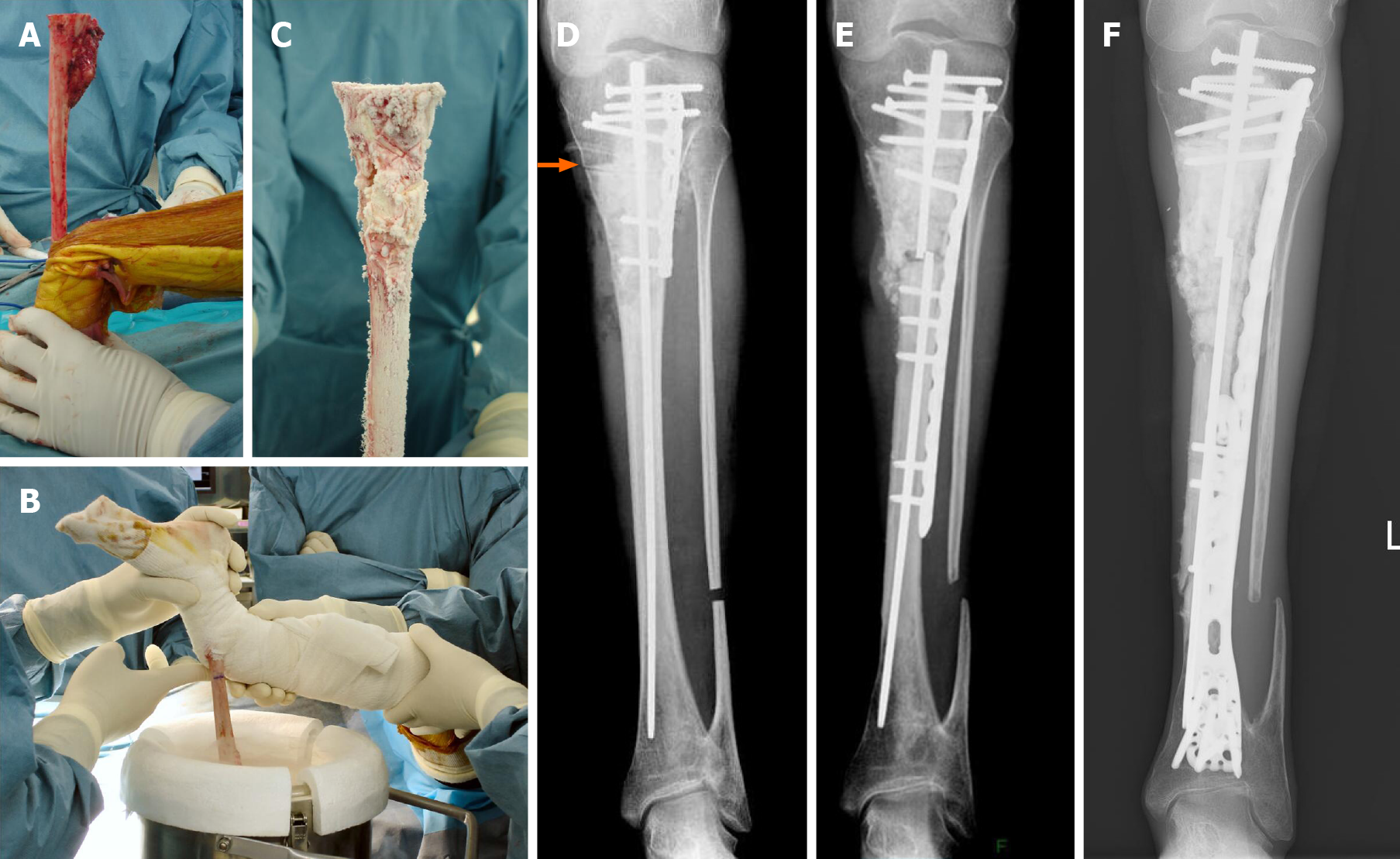
In general, conventional osteosarcoma is treated by chemotherapy and surgery; however, because she also has thyroid cancer with possible lung metastases and HGPS, which does not generally have a long life-span, the osteosarcoma was treated with surgery alone. Wide excision, including skip lesions in the tibia, was planned. With respect to reconstruction after tumor excision, a conventional modular megaprosthesis was not applicable because of the small size of the tibia. Amputation is one of the options, but we attempted to perform limb salvage surgery. The proximal epiphysis of the tibia appeared suitable for preservation. An intercalary endoprosthesis could not be performed because of the short remaining shaft and small diameter. A fibular graft was possible, but donor site morbidity, hypoplasia of the fibula, and length of time using an external fixator were issues. Bone transport was also one of the options, but long-term external fixation and pin tract infection due to existing skin problems of HGPS were also issues. The authors have developed and performed a freezing technique using liquid nitrogen for tumor reconstruction[17]. We also modified the technique to produce pedicle freezing to maintain anatomic continuity on one side[19]. This technique overcame the small size of the tibia and avoided using long-term external fixation for limb salvage surgery. During the surgical procedure, tumor bone was exposed with a wide margin and a proximal tibial osteotomy was performed 3 cm below the knee joint, thus preserving epiphysis (Figure 5A). A distal fibular osteotomy was performed for the pedicle freezing technique. The tibia was rotated and put in a container filled with liquid nitrogen for 20 min (Figure 5B), thawed at room temperature for 15 min (Figure 5C), thawed in distilled water for 15 min, then replaced with reconstruction by a small intramedullary nail, plate, and polymethylmethacrylate. Post-operative X-rays showed a restored reconstruction by the frozen autograft with no displacement (Figure 5D). Walking rehabilitation was started as tolerated post-operatively and the clinical course was good. Three years after surgery; however, an X-ray revealed a fracture of the frozen bone at the end plate and additional plate fixation with autologous bone grafting was performed (Figure 5E). A total of 8 surgeries for complications were performed, such as a pedicle gastrocnemius flap for a skin fistula on the screw head, additional autologous bone grafting for non-union, and debridement for a local infection. During delivery of general anesthesia, mask ventilation and tracheal intubation were difficult because of her small mouth, small mandible, and narrow upper airway related to HGPS[20]. A Berman airway was useful for airway management. During follow-up, she had pyogenic arthritis of the right hip joint of unknown cause that resolved with conservative therapy.
No recurrence or metastases were detected for 13 years post-operatively, limb salvage was successful (Figure 5F), and she walks assisted by crutches.
Thyroid papillary carcinoma was treated by surgical excision. Although the tumor invaded the trachea, esophagus, and right recurrent laryngeal nerve, the tumor was detached from those structures with possible residual tumor and a right lateral cervical dissection was also performed. After surgery, the patient was treated by daily levothyroxine and no primary tumor regrowth or progression of lung metastases was observed for 13 years.
No arterial sclerosis or metabolic disorders were detected at 18 years of age when she first sought evaluation in our hospital. Elevated triglycerides were detected at 21 years of age, a hyperglycemic disorder was treated with voglibose at 22 years of age, and arteriosclerosis was diagnosed at 24 years of age and treated with valsartan. Transient heart failure with pneumonia occurred at 31 years of age, but no coronary artery stenosis was demonstrated on coronary angiography. Her general health was good at the latest follow-up at 31 years of age.
The 5-year survival rate for osteosarcoma patients is approximately 70% and limb salvage surgery is common as an alternative to amputation[21]. King et al[11] first reported an association between osteosarcoma and HGPS. A 13-year-old female presented with a large extra-pleural mass and en bloc resection of the chest wall and ribs, including the tumor, was performed followed by a Marlex closure. Although the surgical margins were negative, positive cervical lymph nodes were identified 6 mo after surgery. Chemotherapy, surgery, and irradiation were used to treat the recurrence; however, the patient eventually died from pulmonary failure. Shalev et al[12] reported another case of a patient with osteosarcoma who was diagnosed at 9 years of age. Chemotherapy and surgery were performed for osteosarcoma in the proximal tibia, but no details of treatment were available in the report. The diagnosis of HGPS was made at 11 years of age. No other cases of osteosarcoma in HGPS have been reported in the literature. Prosthetic replacement following wide resection of an osteosarcoma is the most common method to reconstruct a large bony defect. The advantages of this procedure include the technical simplicity, the immediate post-operative recovery of mechanical stability, and early functional recovery. The incidence of long-term complications, such as infections, loosening, and breakage requiring revision surgery, remains unavoidable[22,23]. Our patient’ s bone was too small to use a regular modular megaprosthesis. To resolve the size of the prosthesis, a custom-made prosthesis has been developed for young patients who have growth potential. When the modular prosthesis is not adequate for a patient with short stature, a custom-made prosthesis is one of the options. A custom-made prosthesis was also inapplicable due to the extremely small diameter of the intramedullary canal (5 mm). Moreover, a megaprosthesis of the tibia has a higher complication rate of infection because of the lack of sufficient soft tissue coverage. Prosthetic reconstruction was avoided because the condition of the skin was not good and the subcutaneous tissue was very thin. Another problem was the patellar tendon could not be reattached on the prosthesis and the extension mechanism was not enough after surgery. Owing to preservation of the epiphysis, there are other choices of biological reconstruction, such as vascularized fibular grafts, allografts, distraction osteogenesis, and tumor-devitalized autografts, in contrast to artificial prostheses. Vascularized bone transfer was limited by length and strength, and her fibula was not available because of hypoplasia (diameter was only 6 mm). Use of an allograft was a problem due to size. It is difficult to obtain bone allografts in our country due to limited numbers in the bone bank. A high incidence of infections or fractures after the use of allografts is also a concern. Distraction osteogenesis is well-known to regenerate living bone of sufficient strength, and osteogenesis, being a biological process, can be expected to form permanent bone, but she would need long-term use of an external fixator because of lower osteogenic potency and pin tract infection cannot be avoided due to the condition of her skin. We have developed a novel method of reconstruction with liquid nitrogen that has been implemented in patients since 1999[17]. The advantages of using frozen autografts are as follows: Simplicity; osteoinduction; osteoconduction; short treatment time; perfect fit; sufficient biochemical strength; no contagion; no need for bone banking; easy attachment of tendons and ligaments; and cryoimmunologic activity[24]. We also modified this technique to produce a pedicle frozen autograft to maintain anatomic continuity on one side. With this method, cutting both sides of the tumor bone is not necessary, thus providing advantages, such as fewer osteotomy sites, a shorter operative time, and early blood flow recovery.
In our case, a pedicle frozen autograft was performed and limb salvage was achieved 13 years post-operatively. The patient had a number of surgical site complications, such as wound dehiscence, and superficial and deep infections due to vulnerable skin in HGPS. If the megaprosthesis was chosen, infection could not be subsided and an amputation would eventually be done. Even though amputation was selected, a prosthetic leg would not be applicable because of the poor condition of the skin. To reduce the complications after frozen autograft surgery, there might be room to facilitate osteogenesis combined with vascularized bone graft or stem cell therapy in the future.
This patient had symptoms of HGPS at birth and was diagnosed at 5 years of age. Cardiovascular disorders did not develop until 24 years of age and thyroid cancer has been successfully managed by surgery and levothyroxine for 13 years. Due to the morphologic characteristics of bone, vulnerable skin condition, and decreased subcutaneous tissue, the treatment strategy for osteosarcoma in HGPS is complicated. A biological reconstruction method is recommended to manage post-operative complications.
A HGPS patient with osteosarcoma was successfully managed, although she had problems due to HGPS such as vulnerable skin, susceptibility to infection and small size of bone which might indicate amputation of affected limb. Limb salvage was achieved 13 years post-operatively owing to biological reconstruction using frozen recycling autograft of tumor-bearing bone. Her general health was good at the latest follow-up at 31 years of age.
Manuscript source: Unsolicited Manuscript
Specialty type: Orthopedics
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim HS, Zhu YF S-Editor: Zhang H L-Editor: A P-Editor: Liu JH
| 1. | Gilford, H. Progeria: a form of senilism. Practitioner 1904; 73: 188-217. |
| 2. | Hennekam RC. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A. 2006;140:2603-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 378] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 3. | Kreienkamp R, Gonzalo S. Metabolic Dysfunction in Hutchinson-Gilford Progeria Syndrome. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Sato-Kawano N, Takemoto M, Okabe E, Yokote K, Matsuo M, Kosaki R, Ihara K. The clinical characteristics of Asian patients with classical-type Hutchinson-Gilford progeria syndrome. J Hum Genet. 2017;62:1031-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Motegi S, Yokoyama Y, Uchiyama A, Ogino S, Takeuchi Y, Yamada K, Hattori T, Hashizume H, Ishikawa Y, Goto M, Ishikawa O. First Japanese case of atypical progeroid syndrome/atypical Werner syndrome with heterozygous LMNA mutation. J Dermatol. 2014;41:1047-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Coppedè F. Premature aging syndrome. Adv Exp Med Biol. 2012;724:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Doubaj Y, De Sandre-Giovannoli A, Vera EV, Navarro CL, Elalaoui SC, Tajir M, Lévy N, Sefiani A. An inherited LMNA gene mutation in atypical Progeria syndrome. Am J Med Genet A. 2012;158A:2881-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1602] [Cited by in RCA: 1566] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 9. | Coppedè F. The epidemiology of premature aging and associated comorbidities. Clin Interv Aging. 2013;8:1023-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Coppedè F, Migliore L. DNA repair in premature aging disorders and neurodegeneration. Curr Aging Sci. 2010;3:3-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | King CR, Lemmer J, Campbell JR, Atkins AR. Osteosarcoma in a patient with Hutchinson-Gilford progeria. J Med Genet. 1978;15:481-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Shalev SA, De Sandre-Giovannoli A, Shani AA, Levy N. An association of Hutchinson-Gilford progeria and malignancy. Am J Med Genet A. 2007;143A:1821-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Hayashi K, Tsuchiya H, Yamamoto N, Takeuchi A, Tomita K. Functional outcome in patients with osteosarcoma around the knee joint treated by minimised surgery. Int Orthop. 2008;32:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Gebert C, Hillmann A, Schwappach A, Hoffmann Ch, Hardes J, Kleinheinz J, Gosheger G. Free vascularized fibular grafting for reconstruction after tumor resection in the upper extremity. J Surg Oncol. 2006;94:114-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Watanabe K, Tsuchiya H, Yamamoto N, Shirai T, Nishida H, Hayashi K, Takeuchi A, Matsubara H, Nomura I. Over 10-year follow-up of functional outcome in patients with bone tumors reconstructed using distraction osteogenesis. J Orthop Sci. 2013;18:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Aponte-Tinao L, Ayerza MA, Muscolo DL, Farfalli GL. Survival, Recurrence, and Function After Epiphyseal Preservation and Allograft Reconstruction in Osteosarcoma of the Knee. Clin Orthop Relat Res. 2015;473:1789-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Tsuchiya H, Wan SL, Sakayama K, Yamamoto N, Nishida H, Tomita K. Reconstruction using an autograft containing tumour treated by liquid nitrogen. J Bone Joint Surg Br. 2005;87:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Takeuchi A, Yamamoto N, Hayashi K, Matsubara H, Miwa S, Igarashi K, Tsuchiya H. Joint-preservation surgery for pediatric osteosarcoma of the knee joint. Cancer Metastasis Rev. 2019;38:709-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Tsuchiya H, Nishida H, Srisawat P, Shirai T, Hayashi K, Takeuchi A, Yamamoto N, Tomita K. Pedicle frozen autograft reconstruction in malignant bone tumors. J Orthop Sci. 2010;15:340-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Kamiutsuri K, Kabata C, Yamada K, Yamamoto K. [Difficult mask ventilation and tracheal intubation in a patient with Hutchinson-Gilford syndrome]. Masui. 2009;58:493-495. [PubMed] |
| 21. | Anderson ME. Update on Survival in Osteosarcoma. Orthop Clin North Am. 2016;47:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 410] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 22. | Henderson ER, O'Connor MI, Ruggieri P, Windhager R, Funovics PT, Gibbons CL, Guo W, Hornicek FJ, Temple HT, Letson GD. Classification of failure of limb salvage after reconstructive surgery for bone tumours: a modified system Including biological and expandable reconstructions. Bone Joint J. 2014;96-B:1436-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 23. | Capanna R, Scoccianti G, Frenos F, Vilardi A, Beltrami G, Campanacci DA. What was the survival of megaprostheses in lower limb reconstructions after tumor resections? Clin Orthop Relat Res. 2015;473:820-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Nishida H, Yamamoto N, Tanzawa Y, Tsuchiya H. Cryoimmunology for malignant bone and soft-tissue tumors. Int J Clin Oncol. 2011;16:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |