Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11475
Peer-review started: August 10, 2021
First decision: September 2, 2021
Revised: September 22, 2021
Accepted: November 14, 2021
Article in press: November 14, 2021
Published online: December 26, 2021
Processing time: 135 Days and 3.7 Hours
Mucinous cystic neoplasm of the liver (MCN-L) is a cyst-forming epithelial neoplasm. The most distinguishing feature is the ovarian-type subepithelial stroma on pathological examination.
An abdominal ultrasound incidentally revealed a liver tumor in a 32-year-old woman. Physical and laboratory examination results did not reveal any abnormalities. Enhanced abdominal computed tomography (CT) revealed a cystic space measuring 7.2 cm × 5.4 cm in the liver. Subsequent CT showed an increase in tumor size. Thus, we performed surgical resection of the tumor and gallbladder. Postoperative histopathological examination confirmed the diagnosis of MCN-L. At the 6-mo of follow-up, no recurrence was observed on ultrasound or CT.
Since preoperative diagnosis of MCN-L is difficult, active surgery is recom
Core Tip: We have presented a case of mucinous cystic neoplasm of the liver that was diagnosed after surgical resection. The patient was asymptomatic and the disease was detected through abdominal imaging. Although we used multiple diagnostic modalities, an accurate diagnosis completely depended on postoperative pathological examination. We believe that surgical resection is an efficient option for cystic tumors of the liver found on imaging examination.
- Citation: Yu TY, Zhang JS, Chen K, Yu AJ. Mucinous cystic neoplasm of the liver: A case report. World J Clin Cases 2021; 9(36): 11475-11481
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11475.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11475
The World Health Organization (WHO) defines mucinous cystic neoplasm of the liver (MCN-L) as a cyst-forming epithelial neoplasm composed of epithelial cells that secrete mucus. The association of MCN-L with ovarian-type subepithelial stroma is characteristic and differentiates it from other cystic tumors of the liver[1]. Due to its low incidence, few studies have been conducted on MCN-L; thus, its etiology and pathogenesis are unclear. Some researchers have proposed that MCN-L contains an ovarian-like stroma due to the location of the liver near the gonads during embryonic development[2]. MCN-L usually has no obvious symptoms and may be incidentally discovered, such as in imaging tests. We report the case of a patient with simultaneous MCN-L, chronic viral hepatitis B infection, and endometrial carcinoma.
An asymptomatic 32-year-old female patient complained of a liver tumor during hospitalization in the Department of Obstetrics and Gynecology 4 mo prior. After follow-up, the tumor had increased in size.
Abdominal ultrasound performed before hysterectomy and adnexectomy revealed a liver mass. The patient had no abdominal pain, abdominal distension, nausea, vomiting, yellow skin and mucosa, or other digestive system symptoms.
The patient had a history of chronic viral hepatitis B infection. The remarkable history was carcinoma of the endometrium, for which she underwent hysterectomy and adnexectomy at our hospital.
The patient did not drink or smoke. Her relatives did not have a similar history of liver disease.
The patient’s body mass index was 17.33 kg/m2, her body temperature was 36 °C, her heart rate was 92 bpm, her respiratory rate was 16 beats per minute, and her blood pressure was 116/81 mmHg. Her abdomen was flat, with a surgical scar measuring approximately 15 cm visible on the lower abdomen. There were no abdominal varicose veins, gastrointestinal patterns, or peristaltic waves. The abdominal wall was soft, without tenderness, rebound pain, or palpable mass. No percussion pain was observed in the liver. The patient did not show shifting dullness or Murphy’s sign.
The patient’s hemoglobin count was 74 g/L, albumin count was 31.1 g/L, and carbohydrate antigen 19-9 (CA19-9) level was 60.30 U/mL. Her alpha-fetoprotein and carcinoembryonic antigen levels were within the normal ranges, while those of alanine aminotransferase, aspartate aminotransferase, and surface antigen of hepatitis B virus were above the normal limits. Hematological examination showed that the patient also had anemia, hypoalbuminemia, and chronic viral hepatitis B infection.
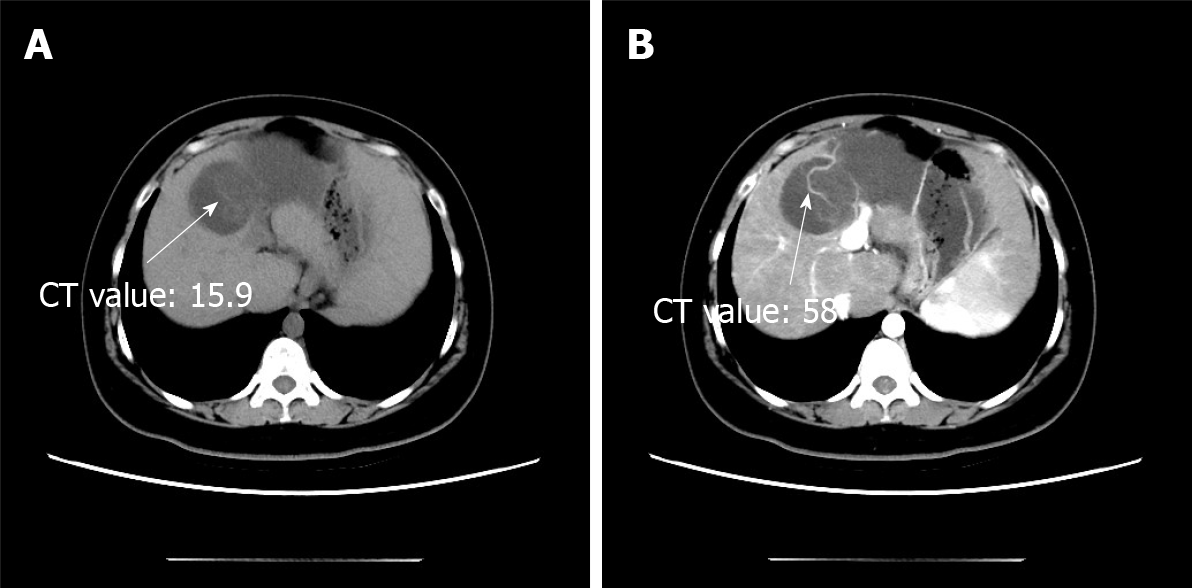
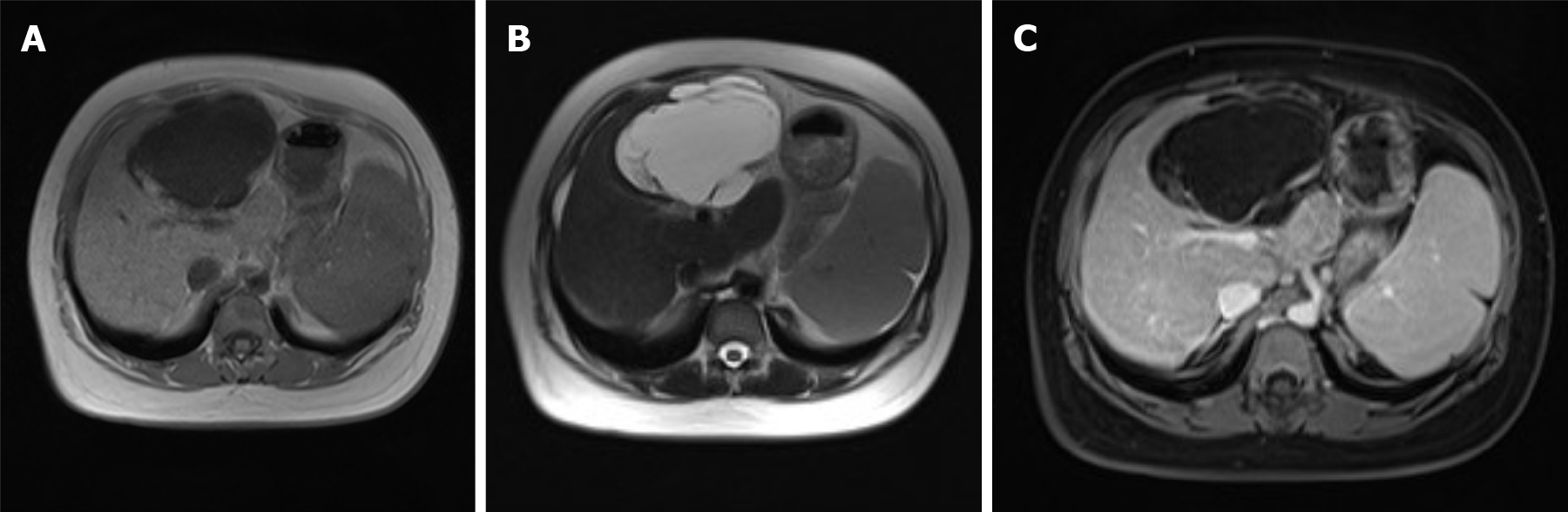
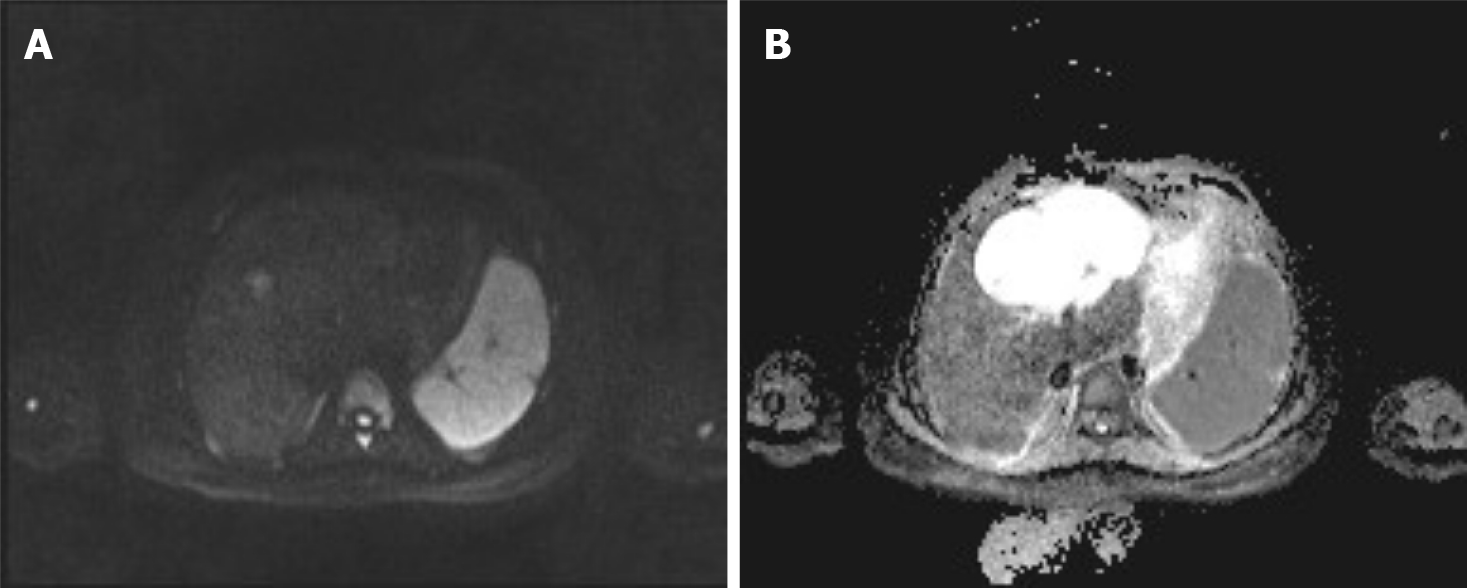
During a previous hospitalization, the patient underwent enhanced computed tomography (CT) of the liver, gallbladder, and spleen, which revealed an irregular multilocular cyst at the border area of the left and right lobes in the liver measuring approximately 7.2 cm × 5.4 cm. The presence of a hepatic hydatid cyst cannot be excluded. Follow-up CT performed 4 mo later showed that the tumor had increased in size by 2 cm (Figure 1). For further examination, the patient underwent enhanced magnetic resonance imaging (MRI) and diffusion-weighted imaging (DWI) (Figure 2). Irregular multilocular cystic shadows were observed in the liver, the cystic wall was not smooth, and nodules were visible. The cystic wall and separations were significantly strengthened on enhanced MRI, and restricted diffusion of the cystic wall nodules was observed on DWI (Figure 3). Multiple larger lymph nodes were observed retroperitoneally.
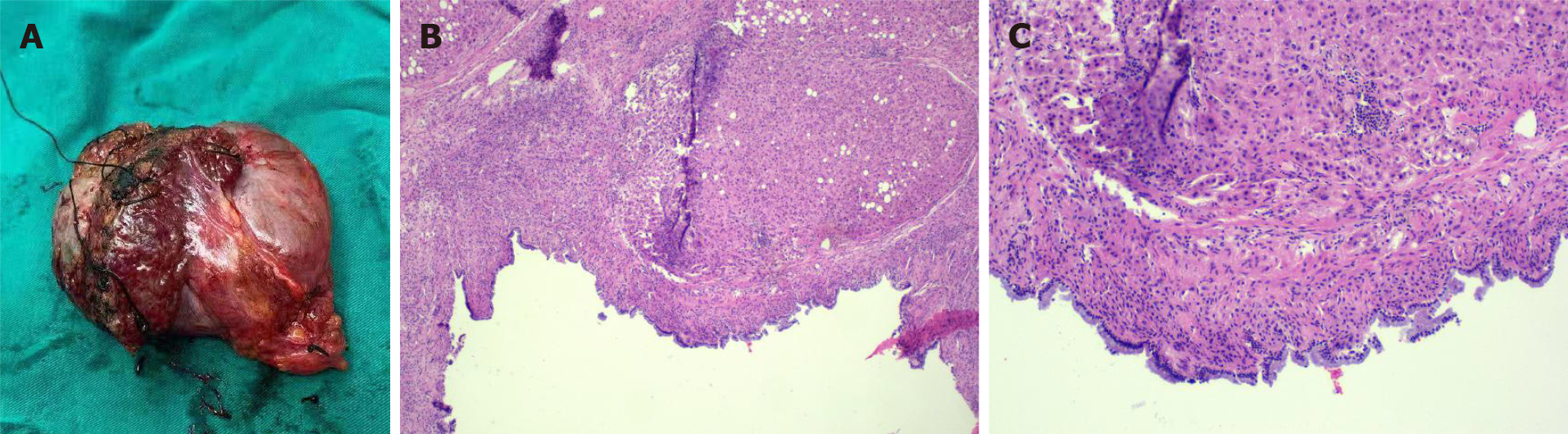
The pathological material was liver tissue measuring 9 cm × 8 cm × 4 cm. Mucus was observed after incision. Light microscopy showed fibrous cyst wall tissue covered by tall columnar epithelium. The diagnosis by the Department of Pathology was MCN-L (Figure 4).
The patient requested surgical treatment. We performed resection of the gallbladder and the tumor. During surgery, we noted a cystic tumor approximately 10 cm in diameter in segment IV of the liver. The tumor was bulging into the abdominal cavity and was densely adhered to the first porta of the liver, gallbladder, and greater omentum. Nodular changes on the liver surface and enlarged spleen were consistent with the appearance of hepatic cirrhosis.
After surgery, the patient’s anemia and hypoalbuminemia were aggravated, therefore she was administered a blood transfusion and albumin infusion.
The patient recovered uneventfully and was discharged from the hospital on day 22 of admission (day 16 after surgery). The patient was followed up for 6 mo postoperatively, with no signs of recurrence or metastasis on CT and ultrasound.
As described above, the 5th edition of the WHO Classification of Digestive System Tumours categorizes MCN-L as a benign tumor of the liver and intrahepatic bile ducts. MCN-L has potential for malignant transformation. The WHO also indicates that MCN-L is rare, with an incidence of one case per 20000-100000 persons[1]. MCN-L tends to occur in middle-aged women and accounts for < 5% of all cases of hepatic cystic diseases with a low malignant transformation rate and good prognosis[3]. MCN-L occurs principally in the left hepatic lobe, but it occasionally occurs in other hepatic lobes and intrahepatic or extrahepatic biliary systems. The International Classification of Diseases for Oncology coding categorizes MCNs as mucinous cystic neoplasms (MCNs) with low-grade intraepithelial neoplasia, MCN with high-grade intraepithelial neoplasia, and MCN with associated invasive carcinoma[1].
It is difficult for patients to identify the disease because the symptoms are not obvious. Patients with larger tumors may develop abdominal discomfort, pain, jaundice, or other symptoms if the tumor compresses the surrounding tissue[4]. Laboratory examination of patients with MCN-L usually does not reveal abnormalities. Traditionally, an increased serum CA19-9 Level was regarded as a sign of MCN-L incidence. However, this correlation was not statistically confirmed in a recent study[5]. There have been no other reports of diagnostic serum biomarkers.
Imaging examination is a valid method for initial diagnosis. On ultrasonography, MCN-L appears as an anechoic mass with thin separation. Some lesions with cystic wall fibrosis, calcification, or intracystic hemorrhage may present as focal hyperechoic areas. On CT and MRI, MCN-L usually presents as a multilocular or multiseptated cystic mass[6]. However, considering the similar imaging features, the distinguishing between the differential diagnoses of MCN-L, such as simple liver cysts, Caroli disease, and hepatic echinococcosis, is difficult[7].
Our patient underwent enhanced CT, enhanced MRI, and DWI. Non-contrast-enhanced CT revealed a hypodense multilocular cystic lesion mainly in the left lobe of the liver, with the longest diameter of 92 mm. The CT values of the lesion were around -5-20 HU. After contrast injection, the cyst wall and intracystic separations were hyperdense in the hepatic arterial, portal venous, and late phases, with CT values of approximately 40-120 HU. On conventional MRI, the lesion appeared hypointense on T1-weighted images and hyperintense on T2-weighted images. The imaging findings of the enhanced MRI were similar to those of the enhanced CT, and nodules were observed in the cyst wall. Diffusion of cystic wall nodules on DWI was restricted. We considered the lesion to be a hepatic cystic disease and could not exclude hepatic hydatid cysts. Based on this consideration, the patient underwent resection, and the nature of the disease was determined by pathological examination.
In general, patients with small tumors may be followed up. The indications for resection are a large lesion, an obvious increase in size over a short follow-up period, and manifestation of symptoms. Surgery, specifically radical resection, is the primary mode of treatment for patients with MCN-L. It should be noted that improper surgical treatment may lead to disease recurrence[8].
Pathologically, the macroscopic appearance of MCN-L is a multilocular cystic lesion with clear boundaries. The cysts usually contain clear mucinous fluid, and the inner surface is smooth or trabeculated. Histopathologically, it is easy to diagnose MCN-L because of its characteristic composition of ovarian stroma. The inner face of the cysts is comprised of mucus-secreting columnar, cuboidal, and flattened epithelial cells arranged in a single, flat row with occasional polypoid or papillary projections. The tissue under the epithelium is a layer of hypercellular ovarian-like stroma surrounded by a dense layer of collagenous fibrous tissue. The mesenchyme resembled an ovarian-like stroma containing a compact arrangement of spindle-shaped cells and oval nuclei[9]. Half of all reported cases showed diffuse ovarian-like stroma, while the other half showed focal ovarian-like stroma[1]. Other MCNs originating in the pancreas, spleen, or retroperitoneum, as well as mucinous neoplasms of the ovary, show similar clinicopathological features to those of MCN-L[10]. Some researchers consider MCN-L to be the counterpart of MCNs of the pancreas[11]. One hypothesis suggests that the ectopic ovarian stroma originates from the gonads because the affected tissues and organs are in close contact with embryonic gonads[12]. Between the fifth and eighth weeks of embryonic development, the gonads are located under the diaphragm at the level of the back of the liver, spleen, and tail of the pancreas. The epithelial cells of the gonads migrate from the posterior wall of the yolk sac to the surface of nearby organs along the wall of the hindgut[2]. It is important to consider that MCN-L can progress to invasive carcinoma, although its degree of malignancy is less than that of primary liver cancer[13].
The ovarian stroma in MCN-L expresses progesterone receptors (PR) and estrogen receptors (ER)[12,14]. However, the immunohistochemical results of this case showed no staining for ER and PR. Considering the patient's history of hysterectomy and oophorectomy, this result may be relevant to her treatment for endometrial cancer.
It has been reported that the steroidogenic features of MCN-L differ from those of ovarian MCN. Ovarian-like stroma in MCN-L is similar to that in postmenopausal ovaries in terms of androgenic dynamics[15]. The feasibility of preoperative biopsy and immunohistochemistry needs to be further studied.
The low incidence of MCN-L leads to unclear recognition of this disease in terms of etiology, pathogenesis, and prognosis, and difficulties in diagnosis before surgery. Multicenter randomized controlled trials or more clinical cases are beneficial for the recognition of MCN-L. At present, radical resection and pathological examinations are reasonable treatment strategies.
We would like to extend thanks to our colleagues, Dr. Zhao Y for sharing and giving permission to pathological images; Dr. Xing QC for providing and giving permission to radiological images and for their continuous guidance and critical comments.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferran JL, Gokce E, Yu R S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | WHO Classification of Tumours Editorial Board. Digestive System Tumours. WHO Classification of Tumours. 5th ed. Lyon, France: IARC publications, 2019: 250-253. |
| 2. | Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, Sessa F, Capella C, Solcia E, Rickaert F, Mariuzzi GM, Klöppel G. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 397] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Nakayama Y, Kato Y, Okubo S, Takahashi D, Okada R, Nishida Y, Kitaguchi K, Gotohda N, Takahashi S, Konishi M. A case of mucinous cystic neoplasm of the liver: a case report. Surg Case Rep. 2015;1:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Ferreira R, Abreu P, Jeismann VB, Segatelli V, Coelho FF, David AI. Mucinous cystic neoplasm of the liver with biliary communication: case report and surgical therapeutic option. BMC Surg. 2020;20:328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Gao J, Zheng J, Cai J, Kirih MA, Xu J, Tao L, Liang Y, Feng X, Fang J, Liang X. Differentiation and management of hepatobiliary mucinous cystic neoplasms: a single centre experience for 8 years. BMC Surg. 2021;21:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Mavilia MG, Pakala T, Molina M, Wu GY. Differentiating Cystic Liver Lesions: A Review of Imaging Modalities, Diagnosis and Management. J Clin Transl Hepatol. 2018;6:208-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Erdogan D, Kloek J, Lamers WH, Offerhaus GJ, Busch OR, Gouma DJ, van Gulik TM. Mucinous cystadenomas in liver: management and origin. Dig Surg. 2010;27:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Muralidhar V, Santhaseelan RG, Ahmed M, Shanmuga P. Simultaneous occurrence of hepatic hydatid cyst and mucinous cystadenoma of the liver in a middle-aged female patient: report of a rare case. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Yamao K, Yanagisawa A, Takahashi K, Kimura W, Doi R, Fukushima N, Ohike N, Shimizu M, Hatori T, Nobukawa B, Hifumi M, Kobayashi Y, Tobita K, Tanno S, Sugiyama M, Miyasaka Y, Nakagohri T, Yamaguchi T, Hanada K, Abe H, Tada M, Fujita N, Tanaka M. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas. 2011;40:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 10. | Shiono S, Suda K, Nobukawa B, Arakawa A, Yamasaki S, Sasahara N, Hosokawa Y, Suzuki F. Pancreatic, hepatic, splenic, and mesenteric mucinous cystic neoplasms (MCN) are lumped together as extra ovarian MCN. Pathol Int. 2006;56:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Sato K, Urakawa H, Sakamoto K, Ito E, Fujita K, Goto K, Honda G, Hamada Y, Yoshimitsu K. Mucinous cystic neoplasm of the liver communicated with intrahepatic duct exhibiting peculiar chronological change in MR imaging appearances: a case report. Abdom Radiol (NY). 2020;45:2257-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Van Treeck BJ, Lotfalla M, Czeczok TW, Mounajjed T, Moreira RK, Allende DS, Reid MD, Naini BV, Westerhoff M, Adsay NV, Kerr SE, Rizvi SH, Smoot RL, Liu Y, Davila J, Graham RP. Molecular and Immunohistochemical Analysis of Mucinous Cystic Neoplasm of the Liver. Am J Clin Pathol. 2020;154:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Usyaky PV, Kubyshkin VA, Vishnevsky VA, Kovalenko YA, Karel'skaya NA, Kalinin DV, Demidova VS, Varlamov AV. [Mucinous cystic liver tumors: diagnosis and surgical treatment]. Khirurgiia (Mosk). 2016;27-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Kumata H, Murakami K, Ishida K, Miyagi S, Arakawa A, Inayama Y, Kinowaki K, Ochiai A, Kojima M, Higashi M, Moritani S, Kuwahara K, Nakatani Y, Kajiura D, Tamura G, Kijima H, Yamakawa M, Shiraishi T, Inadome Y, Suzuki H, Sawai T, Unno M, Kamei T, Sasano H. Steroidogenesis in ovarian-like mesenchymal stroma of hepatic and pancreatic mucinous cystic neoplasms. Hepatol Res. 2018;48:989-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Lam MM, Swanson PE, Upton MP, Yeh MM. Ovarian-type stroma in hepatobiliary cystadenomas and pancreatic mucinous cystic neoplasms: an immunohistochemical study. Am J Clin Pathol. 2008;129:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |