Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11457
Peer-review started: July 22, 2021
First decision: September 2, 2021
Revised: September 8, 2021
Accepted: November 18, 2021
Article in press: November 18, 2021
Published online: December 26, 2021
Processing time: 154 Days and 8.3 Hours
Paraneoplastic syndromes are characterized by atypical clinical manifestations. Several reports of hepatocellular carcinoma (HCC) paraneoplastic phenomena have been reported. They usually manifest as one type in an individual, but it is not common for the two clinical manifestations to occur simultaneously.
A 52-year-old female patient was admitted to hospital with pale skin and numbness of the second to fifth fingers in the left hand, which rapidly developed into severe digital ischemia. Computed tomography angiography revealed uneven thickness of the left ulnar artery with severe local luminal stenosis. Blood analysis during hospitalization showed persistent mild to medium thrombocytopenia and insensitive to hormonal therapy. Antiphospholipid antibody testing showed high titer of IgG anticardiolipin antibodies (aCLs), IgA aCLs, IgG anti-β2-glycoprotein-I (anti-β2 GPI), and IgA anti-β2 GPI. The exact diagnosis was HCC when the high a-fetoprotein levels, computed tomography findings, and the history of chronic hepatitis B came together. This was a rare case of coexisting manifestations as presenting symptoms of malignancy-associated antiphospholipid syndrome. The patient underwent several operations, antithrombotic treatments and hormonal therapy. However, the patient refused chemotherapy and died 8 wk after diagnosis.
This report highlights the importance of atypical clinical changes that could alert the physicians to vigilance for a concomitant underlying malignancy.
Core Tip: Paraneoplastic syndromes, compared with cancer, are characterized by atypical clinical manifestations. This report described a case of hepatocellular carcinoma in which the presenting symptom was severe digital ischemia, mimicking Raynaud’s phenomenon, accompanied with thrombocytopenia, associated with antiphospholipid antibodies. This report highlights the importance of atypical clinical changes that could alert the physicians to be vigilant for concomitant underlying malignancy.
- Citation: Chen JL, Yu X, Luo R, Liu M. Severe digital ischemia coexists with thrombocytopenia in malignancy-associated antiphospholipid syndrome: A case report and review of literature. World J Clin Cases 2021; 9(36): 11457-11466
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11457.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11457
Paraneoplastic syndromes are signs or symptoms that result from tissue damage caused by malignant tumors other than mass effects or metastasis. The most commonly reported paraneoplastic syndromes were hematological followed by rheumatological, dermatological, endocrine and neurological syndromes[1]. Therefore, diseases that feature an advanced age at onset, significant constitutional upset, and otherwise atypical characteristics should increase the index of suspicion for a paraneoplastic syndrome[2]. Like other solid tumors, lymphoproliferative diseases, and hematological cancers, hepatocellular carcinoma (HCC) has a few reports about paraneoplastic phenomena, including hypoglycemia[3], hypertension[4], hypercalcemia[5], Raynaud’s phenomenon (RP)[6], and dermatomyositis[7]. They usually manifest as one type in an individual, but it is not common for the two clinical manifestations to occur simultaneously. This report described one case of HCC in which the presenting symptom was severe digital ischemia accompanied with thrombocytopenia, associated with antiphospholipid antibodies (aPLs).
A 52-year-old female patient was admitted to hospital with pale skin and numbness of the left second to fifth fingers.
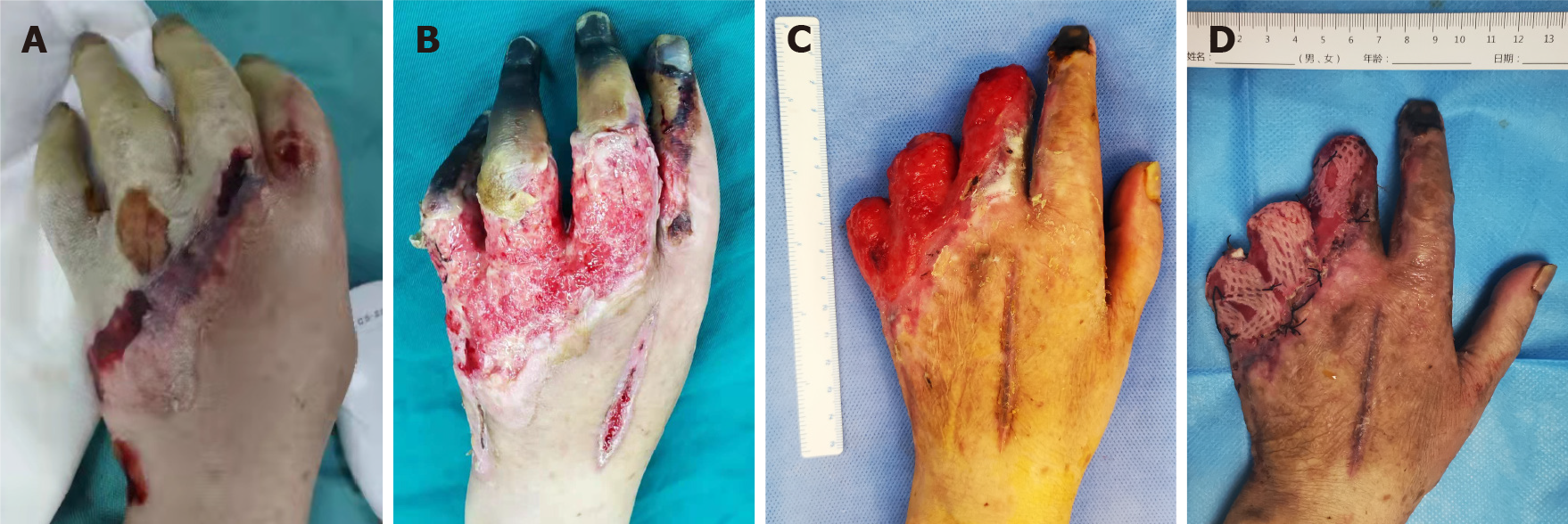
The patient’s symptoms started unprovoked about 1 d before when she rose in the morning. The left hand swelled quickly and showed a clear oblique blood supply boundary line (Figure 1A). Thus, the patient attended the emergency department.
The patient had no other medical history but was an asymptomatic carrier of hepatitis B virus for nearly 10 years. The patient had no history of hypertension or type 2 diabetes.
The patient married at a young age and had two daughters as well as no history of vascular thrombosis and pregnancy morbidity.
The patient’s vital signs were stable and oxygen saturation in room air was 99%, but left digital oxygen saturation was not detectable by the finger pulse oximeter. The skin temperature of the left second to fifth fingers decreased. The pulse of the left radial artery could be felt, but not that of the ulnar artery.
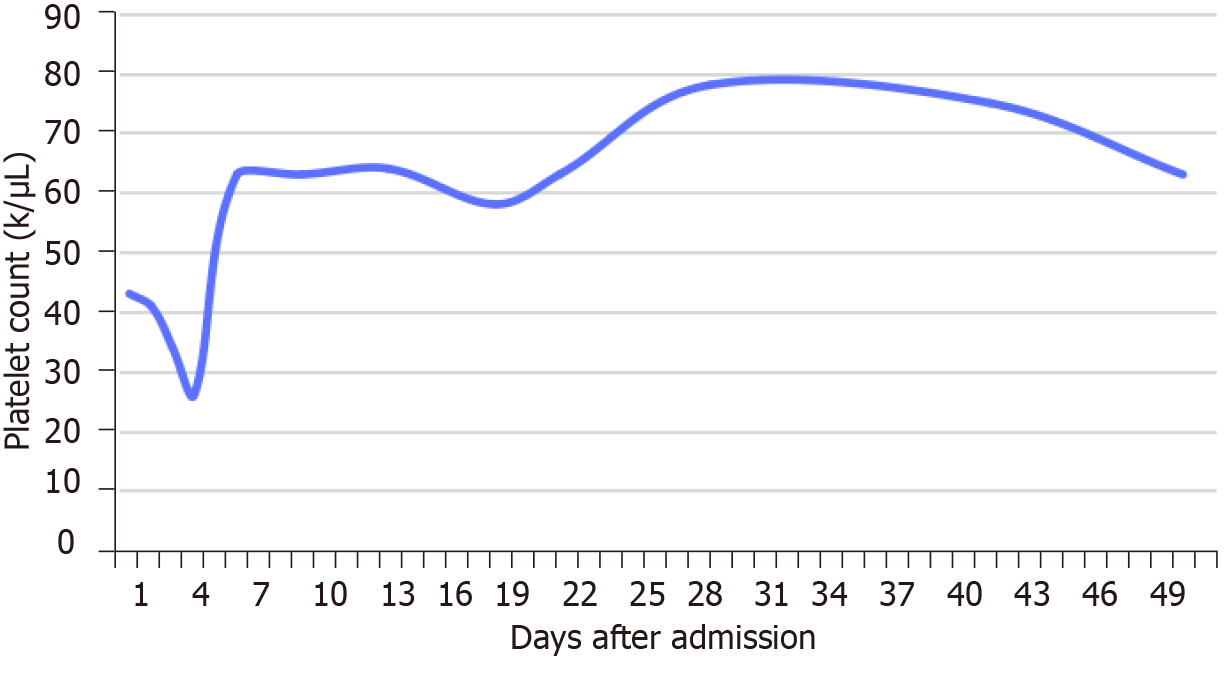
Blood analysis during hospitalization showed a persistent mild to medium thrombocytopenia, with normal red blood cell and white blood cell. The changes in platelet count during hospitalization are presented in Figure 2. D-dimer was increased at 0.82 mg/L, with normal prothrombin time, international normalized ratio, fibrinogen, and activated partial thromboplastin time. Serum C-reactive protein was also normal and erythrocyte sedimentation rate was at 36 mm/h. Serological tests for human immunodeficiency virus and syphilis were negative. Serum complement and immunoglobulins were abnormal. aPL testing showed positive results: High titer (> 120 GPLU/mL) IgG anticardiolipin (aCL) antibodies, high titer (57 APLU/mL) IgA aCL, high titer (> 200 AU/mL) IgG anti-β2-glycoprotein-I (anti-β2 GPI), and high titer (90.10 AU/mL) IgA anti-β2 GPI. However, IgM aCL (4.24 MPLU/mL) and IgM anti-β2 GPI (2 AU/mL) were normal. Antinuclear antibody was also positive. However, lupus anticoagulant (LA) and Coombs’ test were negative. Serum carcinoembryonic antigen and CA 19-9 were within normal limits. However, serum a-fetoprotein level was dramatically increased to 1210 ng/mL. Electrocardiography and chest X-ray were normal. The laboratory values are shown in Table 1.
| Item | Laboratory value | Reference value |
| RBC (1012/L) | 3.68 | 3.8-5.1 |
| WBC (109/L) | 6.81 | 3.5-9.5 |
| Platelet count (109/L) | 41 | 150-450 |
| D-dimer (mg/L) | 0.82 | < 0.55 |
| Prothrombin time (s) | 11.1 | 9.6-12.8 |
| Activated partial thromboplastin time (s) | 28.2 | 24.8-33.8 |
| Fibrinogen (g/L) | 2.82 | 2.0-4.0 |
| International normalized ratio | 0.98 | 0.88-1.15 |
| Serum C-reactive protein (mg/L) | 4.77 | < 5 |
| ESR (mm/h) | 36 | < 38 |
| HIV | Negative | Negative |
| HBV | Positive | Negative |
| Syphilis | Negative | Negative |
| Serum complement C3 (g/L) | 0.899 | 0.785-1.520 |
| Serum complement C4 (g/L) | 0.209 | 0.145-0.360 |
| IgG (g/L) | 10.6 | 8.0-15.5 |
| IgA (mg/L) | 950 | 836-2900 |
| IgM (mg/L) | 1240 | 700-2200 |
| IgG anticardiolipin antibodies (GPLU/mL) | > 120 | < 10 |
| IgA anticardiolipin antibodies (APLU/mL) | 57 | < 10 |
| IgM anticardiolipin antibodies (MPLU/mL) | 4.24 | < 10 |
| IgG anti-β2 -glycoprotein-I (AU/mL) | > 200 | < 20 |
| IgA anti-β2 -glycoprotein-I (AU/mL) | 90.1 | < 20 |
| IgM anti-β2 -glycoprotein-I (AU/mL) | 2 | < 20 |
| Antinuclear antibody | +1:100 | Negative |
| Lupus anticoagulant | Negative | Negative |
| Coombs’ test | Negative | Negative |
| Carcinoembryonic antigen (ng/mL) | 1.87 | < 5 |
| α-fetoprotein | > 1210 | < 7 |
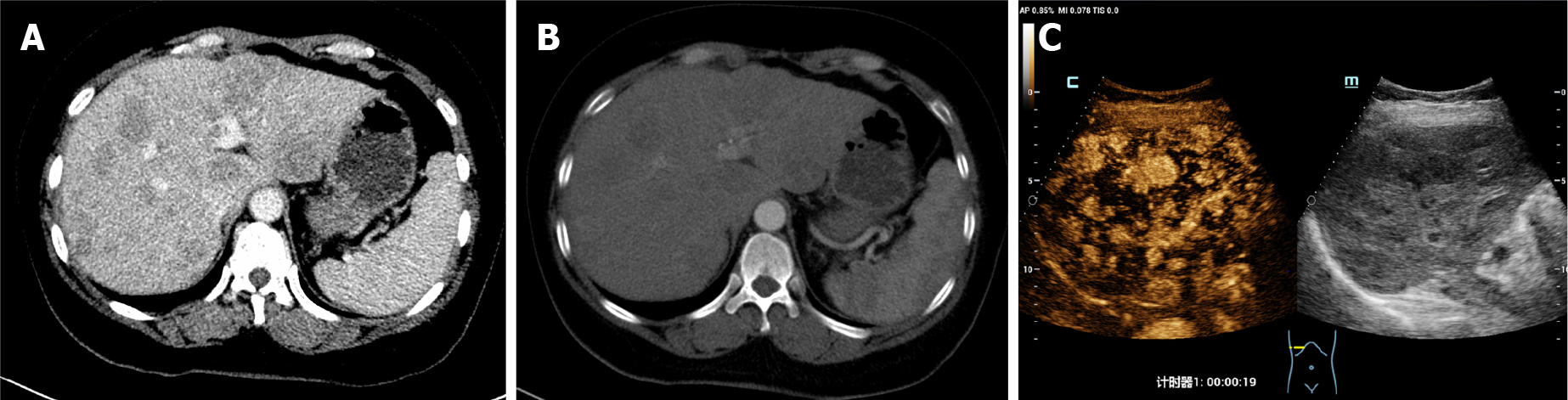
Arteriovenous color Doppler ultrasound of the upper extremity revealed that the blood flow signal of the proper digital artery was poor. The left upper brachial artery was further evaluated with computed tomography (CT) angiography. The latter revealed uneven thickness at the bifurcation of the left ulnar artery with severe local lumen stenosis, leading to slender branches, delayed imaging, and light imaging. It suggested that digital necrosis was mainly caused by a decrease in blood supply. Left hand X-ray showed no obvious bone destruction. On abdominal CT, dimensions of the liver were normal but contours were irregular. There were several mass lesions that appeared as mixed contrast in the arterial phase (Figure 3A) and lost their contrast in the portal venous phase (Figure 3B). Color Doppler ultrasound in the abdomen showed liver cirrhosis with multiple solid masses in the liver, and high enhancement in the arterial phase (Figure 3C).
The final diagnosis was HCC, left digital ischemia, thrombocytopenia, non-criteria antiphospholipid syndrome (APS), and chronic hepatitis B (old).
The patient underwent three operations: emergency fasciotomy, elective finger amputation and skin grafting. Severe swelling of the hand leads to osteofascial syndrome, which can cause continuous deterioration of local capillary blood supply. Therefore, fasciotomy was performed upon admission to relieve the pressure within the fascial compartment. About 1 wk after admission, the proximal skin of the third to fifth fingers of the left hand were necrotic and exfoliated, and dry gangrene occurred in the fingertips (Figure 1B). Therefore, finger amputation was performed to completely remove the necrotic tissue (Figure 1C). Finally, after the wound was fresh and up to standard, the patient underwent autologous skin grafting (Figure 1D).
Intensified antithrombotic treatments with antiplatelets (aspirin 100 mg/d), and vascular microcirculation disorders improvement (papaverine hydrochloride 120 mg/d, cinepazide maleate 320 mg/d, alprostadil 2 mL/d), anticoagulants (low molecular weight heparin 4000 IU/d) and hormonal therapy (prednisone 30 mg/d) were performed.
For anticoagulant therapy, heparin was replaced with rivaroxaban (10 mg/d) after discharge. In addition, the patient refused chemotherapy for HCC and died 8 wk after diagnosis.
Paraneoplastic phenomena may be the first sign of undiagnosed cancer, as in this case. Although the clinical manifestations of tumors and paraneoplastic syndromes are different, clinicians should realize that paraneoplastic phenomena are not caused by tumor mass effects or metastasis[2].
Paraneoplastic syndromes of HCC are not uncommon, and the incidence was reported to range from 20% to 31%[8,9]. Most reports indicate that paraneoplastic syndromes are associated with poor outcomes, but whether it is an independent prognostic factor for reduced HCC survival remains unclear[9,10]. The published case reports of paraneoplastic syndromes in HCC are summarized in Table 2. Although the reported cases of paraneoplastic syndromes are diverse, they can be grouped into six categories: Paraneoplastic hematologic syndrome, paraneoplastic rheumatologic syndrome, paraneoplastic dermatologic syndrome, paraneoplastic endocrine syndrome, paraneoplastic neurologic/neuropsychiatric syndrome, and paraneoplastic miscellaneous syndrome. In addition, the paraneoplastic syndromes usually appear before an HCC diagnosis. A study further found that erythrocytosis and hypercholesterolemia often developed earlier in the clinical course while hypoglycemia and hypercalcemia were usually terminal events[8]. In addition, most patients present one paraneoplastic syndrome and the occurrence of coexisting syndromes is uncommon. To our knowledge, this was a rare case and the first report of HCC accompanied with paraneoplastic thrombocytopenia and severe digital ischemia, mimicking RP, as well as being associated with APS.
| Category | Associated diseases/condition | Ref. |
| Hematologic syndromes | Severe eosinophilia | Yuen et al[23], 1995 |
| Hemophagocytic syndrome | Sakai et al[24], 2001 | |
| Erythrocytosis | Tsuchiya et al[25], 2009 | |
| Leukemoid reaction | Shin et al[26], 2011 | |
| Thrombocytosis | Abbas et al[27], 2019 | |
| Rheumatologic syndromes | Raynaud's phenomenon | Sahan et al[6], 2006 |
| Polymyositis | Thanapirom et al[28], 2014 | |
| Dermatomyositis | Chou et al[7], 2017 | |
| Polyarthritis | Sathiyapalan et al[29], 2021 | |
| Dermatologic syndromes | Symptomatic porphyria | Ochiai et al[30], 1997 |
| Disseminated superficial porokeratosis | Kono et al[31], 2000 | |
| Cutaneous lupus erythematosus | Ho et al[32], 2001 | |
| Erythema nodosum | Glinkov et al[33], 2003 | |
| Pemphigus | Yokokura et al[34], 2006 | |
| Generalized granuloma annulare | Cho et al[35], 2018 | |
| Endocrine syndromes | Hypercholinesterasemia | Tajiri et al[36], 1983 |
| Hyperlipidemia | Makino et al[37], 1986 | |
| Hyperestrogenia | Salles et al[38], 1987 | |
| Hyperthyroidism | Carri et al[39], 1989 | |
| Hyperthyroxinemia | Nizam et al[40], 1995 | |
| Carcinoid syndrome | Nwokediuko et al[41], 2010 | |
| Hypercalcemia | Newman et al[5], 2015 | |
| Hyperglycemia | Kim et al[42], 2015 | |
| Neurologic or neuropsychiatric syndromes | Necrotizing myelopathy | Misumi et al[43], 1988 |
| Neurologic manifestations | Norris et al[44], 1997 | |
| Demyelinating polyneuropathy | Walcher et al[45], 2002 | |
| Peripheral neuropathy | Matsui et al[46], 2015 | |
| Neuropsychiatric manifestations | Karam et al[47], 2020 | |
| Hyperammonemic encephalopathy | Lee et al[48], 2021 | |
| Miscellaneous syndromes | Hypertension | Arai et al[4], 1999 |
| Membranous glomerulonephritis | Texier et al[49], 2004 | |
| Nummular loss of the retinal pigment epithelium | Lee et al[50], 2007 | |
| Myasthenia gravis | Vautravers et al[51], 2008 | |
| Rhabdomyolysis | Bárdos et al[52], 2021 |
RP mainly involves bilateral fingers and mostly presents in young female patients. It is an episodic disease with short pallor phase, but not trophic changes and endothelial damage[6]. However, severe, asymmetric RP that occurs after age 50 years may be a paraneoplastic phenomenon. RP has been found in various malignancies including lung cancer, renal cell carcinoma, and melanoma. In addition, > 80% of patients may progress to gangrene and ischemic necrosis[11].
In a French study, 15% of patients admitted for an initial occurrence of digital ischemia had an underlying cancer, including adenocarcinoma, squamous cell carcinoma, and lymphoid neoplasia[12]. However, the mechanism of paraneoplastic RP is still unknown, and its possible physiological mechanism includes a vasoconstrictive substance secreted by the tumor cells[13]. In the French study, it was suggested that paraneoplastic RP was mainly related to thrombocytosis[12]. However, this view contradicts the significant decrease in platelet count in our case, indicating the heterogeneous effects on the blood system (especially platelets) in different tumors. The etiology of thrombocytopenia can be categorized as problems of sequestration, decreased platelet production, increased platelet destruction, or increased platelet consumption. Corticosteroids can serve both therapeutic and diagnostic purposes, with positive response to these interventions lending support to an immune-mediated process[14]. In our case, the above view is also supported by the fact that the patient’s platelet count gradually recovered after oral prednisone treatment.
Based on the Sydney criteria, the core clinical manifestations of APS are arterial or venous thrombosis and obstetric complications and aPLs confirmed by laboratory detection[15]. Among them, the aPLs are defined as LA, aCL, and anti-β2GPI antibodies.
In addition, according to the Euro-Phospholipid Project, there are many common manifestations of APS, such as arthralgia (38.7%), immune thrombocytopenia (29.6%), arthritis (27.1%), migraine (20.2%), stroke (19.8%), but digital ischemia was rare, in only 3.3% of APS patients[16]. APS may be primary or related to a variety of diseases, such as systemic lupus erythematosus, Sjogren’s syndrome, infectious agents, and medication[17]. In particular, APS may be associated with various solid and hematological malignancies[18]. There are many case reports about aPLs antibodies related to malignant tumors, and their manifestations are also diverse. A Malaysian review has been fully summarized and reported[18]. However, the role and clinical significance of aPLs in the occurrence and development of malignant tumors are not clear. Since most cancer patients have thromboembolism, and aPLs are responsible for thrombus formation, it is speculated that aPLs may have a direct impact on thrombosis or contribute to the pathogenesis of cancer[18]. In addition, several mechanisms have been suggested to explain the association between aPLs and cancer including: (1) Production of autoantibodies as a response to tumor antigens; (2) Secretion of aCL antibodies from tumor cells; and (3) Production of monoclonal immunoglobulins with LA and aCL activities[19].
The relationship between RP and aPLs is also lacking, because vasospasm in small muscular arteries and finger arterioles rather than aPLs-mediated thrombosis is the underlying mechanism of the phenomenon[20].
Based on the APS criteria revision in the report of the 14th international congress on aPLs technical task force on APS clinical features[21], non-criteria manifestations of APS were considered as those suggested, recommended, or strongly recommended to be included. Thrombocytopenia is one of the major non-obstetric manifestations of APS, whereas RP is a minor one[22].
In elderly patients, the atypical clinical manifestations could alert the physicians to be vigilant for a concomitant underlying malignancy. The causality or interaction between cancer and paraneoplastic syndromes should be further studied.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gaman MA S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | El Rassy E, Assi T, Kattan J, Pavlidis N. Paraneoplastic syndromes in cancers of unknown primary: An unknown field for oncologists. Bull Cancer. 2019;106:590-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Khan F, Kleppel H, Meara A. Paraneoplastic Musculoskeletal Syndromes. Rheum Dis Clin North Am. 2020;46:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Saigal S, Nandeesh HP, Malhotra V, Sarin SK. A case of hepatocellular carcinoma associated with troublesome hypoglycemia: management by cytoreduction using percutaneous ethanol injection. Am J Gastroenterol. 1998;93:1380-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Arai H, Saitoh S, Matsumoto T, Makita F, Mitsugi S, Yuasa K, Takagi H, Mori M. Hypertension as a paraneoplastic syndrome in hepatocellular carcinoma. J Gastroenterol. 1999;34:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Newman NB, Jabbour SK, Hon JD, Berman JJ, Malik D, Carpizo D, Moss RA. Hepatocellular Carcinoma Without Cirrhosis Presenting With Hypercalcemia: Case Report and Literature Review. J Clin Exp Hepatol. 2015;5:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Sahan C, Ucer T, Aksakal E. A case of hepatocellular carcinoma who admitted with Raynaud's phenomenon. Rheumatol Int. 2006;27:87-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Chou JW, Lin YL, Cheng KS, Wu PY, Reanne Ju T. Dermatomyositis Induced by Hepatitis B Virus-related Hepatocellular Carcinoma: A Case Report and Review of the Literature. Intern Med. 2017;56:1831-1837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Luo JC, Hwang SJ, Wu JC, Lai CR, Li CP, Chang FY, Chiang JH, Lui WY, Chu CW, Lee SD. Clinical characteristics and prognosis of hepatocellular carcinoma patients with paraneoplastic syndromes. Hepatogastroenterology. 2002;49:1315-1319. [PubMed] |
| 9. | Qu Q, Wang S, Chen S, Zhou L, Rui JA. Prognostic role and significance of paraneoplastic syndromes in hepatocellular carcinoma. Am Surg. 2014;80:191-196. [PubMed] |
| 10. | Chang PE, Ong WC, Lui HF, Tan CK. Epidemiology and prognosis of paraneoplastic syndromes in hepatocellular carcinoma. ISRN Oncol. 2013;2013:684026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Paw P, Dharan SM, Sackier JM. Digital ischemia and occult malignancy. Int J Colorectal Dis. 1996;11:196-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Le Besnerais M, Miranda S, Cailleux N, Girszyn N, Marie I, Lévesque H, Benhamou Y. Digital ischemia associated with cancer: results from a cohort study. Medicine (Baltimore). 2014;93:e47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Poszepczynska-Guigné E, Viguier M, Chosidow O, Orcel B, Emmerich J, Dubertret L. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Krauth MT, Puthenparambil J, Lechner K. Paraneoplastic autoimmune thrombocytopenia in solid tumors. Crit Rev Oncol Hematol. 2012;81:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4924] [Cited by in RCA: 4699] [Article Influence: 247.3] [Reference Citation Analysis (0)] |
| 16. | Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Lakos G, Tincani A, Kontopoulou-Griva I, Galeazzi M, Meroni PL, Derksen RH, de Groot PG, Gromnica-Ihle E, Baleva M, Mosca M, Bombardieri S, Houssiau F, Gris JC, Quéré I, Hachulla E, Vasconcelos C, Roch B, Fernández-Nebro A, Boffa MC, Hughes GR, Ingelmo M; Euro-Phospholipid Project Group. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1425] [Cited by in RCA: 1347] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 17. | Gómez-Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. J Autoimmun. 2014;48-49:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Islam MA. Antiphospholipid antibodies and antiphospholipid syndrome in cancer: Uninvited guests in troubled times. Semin Cancer Biol. 2020;64:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Zuckerman E, Toubi E, Golan TD, Rosenvald-Zuckerman T, Sabo E, Shmuel Z, Yeshurun D. Increased thromboembolic incidence in anti-cardiolipin-positive patients with malignancy. Br J Cancer. 1995;72:447-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Grossman A, Gafter-Gvili A, Green H, Ben Aharon I, Stemmer SM, Molad Y, Krause I. Severe digital ischemia-a presenting symptom of malignancy-associated antiphospholipid syndrome. Lupus. 2008;17:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Abreu MM, Danowski A, Wahl DG, Amigo MC, Tektonidou M, Pacheco MS, Fleming N, Domingues V, Sciascia S, Lyra JO, Petri M, Khamashta M, Levy RA. The relevance of "non-criteria" clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmun Rev. 2015;14:401-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Pires da Rosa G, Bettencourt P, Rodríguez-Pintó I, Cervera R, Espinosa G. "Non-criteria" antiphospholipid syndrome: A nomenclature proposal. Autoimmun Rev. 2020;19:102689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Yuen BH, Reyes CV, Rawal PA, Sosman J, Jensen J. Severe eosinophilia and hepatocellular carcinoma: an unusual association. Diagn Cytopathol. 1995;13:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Sakai T, Shiraki K, Deguchi M, Itoh N, Konishi T, Takase K, Nakano T. Hepatocellular carcinoma associated with hemophagocytic syndrome. Hepatogastroenterology. 2001;48:1464-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Tsuchiya A, Kubota T, Takizawa K, Yamada K, Wakai T, Matsuda Y, Honma T, Watanabe M, Shirai Y, Maruyama H, Nomoto M, Aoyagi Y. Successful Treatment in a Case of Massive Hepatocellular Carcinoma with Paraneoplastic Syndrome. Case Rep Gastroenterol. 2009;3:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Shin HP, Jeon JW, Park JJ, Cha JM, Joo KR, Lee JI, Kim GY, Kang SY. A case of leukemoid reaction in a patient with sarcomatous hepatocellular carcinoma. Korean J Hepatol. 2011;17:226-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Abbas H, Hanif S, Tariq H, Chilimuri S. Thrombocytosis as a Rare Paraneoplastic Syndrome Occurring in Hepatocellular Carcinoma: A Case Report. Gastroenterology Res. 2019;12:96-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Thanapirom K, Aniwan S, Treeprasertsuk S. Polymyositis Associated with Hepatitis B Virus Cirrhosis and Advanced Hepatocellular Carcinoma. ACG Case Rep J. 2014;1:167-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Sathiyapalan A, Legault K, van der Pol CB, Meyers BM. Paraneoplastic Polyarthritis in Hepatocellular Carcinoma Treated With Lenvatinib. Hepatology. 2021;74:1705-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Ochiai T, Morishima T, Kondo M. Symptomatic porphyria secondary to hepatocellular carcinoma. Br J Dermatol. 1997;136:129-131. [PubMed] |
| 31. | Kono T, Kobayashi H, Ishii M, Nishiguchi S, Taniguchi S. Synchronous development of disseminated superficial porokeratosis and hepatitis C virus-related hepatocellular carcinoma. J Am Acad Dermatol. 2000;43:966-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Ho C, Shumack SP, Morris D. Subacute cutaneous lupus erythematosus associated with hepatocellular carcinoma. Australas J Dermatol. 2001;42:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Glinkov S, Krasnaliev I, Atanassova M, Arnaudov P, Kirov K, Glinkova V. Hepatocellular carcinoma associated with paraneoplastic erythema nodosum and polyarthritis. J Hepatol. 2003;39:656-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Yokokura H, Demitsu T, Kakurai M, Umemoto N, Azuma R, Yamada T, Suzuki M, Jimbu Y, Yoneda K, Ishii N, Hashimoto T. Paraneoplastic pemphigus mimicking erosive mucosal lichen planus associated with primary hepatocellular carcinoma. J Dermatol. 2006;33:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Cho SI, Yu DA, Lee JH, Cho KH, Mun JH. Paraneoplastic Generalized Granuloma Annulare in a Patient with Hepatocellular Carcinoma. Ann Dermatol. 2018;30:503-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Tajiri J, Nishizono Y, Fujiyama S, Sagara K, Sato T, Shibata H. Hypercholinesterasemia in patients with hepatocellular carcinoma: a new paraneoplastic syndrome. Gastroenterol Jpn. 1983;18:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Makino H, Takazakura E, Nakamura S, Kobayashi K, Hattori N, Nonomura A, Ohta G. Hepatocellular carcinoma with metastatic gastric cancer simulating Borrmann type 2 and hyperlipidemia. Acta Pathol Jpn. 1986;36:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Salles G, Vital Durand D, Mackiewicz R, Pugeat M, Levrat R. [Hepatocellular carcinoma and hyperestrogenia in a male]. Gastroenterol Clin Biol. 1987;11:607-609. [PubMed] |
| 39. | Carri J, Peral F, Surreco M, Luján A, Leguizamón R, Martínez G, Salvucci M. [Fibrolamellar hepatocellular carcinoma: a clinical report with paraneoplastic hyperthyroidism (apropos of a case)]. Acta Gastroenterol Latinoam. 1989;19:155-164. [PubMed] |
| 40. | Nizam R, Ahmed F. Hyperthyroxinemia and elevated lipids as paraneoplastic phenomena in hepatocellular carcinoma. A case report. J Clin Gastroenterol. 1995;21:246-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Nwokediuko SC, Uchenna I, Esther O, Okechukwu O, Augustine O, Charity A. Relatively Long Survival in Hepatocellular Carcinoma Presenting With Carcinoid Syndrome. Gastroenterology Res. 2010;3:46-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Kim EK, Kim JS, Shin KC, Lee GT, Han CJ, Kim SB, Ku YH. Complete Tumor Resection for a Hepatocellular Carcinoma Secreting Parathyroid Hormone-related Peptide. Korean J Gastroenterol. 2015;66:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Misumi H, Ishibashi H, Kanayama K, Kajiyama W, Nomura H, Sugimoto T, Hiroshige K, Niho Y. Necrotizing myelopathy associated with hepatocellular carcinoma. Jpn J Med. 1988;27:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Norris S, Rajendiran S, Sheahan K, Murphy S, Royston D, Alyusuf R, Farrell M, McCormick PA. Noncirrhotic hepatoma presenting with paraneoplastic neurologic manifestations: two cases. Am J Gastroenterol. 1997;92:1923-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Walcher J, Witter T, Rupprecht HD. Hepatocellular carcinoma presenting with paraneoplastic demyelinating polyneuropathy and PR3-antineutrophil cytoplasmic antibody. J Clin Gastroenterol. 2002;35:364-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Matsui T, Hori Y, Nagano H, Eguchi H, Marubashi S, Wada H, Wada N, Ikeda J, Sakamoto M, Morii E. Poorly differentiated hepatocellular carcinoma accompanied by anti-Hu antibody-positive paraneoplastic peripheral neuropathy. Pathol Int. 2015;65:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Karam C, Haque IU, Fewtrell M, Das A. Fibrolamellar hepatocellular carcinoma with paraneoplastic neuropsychiatric manifestations. ANZ J Surg. 2020;90:2114-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Lee JS, Jin HY, Ko JM, Kim SH, Han N, Park BK, Park M, Park HJ, Lee JA. Hyperammonemic Encephalopathy Mimicking Ornithine Transcarbamylase Deficiency in Fibrolamellar Hepatocellular Carcinoma: Successful Treatment with Continuous Venovenous Hemofiltration and Ammonia Scavengers. Cancer Res Treat. 2021;53:283-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Texier F, Dharancy S, Provot F, Augusto D, Mortier PE, Mathurin P, Copin MC, Paris JC. [Membranous glomerulonephritis complicating hepatocellular carcinoma]. Gastroenterol Clin Biol. 2004;28:605-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 50. | Lee JM, Seong HK, Nam WH, Kim HK. Cancer-associated nummular loss of the retinal pigment epithelium. Korean J Ophthalmol. 2007;21:261-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Vautravers C, Rat P, Cercueil JP, Moreau T, Horiot JC, Chauffert B. Hepatocellular carcinoma presenting as paraneoplastic myasthenia gravis. Eur J Intern Med. 2008;19:e86-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Bárdos D, Molnár MJ, Dudás I, Tuza S, Szijártó A, Hahn O. Polymyositis and rhabdomyolysis caused by hepatocellular carcinoma - Case report and literature review. Ann Med Surg (Lond). 2021;65:102269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |