Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11346
Peer-review started: April 25, 2021
First decision: June 24, 2021
Revised: July 8, 2021
Accepted: August 24, 2021
Article in press: August 24, 2021
Published online: December 26, 2021
Processing time: 242 Days and 0.9 Hours
Gastrointestinal (GI) metastasis from breast cancer (BC) is rarely encountered in clinical practice. Nonspecific symptoms and long intervals make early diagnosis difficult. Therefore, increased awareness of GI metastasis secondary to BC and a deep understanding of the clinical and pathological features, and intervention for GI metastasis are fundamental to avoid delay in correct diagnosis and mana
The present report discusses the case of a Chinese female patient aged 36 years. The patient presented with difficult defecation along with bloody stools and hypogastralgia. In 2015, she had undergone right modified radical mastectomy and axillary lymph node dissection in another hospital to treat the infiltrating ductal breast carcinoma pT1N1M0. The presenting symptoms were investigated by colonoscopy, which indicated a circumferential stricture in the lower rectum at 3 cm from the anal edge. Further investigation with positron emission tomography-computed tomography revealed an uptake of fluorodeoxyglucose within the distal rectum as well as in the left acetabulum. The samples from laparoscopic exploration were biopsied, which revealed metastases of BC. Immunohistochemical analysis of the tumor confirmed that the patient had rectal metastasis of infiltrating ductal BC.
Rectal metastasis should be considered when patients with a history of BC present with changed bowel habits.
Core Tip: Rectal metastasis of infiltrating ductal breast cancer has a low incidence. For patients with a history of breast cancer with digestive complaints, bowel metastasis must be considered.
- Citation: Ban B, Zhang K, Li JN, Liu TJ, Shi J. Ductal breast carcinoma metastasized to the rectum: A case report and review of the literature. World J Clin Cases 2021; 9(36): 11346-11354
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11346.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11346
Breast cancer (BC) is the most frequent malignant tumor among women and is associated with significant morbidity and mortality rates[1]. Metastatic tumors account for 30% of BC cases with a 5-year survival rate of 22%, and those metastatic cases are responsible for 90% of BC deaths[2]. BC metastasis includes contiguous, lymphatic and hematogenous forms of spread. While hematogenous spread of BC can target any site, the most common sites are bone, lung, liver and brain[3]. Metastasis to the gastro
A 36-year-old Chinese woman presented with a complaint of difficult defecation, along with bloody stools and hypogastralgia. The symptoms had appeared 2 mo earlier and had progressively worsened within 2 wk, which led to hospitalization for a complete medical examination.
A patient with no history of colorectal surgery or irradiation presented with clinical symptomatology characterized by difficult defecation, bloody stools, and hypogastralgia.
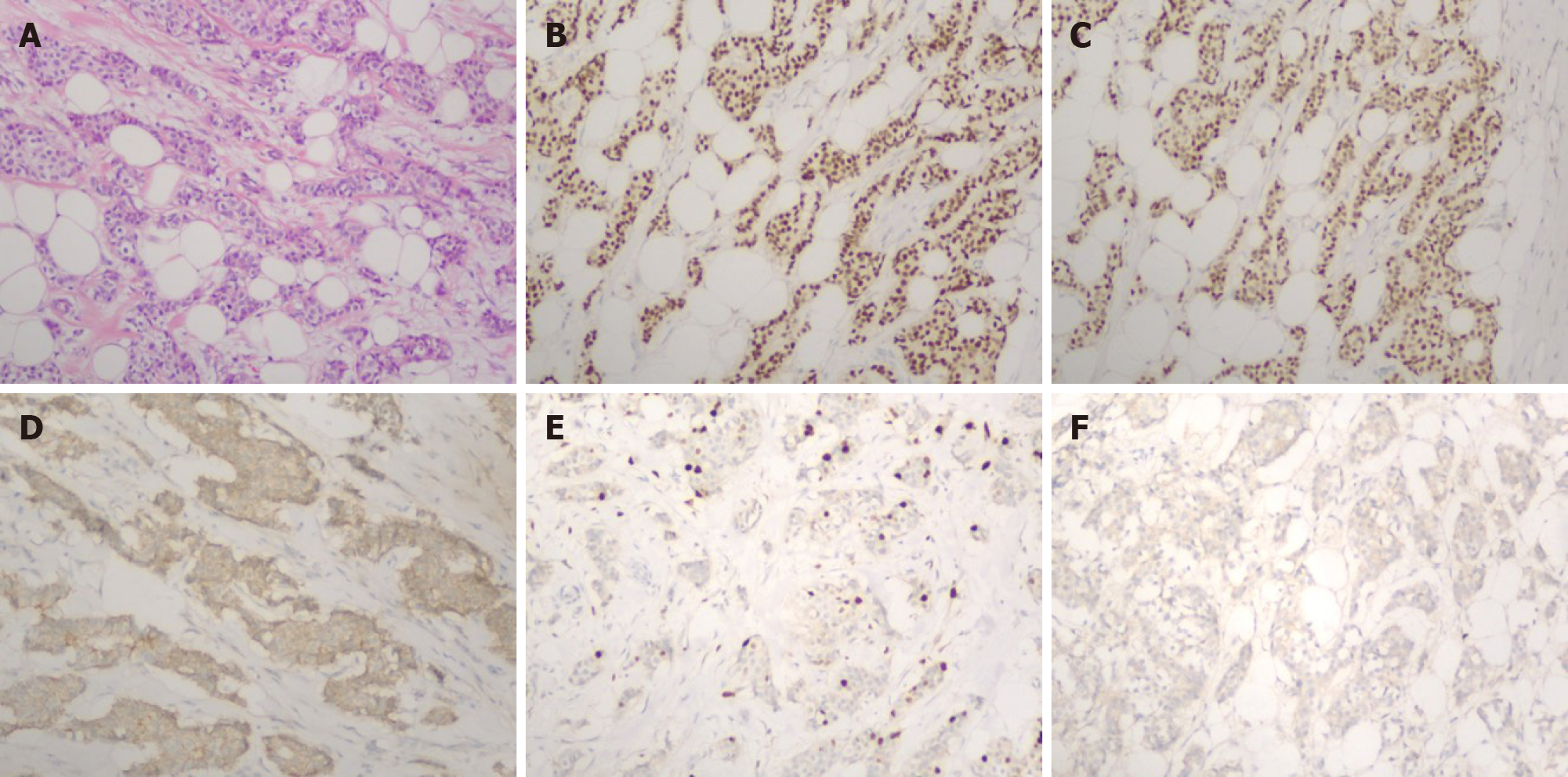
In 2015, the patient had undergone right modified radical mastectomy and axillary lymph node dissection in another hospital for the treatment of infiltrating ductal breast carcinoma pT1N1M0. Her histopathological examination had revealed an infiltrating ductal BC 1.5 cm in size and histological grade 2. Moreover, among the 20 lymph nodes resected, four were infiltrated with cancer cells. Immunohistochemical staining revealed a positive estrogen receptor (ER) rate of 70%, positive progesterone receptor (PR) rate of 70%, and positive Ki-67 rate of 15%-30%. Furthermore, human epidermal growth factor receptor 2 (Her-2) was negative and E-cadherin was positive (Figure 1). DM was not detected at the time of diagnosis. Postoperatively, the patient underwent six cycles of adjuvant chemotherapy (TC protocol), followed by endocrine therapy with tamoxifen (20 mg/d). The patient discontinued the endocrine therapy after 1 year.
The patient denied any family history of BC or rectal cancer.
The patient’s vital signs were as follows: Body temperature, 36.3 °C; heart rate, 74 beats/min; blood pressure, 116/76 mmHg; respiratory rate, 15 breaths/min; and in-room oxygen saturation, 100%. Abdominal examination revealed no obvious abnormality. However, digital rectal examination revealed a tumor with a smooth surface at the knee-chest position, which was located 3 cm above the anal edge. It was a circumferential tumor with swelling. The tumor root could not be moved with palpation. No pus or dark-red blood residue was observed on the glove after rectal examination.
Results of routine laboratory tests were normal. Blood analysis revealed no abnormality in any of the blood counts. White blood cell count was 5.9 × 109 cells/L (normal limit: 3.5 × 1012-9.5 × 1012 cells/L), red blood cell count was 4.27 × 1012 cells/L (normal limit: 3.80 × 1012-5.10 × 1012 cells/L), and platelet count was 313 × 109 cells/L (normal limit: 125 × 109-350 × 109 cells/L). However, both carbohydrate antigen (CA)-125 and CA-153 were elevated, with values of 41.10 U/mL (normal range: 0-35 U/mL) and 36.80 U/mL (normal range: 0-31.3 U/mL), respectively.
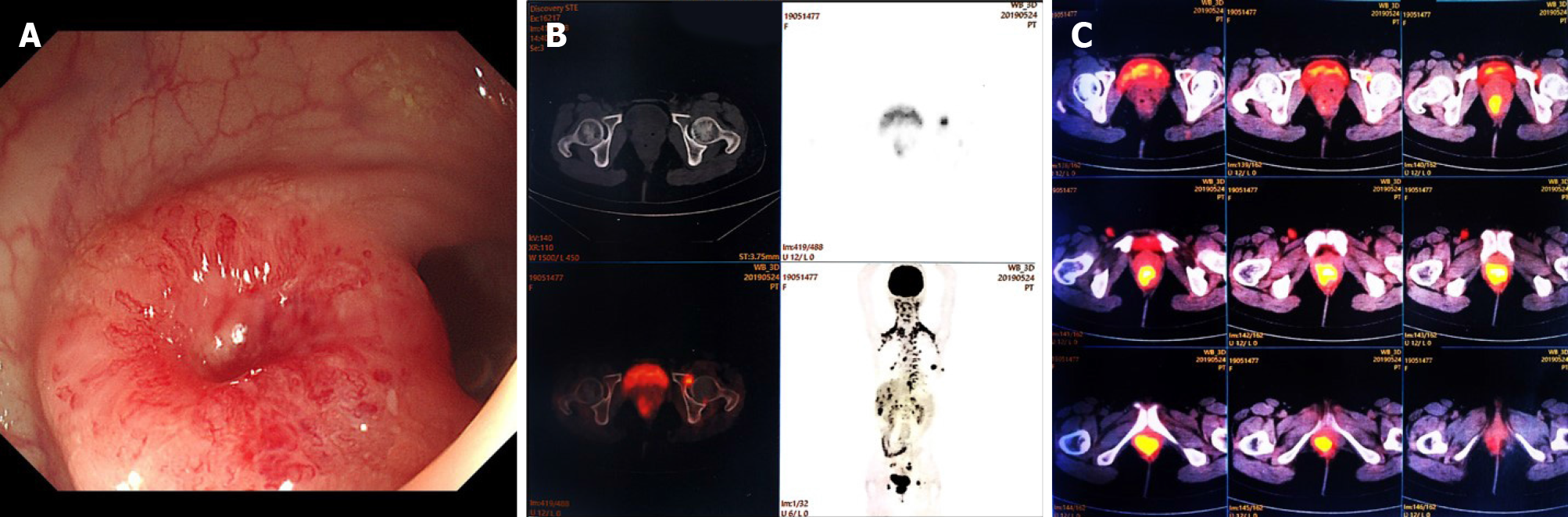
Colonoscopic examination revealed a circumferential stricture in the lower rectum at a distance of 3 cm from the anal edge. Its surface was smooth and red, which was suggestive of a submucosal tumor (Figure 2A). Multiple biopsies of the tumor were performed, which detected no malignancy. The patient was recommended to undergo positron emission tomography-computed tomography (PET-CT) for further investigation, which revealed an uptake of fluorodeoxyglucose within the left acetabulum and distal rectum, with the maximal standardized uptake values of 5.5 and 11.2, respectively, suggesting suspicious metastasis at these positions (Figure 2B and 2C).
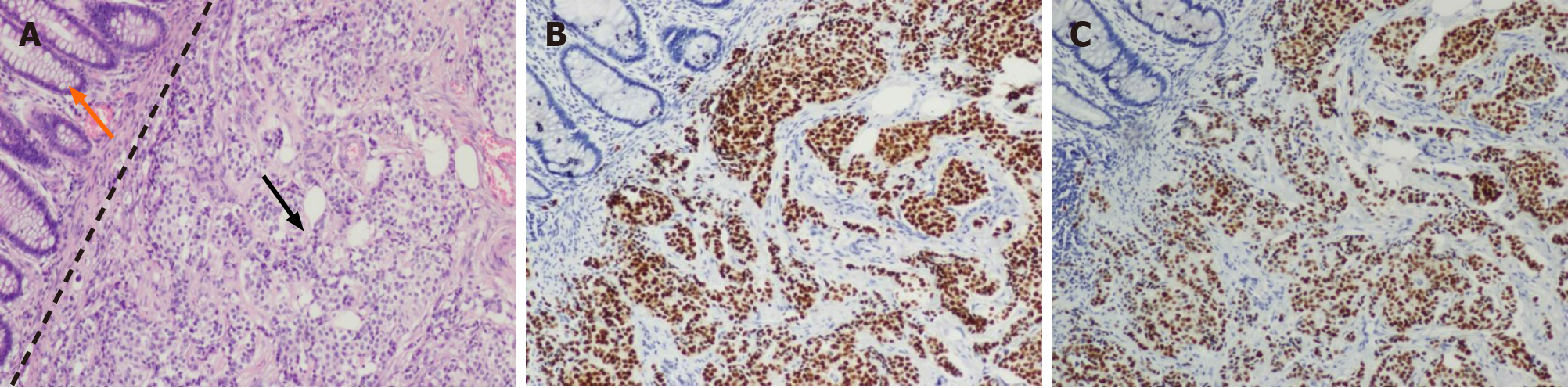
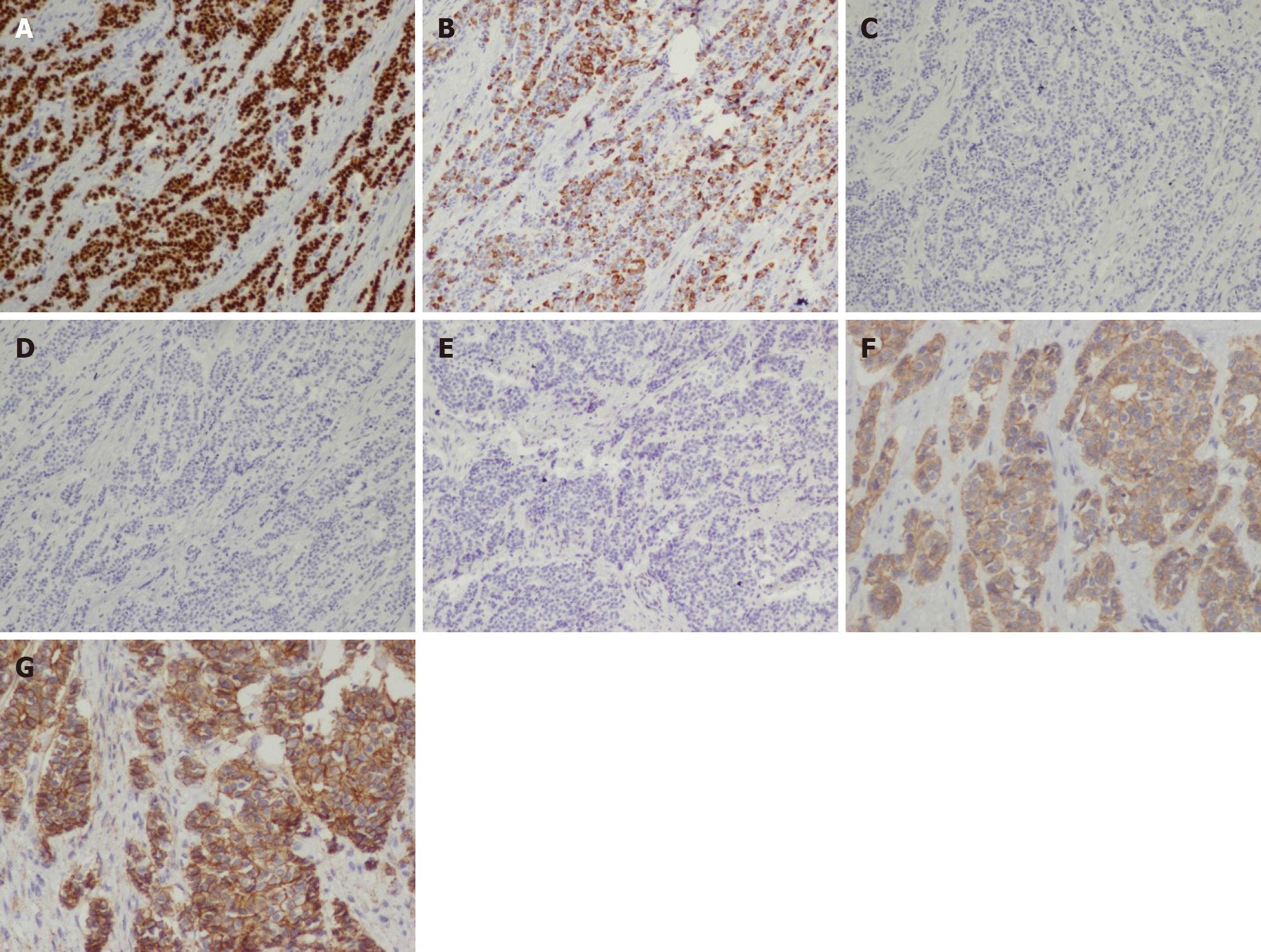
In the laparoscopic exploration, a circumferential tumor was detected in the distal rectum, following which a biopsy of the tumor was performed. Fast frozen pathology of the specimen revealed that the tumor was morphologically consistent with ductal breast carcinoma and that nests of tumor cells extensively infiltrated the muscular layer. Tumor cells were PR and ER positive, with a positivity rate of 90% for both (Figure 3). In contrast, the background rectal epithelial cells tested PR and ER negative. Since the patient had a history of ductal BC, she was intraoperatively diagnosed with BC rectal metastasis. Given that the tumor had caused bleeding and incomplete intestinal obstruction, which were surgical indications, a colostomy was performed laparoscopically. The surgical dissected biopsy revealed a neoplasm in the submucosal layer, while no abnormality was observed in the mucosal layer. Further immunohistochemical analysis of the tumor-infiltrating part was performed, and the results confirmed this rare metastasis. In addition, the tumor cells tested positive for cytokeratin (CK)7, GATA3, P120 and E-cadherin and negative for CK20, caudal type homeobox (CDX)2 and stabilin (STAB)2 (Figure 4).
No special event was noted after surgery. At 12 d after surgery, the patient was discharged from hospital. Afterwards, local radiotherapy was given to a total dose of 39 Gy in 13 sessions of 3 Gy, and a chemotherapy plan of gemcitabine combined with cisplatin was also applied.
The patient was followed-up for 10 mo after discharge from hospital. Tamoxifen administration was continued, and she remained in a stable condition.
GI metastasis from breast carcinoma is rare and has been reported to occur in 6%-18% of disseminated BC patients[6]. Metastasis may occur in all the regions of the GI tract, with the rectum being an infrequently affected site[7]. Infiltrating ductal cancer is the most frequently occurring subtype of BC, and accounts for 75% of all primary BC[1]. However, it metastasizes to the GI tract at a rate of only 0.2%, in contrast to the 4.5% metastatic rate of invasive lobular carcinoma[8,9]. Infiltrating lobular carcinoma, which accounts for only 12% of all primary BC cases, contributes 64% of the GI metastases from primary BC[10]. The different clinical metastatic patterns between infiltrating ductal carcinoma and infiltrating lobular carcinoma may be interpreted based on unique biological and histological characteristics. E-cadherin, the molecule responsible for intercellular adhesion, is present in ductal carcinoma but absent in lobular carcinoma, which possibly explains the different metastatic patterns[11]. Typically, the venous vertebral plexus (Batson’s plexus) is the probable route for BC metastasis through the veins. This plexus extends from the skull to the scrum without any valves, thereby providing an unrestricted channel for the transport of the metastatic emboli into the ribs, the distant organs, and the vertebral bones[12].
Early diagnosis is challenging for several reasons. First, the tumors metastasizing from primary BC to the GI tract manifest no specific symptoms. Montagna et al[8] reviewed 40 patients, among whom, 80% complained of vomiting, nausea, stomach ache, altered bowel habits, fatigue, and unsuspected weight loss; all of which are commonly observed symptoms in primary as well as secondary intestinal tumors. McLemore et al[10] studied 12001 patients with BC, and metastasis detected in 11 patients remained undiagnosed until an exploratory laparotomy was conducted. Second, the long disease-free interval of BC renders the early diagnosis of metastasis difficult. Therefore, exploring the history of BC is crucial for establishing the diagnosis of bowel metastasis. As suggested by Schwarz et al[13], the median interval from BC to GI metastasis was between 0.25 and 12.5 years (median: 6 years). McLemore et al[10] reported an average interval of 7 years. López Deogracias et al[14] presented a case with invasive lobular carcinoma developing a metastatic rectal lesion, which caused urethral dilation 15 years later. Mistrangelo et al[15] presented a patient who developed sigmoid colon metastasis from primary lobular BC after an interval of 25 years; the longest so far among the reported cases. In our case, the disease-free interval was 4 years and the time for the occurrence of metastasis was inside the highest risk window. Therefore, it is critical to include the possibility of intestinal metastasis in the early diagnosis of cases presenting with digestive complaints along with a history of BC.
At the early stage of metastasis, the general endoscopic appearance is normal mucosa, as the lesions often involve the submucosal layer rather than the mucosal layer. Therefore, superficial biopsy seems to have a limited role. Szabó et al[16] reported a case of infiltrating lobular carcinoma mimicking Crohn’s disease, for which biopsy suggested necrosis and not cancer. However, the histopathological examinations conducted after surgery indicated terminal ileal metastasis of invasive lobular carcinoma. Carcoforo et al[17] reported the case of a female patient aged 73 years with no history of cancer, who presented with vomiting, nausea and abdominal pain. Colonoscopy revealed a stricture at 15 cm on the top of the anal verge. Moreover, negative results were obtained in repeated biopsies, while biopsies combined with exploratory laparotomy revealed intestinal metastases of invasive BC. Deep biopsy or endoscopic ultrasound-guided fine-needle aspiration may be conducive to prompt an accurate diagnosis of GI tract metastasis. Matsumoto et al[18] reported the case of an 84-year-old woman with progressive dysphagia. Her endoscopy revealed esophageal stenosis located 30 cm away from the incisors, although no abnormality was observed in the overlying mucosa. In addition, no abnormality was detected in the biopsies. Finally, esophageal metastasis from BC was confirmed through fine-needle biopsy cytology conducted endoscopically under the guidance of ultrasound. Late metastasis may affect all the intestinal layers and manifest in linitis plastica lesions, ulcers, and bleeding, thus mimicking primary intestinal tumor or inflammatory bowel disease[16,19].
The radiologist plays a crucial role in examining the patients with BC for detecting metastasis. In abdominal CT, the common identifications are mural thickening, bowel dilation, rigidity of the colorectum, and linitis-plastica-type lesion of the stomach[20]. These macroscopic characteristics are nonspecific and indistinguishable from lowly differentiated cancer that is observed frequently in the stomach. Magnetic resonance imaging (MRI), in comparison, provides better soft-tissue contrast and a high-level description of the various histological layers of the GI wall. In a study by Lau et al[21], concentric mural thickening was concluded as the MRI feature of breast metastases to the rectum, while eccentric wall thickening and an obvious invasive margin were reported as the more frequently observed features in primary rectal carcinoma. PET-CT could be used to detect DM, such as in the case analyzed in the present study, as PET-CT presents high specificity and sensitivity in the detection of DM compared to conventional imaging[22]. However, PET-CT is not the preferred diagnostic tool in BC due to its low sensitivity and specificity, which are in the range of 48%-96% and 73%-100%, respectively[23].
Immunohistochemistry plays a decisive role in the establishment of a diagnosis. CK7 and CK20 are two effective cytokeratins among the 20 intermediate filament subtypes. CK7 expression is observed in glandular and ductal epithelial tissues in breast and lung cancers. CK20 positivity is observed in the GI epithelium[24]. CK7 positivity and CK20 negativity favor metastasis, as in our case, while a CK7−/CK20+ pattern is suggestive of a large bowel primary tumor[14,25]. CDX2 is the caudal homeobox gene that encodes the transcription factor (TF), which plays a vital role in intestinal epithelial differentiation and proliferation. CDX2 may be expressed in gastric cancer, primary urinary bladder adenocarcinoma, and mucinous ovarian adenocarcinoma[26]. Bayrak et al[24] analyzed 118 colorectal, 59 gastric and 32 pancreatic adenocarcinoma resection specimens and concluded that in colorectal adenocarcinoma, CDX2 expression and the CK7−/CK20+ pattern were highly sensitive and specific. SATB2, a recently described transcriptional regulator, is reported as a highly specific and sensitive marker of colorectal cancer (CRC)[27]. Magnusson et al[28] reported that SATB2 plus CD20+ could detect > 95% of the CRC cases. E-cadherin, the transmembrane glycoprotein, regulates intercellular adhesion in a calcium-dependent manner and participates in the adhesion of epithelial cells[29]. E-cadherin has also been frequently used as a marker to distinguish ductal carcinoma from the lobular one. E-cadherin is expressed within the cell membrane in most ductal carcinomas. On the contrary, E-cadherin is absent in several lobular carcinomas[30]. P120 catenin is stained intensely in the membrane of ductal carcinomas and strongly and diffusely stained in the cytoplasm of lobular carcinomas. Furthermore, 10%-16% of ductal carcinomas test negative for E-cadherin, and in these cases, P120 catenin maintains its membrane localization[30,31]. GATA3, one of the TF proteins, plays a vital role in enhancing the differentiation and proliferation of mammary ductal epithelial cells. GATA3 is regarded as the most sensitive single marker of invasive BC, with an estimated expression rate of > 90%. When confronting a neoplasm with unclear origin, particularly in the case of BC, routine assessment of GATA3 is recommended[32]. In the present report, negativity for both CDX2 and STAB2, along with a CK7+/CK20− profile assisted in excluding the diagnosis of primary tumor of the rectum. Positivity for E-cadherin, P120 and GATA3 indicates metastasis of infiltrating ductal BC. In addition, for the original breast carcinoma treated 3 years ago in our case, 70% ER and PR positive rates were observed, while for the rectal metastatic lesion, the rates were 90%. This difference has also been reported by other reviewers, which suggests that BC presents with different biological features in the primary tumors compared to metastases[33,34].
The cases with GI tract metastases are frequently treated with systemic treatment (endocrine therapy and/or chemotherapy), as the GI metastasis is generally associated with extensive metastases[35]. However, the unique role of surgical intervention cannot be ignored. Surgical intervention includes GI resection, diverting ostomy, and GI bypass. In patients with GI metastasis alone, radical surgical resection along with systemic treatment is reported to have a better prognosis[10]. In the patients presenting disseminated disease, surgery has no prolonging effect on the overall survival, although these patients do benefit from palliative surgery, as reported previously, for relief from the symptoms[10]. Typically, perforation, bleeding, and intestinal obstruction are the surgical indications for such cases, and surgery should, therefore, be performed to avoid severe complications and improve supportive care. Moreover, surgery plays a crucial role in obtaining a timely and accurate diagnosis of bowel metastasis, as stated earlier. In summary, for such cases, the decision for surgery should be undertaken on the basis of the general condition, symptoms, clinical presentations, and a quality-of-life assessment. In our case, the metastatic tumor had caused bleeding and incomplete intestinal obstruction which were surgical indications. Therefore, colostomy was performed along with chemotherapy, endocrine treatment, and radiotherapy. This therapeutic strategy was expected to achieve long-term survival.
BC has been shown to metastasize to the GI tract, although it may have a long interval. We should also be aware that the presenting symptoms can be nonspecific and it may be difficult to diagnose metastasis on biopsy or endoscopy. Comprehensive analysis of imaging manifestations is helpful in correct diagnosis. Histopathology and immunohistochemistry play important roles in the verification of metastasis while excluding primary rectal cancer. Surgery also plays a unique and important role in its diagnosis and treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Al Khader A, Fakhr I, Lieto E S-Editor: Ma YJ L-Editor: Kerr C P-Editor: Liu JH
| 1. | Coughlin SS. Epidemiology of Breast Cancer in Women. Adv Exp Med Biol. 2019;1152:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (2)] |
| 2. | Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer. Nat Rev Dis Primers. 2019;5:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 1768] [Article Influence: 294.7] [Reference Citation Analysis (0)] |
| 3. | Wang L, Zhang S, Wang X. The Metabolic Mechanisms of Breast Cancer Metastasis. Front Oncol. 2020;10:602416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 4. | Taal BG, den Hartog Jager FC, Steinmetz R, Peterse H. The spectrum of gastrointestinal metastases of breast carcinoma: II. The colon and rectum. Gastrointest Endosc. 1992;38:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Taal BG, den Hartog Jager FC, Steinmetz R, Peterse H. The spectrum of gastrointestinal metastases of breast carcinoma: I. Stomach. Gastrointest Endosc. 1992;38:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Invento A, Mirandola S, Pellini F, Pollini GP, Grigolato D. Breast cancer and gastrointestinal metastasis. A case report and review of the literature. Ann Ital Chir. 2018;89:153-156. [PubMed] |
| 7. | Bamias A, Baltayiannis G, Kamina S, Fatouros M, Lymperopoulos E, Agnanti N, Tsianos E, Pavlidis N. Rectal metastases from lobular carcinoma of the breast: report of a case and literature review. Ann Oncol. 2001;12:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Montagna E, Pirola S, Maisonneuve P, De Roberto G, Cancello G, Palazzo A, Viale G, Colleoni M. Lobular Metastatic Breast Cancer Patients With Gastrointestinal Involvement: Features and Outcomes. Clin Breast Cancer. 2018;18:e401-e405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Haberstich R, Tuech JJ, Wilt M, Rodier JF. Anal localization as first manifestation of metastatic ductal breast carcinoma. Tech Coloproctol. 2005;9:237-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | McLemore EC, Pockaj BA, Reynolds C, Gray RJ, Hernandez JL, Grant CS, Donohue JH. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol. 2005;12:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Critchley AC, Harvey J, Carr M, Iwuchukwu O. Synchronous gastric and colonic metastases of invasive lobular breast carcinoma: case report and review of the literature. Ann R Coll Surg Engl. 2011;93:e49-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Carpenter K, Decater T, Iwanaga J, Maulucci CM, Bui CJ, Dumont AS, Tubbs RS. Revisiting the Vertebral Venous Plexus-A Comprehensive Review of the Literature. World Neurosurg. 2021;145:381-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Schwarz RE, Klimstra DS, Turnbull AD. Metastatic breast cancer masquerading as gastrointestinal primary. Am J Gastroenterol. 1998;93:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | López Deogracias M, Flores Jaime L, Arias-Camisón I, Zamacola I, Murillo Guibert J, Suescun García R, Querejeta Usabiaga J, Martínez García F. Rectal metastasis from lobular breast carcinoma 15 years after primary diagnosis. Clin Transl Oncol. 2010;12:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Mistrangelo M, Cassoni P, Mistrangelo M, Castellano I, Codognotto E, Sapino A, Lamanna G, Cravero F, Bianco L, Fora G, Sandrucci S. Obstructive colon metastases from lobular breast cancer: report of a case and review of the literature. Tumori. 2011;97:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 16. | Szabó J, Falkus B, Simon E, Brünner S, Baranyay F. [Late gastrointestinal metastases of invasive lobular breast carcinoma mimicking Crohn's disease]. Orv Hetil. 2010;151:1666-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Carcoforo P, Raiji MT, Langan RC, Lanzara S, Portinari M, Maestroni U, Palini GM, Zanzi MV, Bonazza S, Pedriali M, Feo CV, Stojadinovic A, Avital I. Infiltrating lobular carcinoma of the breast presenting as gastrointestinal obstruction: a mini review. J Cancer. 2012;3:328-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Matsumoto Y, Matsukawa H, Seno H, Ono S. Education and imaging. Gastrointestinal: breast cancer metastasis to the esophagus diagnosed using endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol Hepatol. 2015;30:233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Matsuda I, Matsubara N, Aoyama N, Hamanaka M, Yamagishi D, Kuno T, Tsukamoto K, Yamano T, Noda M, Ikeuchi H, Tomita N, Hirota S. Metastatic lobular carcinoma of the breast masquerading as a primary rectal cancer. World J Surg Oncol. 2012;10:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Winston CB, Hadar O, Teitcher JB, Caravelli JF, Sklarin NT, Panicek DM, Liberman L. Metastatic lobular carcinoma of the breast: patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am J Roentgenol. 2000;175:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Lau LC, Wee B, Wang S, Thian YL. Metastatic breast cancer to the rectum: A case report with emphasis on MRI features. Medicine (Baltimore). 2017;96:e6739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Pesapane F, Downey K, Rotili A, Cassano E, Koh DM. Imaging diagnosis of metastatic breast cancer. Insights Imaging. 2020;11:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Warning K, Hildebrandt MG, Kristensen B, Ewertz M. Utility of 18FDG-PET/CT in breast cancer diagnostics--a systematic review. Dan Med Bull. 2011;58:A4289. [PubMed] |
| 24. | Bayrak R, Haltas H, Yenidunya S. The value of CDX2 and cytokeratins 7 and 20 expression in differentiating colorectal adenocarcinomas from extraintestinal gastrointestinal adenocarcinomas: cytokeratin 7-/20+ phenotype is more specific than CDX2 antibody. Diagn Pathol. 2012;7:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 637] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 26. | Yu J, Li S, Xu Z, Guo J, Li X, Wu Y, Zheng J, Sun X. CDX2 inhibits epithelial-mesenchymal transition in colorectal cancer by modulation of Snail expression and β-catenin stabilisation via transactivation of PTEN expression. Br J Cancer. 2021;124:270-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Hoskoppal D, Epstein JI, Gown AM, Arnold Egloff SA, Gordetsky JB, Shi CJ, Giannico GA. SATB2 protein expression by immunohistochemistry is a sensitive and specific marker of appendiceal and rectosigmoid well differentiated neuroendocrine tumours. Histopathology. 2020;76:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Magnusson K, de Wit M, Brennan DJ, Johnson LB, McGee SF, Lundberg E, Naicker K, Klinger R, Kampf C, Asplund A, Wester K, Gry M, Bjartell A, Gallagher WM, Rexhepaj E, Kilpinen S, Kallioniemi OP, Belt E, Goos J, Meijer G, Birgisson H, Glimelius B, Borrebaeck CA, Navani S, Uhlén M, O'Connor DP, Jirström K, Pontén F. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. Am J Surg Pathol. 2011;35:937-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 29. | Margan MM, Cimpean AM, Ceausu AR, Raica M. Differential Expression of E-Cadherin and P-Cadherin in Breast Cancer Molecular Subtypes. Anticancer Res. 2020;40:5557-5566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Bonacho T, Rodrigues F, Liberal J. Immunohistochemistry for diagnosis and prognosis of breast cancer: a review. Biotech Histochem. 2020;95:71-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Venhuizen JH, Span PN, van den Dries K, Sommer S, Friedl P, Zegers MM. P120 Catenin Isoforms Differentially Associate with Breast Cancer Invasion and Metastasis. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Aphivatanasiri C, Li J, Chan R, Jamidi SK, Tsang JY, Poon IK, Shao Y, Tong J, To KF, Chan SK, Tam F, Cheung SY, Shea KH, Tse GM. Combined SOX10 GATA3 is most sensitive in detecting primary and metastatic breast cancers: a comparative study of breast markers in multiple tumors. Breast Cancer Res Treat. 2020;184:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Guarneri V, Giovannelli S, Ficarra G, Bettelli S, Maiorana A, Piacentini F, Barbieri E, Dieci MV, D'Amico R, Jovic G, Conte P. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: impact on patient management. Oncologist. 2008;13:838-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Lower EE, Glass EL, Bradley DA, Blau R, Heffelfinger S. Impact of metastatic estrogen receptor and progesterone receptor status on survival. Breast Cancer Res Treat. 2005;90:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Tang T, Zhang L, Li C, Zhou T. Gastric and adrenal metastasis from breast cancer: Case report and review of literature. Medicine (Baltimore). 2020;99:e18812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |