Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11183
Peer-review started: January 20, 2021
First decision: September 28, 2021
Revised: October 15, 2021
Accepted: November 3, 2021
Article in press: November 3, 2021
Published online: December 26, 2021
Processing time: 337 Days and 9.3 Hours
Prostate cancer (PCa) is one of the most common cancers among men. Various strategies for targeted biopsy based on multiparametric magnetic resonance imaging (mp-MRI) have emerged, which may improve the accuracy of detecting clinically significant PCa in recent years.
To investigate the diagnostic efficiency of a template for cognitive MRI-ultrasound fusion transperineal targeted plus randomized biopsy in detecting PCa.
Data from patients with an increasing prostate-specific antigen (PSA) level but less than 20 ng/mL and at least one lesion suspicious for PCa on MRI from December 2015 to June 2018 were retrospectively analyzed. All patients un
PCa was detected in 66 of 127 patients, and 56 cases presented clinically significant PCa. Cognitive fusion targeted biopsy alone detected 59/127 cases of PCa, specifically 52/59 cases with clinically significant PCa and 7/59 cases with clinically insignificant PCa. A randomized biopsy detected seven cases of PCa negative on targeted biopsy, and four cases had clinically significant PCa. PSA density (OR: 1.008, 95%CI: 1.003-1.012, P = 0.001; OR: 1.006, 95%CI: 1.002-1.010, P = 0.004) and Prostate Imaging-Reporting and Data System (PI-RADS) scores (both P < 0.001) were independently associated with the results of cognitive fusion targeted biopsy combined with randomized biopsy and targeted biopsy alone.
This single-centered study proposed a feasible template for cognitive MRI-ultrasound fusion transperineal targeted plus randomized biopsy. Patients with higher PSAD and PI-RADS scores were more likely to be diagnosed with PCa.
Core Tip: Prostate biopsy remains the standard diagnostic modality before curative treatment. Cognitive magnetic resonance imaging (MRI)-ultrasound fusion biopsy is a more accessible and economical biopsy technique for small-sample institutions to realize imaging-guided targeted biopsy. In this study, we proposed a customized template and reported a feasible approach for cognitive MRI-ultrasound fusion biopsy with our single institutional experience. The results from this retrospective study revealed that a high yield of cancer, and that patients with higher prostate-specific antigen density and Prostate Imaging-Reporting and Data System scores are more likely to be diagnosed with prostate cancer under this biopsy strategy.
- Citation: Pang C, Wang M, Hou HM, Liu JY, Zhang ZP, Wang X, Zhang YQ, Li CM, Zhang W, Wang JY, Liu M. Cognitive magnetic resonance imaging-ultrasound fusion transperineal targeted biopsy combined with randomized biopsy in detection of prostate cancer. World J Clin Cases 2021; 9(36): 11183-11192
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11183.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11183
Prostate cancer (PCa) is one of the most common cancers and the second leading cause of cancer-related deaths among men in the United States[1]. Prostate biopsy remains the standard modality for PCa diagnosis. Traditionally, a biopsy is primarily conducted under transrectal and systematic ultrasound guidance[2]. However, the detection rate of the initial biopsy is unsatisfactory, with an overall yield of only 22%-29% reported in previous studies[3,4] due to sampling error or technical limitations resulting from the location of the tumor (i.e., anterior tumor, which accounts for approximately 21% of all PCa). Moreover, a higher risk of detecting lower-grade cancer while missing clinically significant PCa (csPCa) occurs by traditional systematic biopsy (SBx), which may increase the probability of overtreatment or underestimation of PCa burden and aggressiveness.
Targeted biopsy (TBx) based on multiparametric magnetic resonance imaging (mp-MRI), which has emerged as a potential method for the detection, localization, stratification, and staging of PCa, is promising in overcoming the above challenges of traditional SBx[5-8].Various strategies of TBx have been reported in previous studies, with the software registration mp-MRI-ultrasound fusion TBx and mp-MRI in-bore TBx studied most. Cognitive MRI-ultrasound fusion TBx (COG-TB) is a more economical and accessible targeted biopsy strategy, especially for small institutions or those without fusion software or equipment for MRI in-bore biopsy; however, primarily based on the operator’s tumor identification, COG-TB requires a higher level of experience and more easily followed template to reduce operator variability, and there are sparse data on the optimal template and predictors for the detection rate of COG-TB. Moreover, patients with high prostate-specific antigen (PSA) levels (i.e., PSA > 20 ng/mL) were included in previous studies on the influencing factors of the detection rate, which may cause selection bias and result in a detection rate.
Thus, this retrospective study was conducted to propose a feasible template for COG-TB with our single institutional experience on a biopsy-naïve cohort with a PSA level that was elevated but < 20 ng/mL to evaluate the detection rate for csPCa of COG-TB followed by randomized biopsy (SBx) and to investigate potential influencing factors.
We retrospectively studied a total of 127 biopsy-naïve men from December 2015 to June 2018, with increasing PSA levels < 20 ng/mL and detectable lesions suspicious for PCa on mp-MRI undergoing transperineal template-guided COG-TB followed by SBx outside the targeted area. The study was approved by the Institutional Review Board of Beijing Hospital (2018BJYYEC-028-02).
All patients underwent pelvic MRI approximately 1 wk before the biopsy. All mp-MRI examinations were performed using a 3.0 T scanner (c 3T; GE, Discovery 750, America), including multiplanar turbo spin-echo T2-weighted imaging (T2WI, TR/TE = 4800/90 ms, slice thickness: 4 mm, interslice gap: 1 mm, FOV = 28 cm, matrix = 334 × 336) and axial diffusion-weighted imaging (DWI, TR/TE = 4000/80 ms, slice thickness: 4 mm, interslice gap: 1 mm, FOV = 22 cm, NEX = 3, matrix = 128 × 128, B values of 0, 1000, 1400, and 2000 s/mm2).
Two experienced (at least 3 years) radiologists who were blinded to the biopsy results evaluated the mp-MRI data separately and independently located each suspicious lesion based on the Prostate Imaging-Reporting and Data System version 2.1 (PI-RADS v2.1)[9,10]. Additionally, the maximum dimensions of the suspicious lesion were measured on axial T2WI, and the prostate volume was calculated by multiplying the dimensions of the prostate gland in all three different planes × 0.52. The two radiologists independently reviewed all data to achieve consensus.
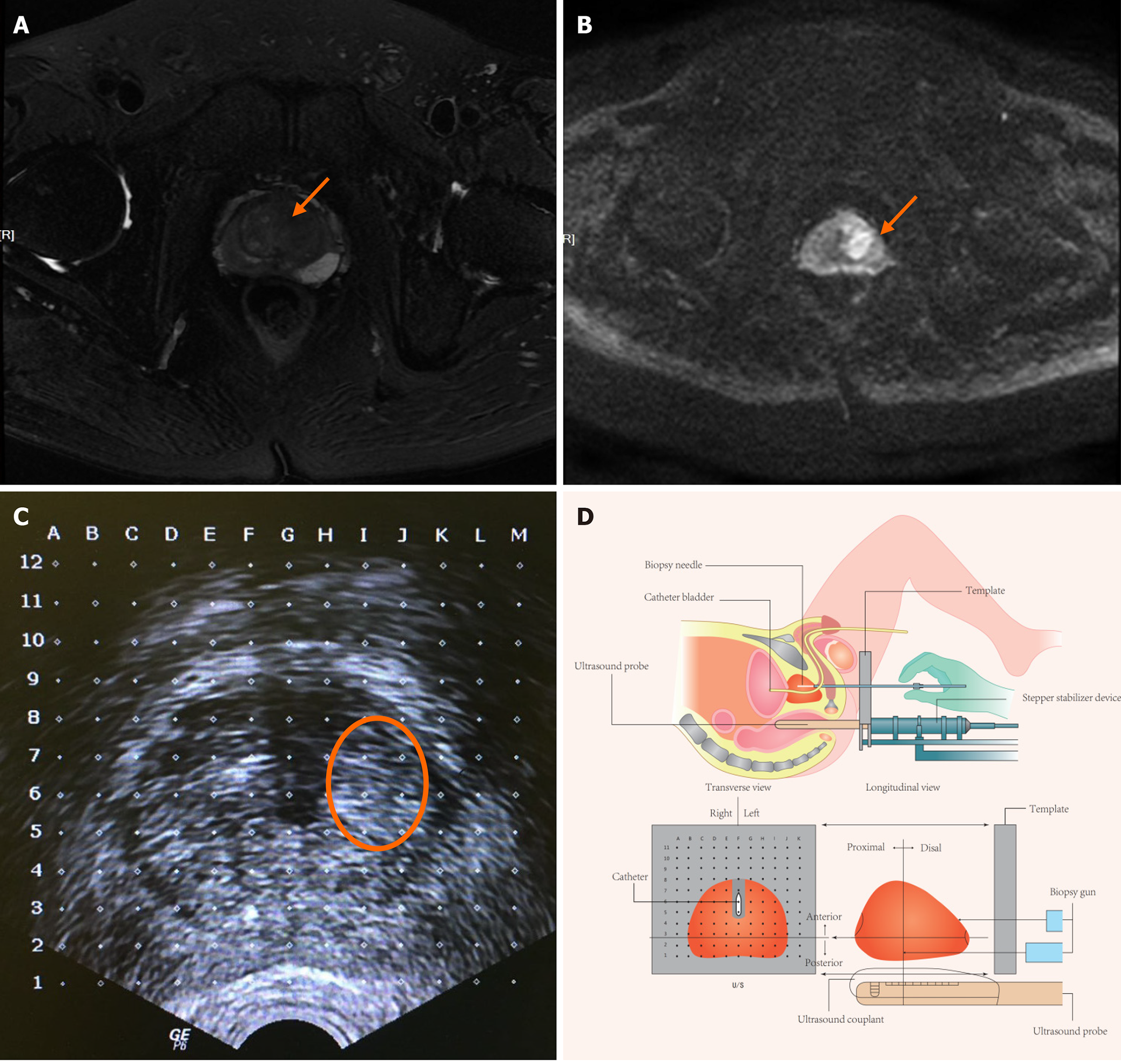
First, general anesthesia was administered, then positioned the patient in a lithotomy with the scrotum elevated anteriorly using microporous tape to expose the perineum. Next, the biplanar TRUS probe was fixed on a stepper stabilizer device, such that the TRUS probe could be propelled forward and backward by a specific distance to localize the targeted layer to be consistent with images on mp-MRI. A grid was then placed on the stepper stabilizer device ahead of the perineum to guide the biopsy gun. The urethra was visible on TRUS images using an indwelling Foley catheter (Figure 1).
The urologist reviewed the MRI and the report before the biopsy. MRI transverse images were obtained every 5 mm, and the layer intervals on the TRUS images were set to 5 mm using the stepper stabilizer device. The first step of cognition was to identify the apex and base of the prostate and then determine the corresponding layer containing the targeted lesion. The second step was to target the lesion on TRUS images using the urethra, the outline of the prostate, and the boundary between the peripheral and transitional zones. The targeted biopsy was administered first, with approximately two to four cores obtained per targeted lesion. Transperineal SBx outside the targeted areas was subsequently performed using a custom nine-region template, in which the prostate gland was divided into eight regions in a single plane with the apex of the prostate as an additional ninth region. Generally, two to four cores were obtained within each region according to the prostate gland volume.
Biopsy specimens were collected in formalin and sent for pathological analysis. Finally, grades were determined for each core by a uropathologist based on the International Society of Urological Pathology (ISUP) grading system[11].
Suspicious lesions on mp-MRI were defined as lesions with an overall PI-RADS score of 3-5, which could be considered candidate lesions for targeted biopsy.
csPCa was defined as a PCa lesion with a Gleason score ≥ 7 (ISUP ≥ 2), maximum cancer core length ≥ 4 mm, or both[12].
The age, BMI, prostate volume, PSA, PSA density for each patient, tumor dimension, location, and PI-RADS score for each lesion were recorded. Student’s t-test and Mann-Whitney U test were used for continuous variables. The chi-squared test was used for categorical variables, and a two-sided P < 0.05, was considered statistically significant. Univariate and multivariate logistic regression analyses were then conducted to screen for the influencing factors. All analyses were performed using SPSS statistical software (Version 24, IBM, Armonk, NY, United States).
The basic characteristics of patients, dichotomized by biopsy results are shown in Table 1. The median age was 68 (IQR: 63-74) years with a median PSA level of 8.51 (IQR: 5.43-11.40) ng/mL and a median tumor dimension of 1.10 (IQR: 0.70-1.30) cm. The overall operation time was 30 (20-45) min. After the biopsy, most patients had mild self-limited hematuria and perineal ecchymoses for < 7 days. Only one patient was treated with seminal vesiculoscopy for hemospermia. All procedures were well tolerated without high-grade complications or adverse events (defined as Clavien II or greater)[13] in the remaining cohort of patients.
| Total cohort | Negative biopsy | Positive biopsy | P value | |
| Patients, n | 127 | 60 | 67 | - |
| Age (yr), median (IQR) | 68 (63-74) | 66 (61-70) | 70 (65-78) | < 0.001 |
| BMI (kg/m2), median (IQR) | 24.8 (22.64-26.54) | 24.48 (23.03-26.56) | 24.77 (22.49-26.26) | 0.466 |
| PSA (ng/ml), median (IQR) | 8.51 (5.43-11.40) | 7.04 (5.06-11.01) | 8.96 (5.81-12.01) | 0.056 |
| Prostate volume (cm3), median (IQR) | 36.30 (26.80-46.20) | 42.67 (31.58-58.23) | 32.10 (23.20-39.65) | < 0.001 |
| PSAD (ng/ml/cm3), median (IQR) | 0.23 (0.14-0.34) | 0.16 (0.12-0.25) | 0.30 (0.18-0.43) | < 0.001 |
| Largest dimention (cm), median (IQR) | 1.10 (0.70-1.30) | 1.05 (0.53-1.28) | 1.10 (0.80-1.40) | |
| PI-RADS | 4 (3-5) | 3 (3-5) | 4(3-5) | < 0.001 |
| 3 (n/%) | 57 (44.9) | 46 (76.7) | 11 (16.4) | |
| 4 (n/%) | 41 (32.3) | 12 (20.0) | 29 (43.3) | |
| 5 (n/%) | 29 (22.8) | 2 (3.3) | 27 (40.3) | |
| Location, n (%) | 0.054 | |||
| TZ | 41 (32.3) | 23 (38.3) | 18 (26.9) | |
| PZ | 75 (59.1) | 35 (58.3) | 49 (59.7) | |
| Both | 0.054 | 2 (3.3) | 9 (13.4) | |
| Total cores, n, median (IQR) | 19 (17-22) | 20 (18-22) | 18 (15-22) | 0.046 |
| Targeted | 5 (4-8) | 5 (3-8) | 6 (4-8) | |
| Randomized | 14 (11-16) | 14 (12-16) | 13 (10-16) |
PCa was detected in 66 of the 127 patients (51.9%), of which 56 (44.1%) were csPCa. Urothelial carcinoma was detected in one case and was found to be positive only for COG-TB, which was not included in further analysis stratified by csPCa status. COG-TB alone detected 59/127 cases with PCa (46.5%), specifically 52/59 cases with csPCa (88.1%) and 7/59 cases with clinically insignificant PCa (11.9 %). Transperineal SBx detected 7 cases of PCa negative on COG-TB, of which 4 were csPCa, suggesting an approximate 7.1% added value to the result of COG-TB alone.
No significant differences in BMI, PSA level, tumor location, or total biopsy cores (all P > 0.05), whereas differences were found in age, prostate volume, PSA density (PSAD), maximum dimension, and PI-RADS scores were noted between patients with positive and negative results (all P < 0.05) (Table 1). Regarding csPCa status, age, prostate volume, PSAD, tumor dimension, and PI-RADS score also differed significantly (all P < 0.05) between patients with or without a diagnosis of csPCa (Table 2).
| Non-csPca | csPCa | P value | |
| Patients, n | 71 | 56 | - |
| Age (yr), median (IQR) | 66 (62-70) | 70 (65-78) | 0.001 |
| BMI (kg/m2), median (IQR) | 24.50 (23.05-26.70) | 24..75 (22.48-25.95) | 0.144 |
| PSA (ng/mL), median (IQR) | 7.32 (5.16-11.69) | 8.88 (5.76-11.14) | 0.277 |
| Proatate Volume (mL), median (IQR) | 41.02 (30.90-53.50) | 31.95 (23.39-39.65) | 0.001 |
| PSAD (ng/mL/cm3), median (IQR) | 0.18 (0.13-0.26) | 0.30 (0.21-0.40) | 0.003 |
| Largest dimention (cm), median(IQR) | 1.00 (0.60-1.30) | 1.10 (0.83-1.48) | 0.04 |
| PI-RADS | 3 (3-5) | 4 (3-5) | 0.001 |
| 3, n (%) | 47 (66.20) | 10 (17.86) | |
| 4, n (%) | 19 (26.76) | 22 (39.29) | |
| 5, n (%) | 5 (7.04) | 24 (42.86) | |
| Total cores, n (IQR) | 19 (17-22) | 18 (15-21) | 0.097 |
The results of logistic regression analysis showed that PSAD (OR, 1.008; 95%CI: 1.003-1.012, P = 0.001) and PI-RADS score (P < 0.001) were independent risk factors for COG-TB with SBx (Table 3). For COG-TB alone, PSAD (OR: 1.006, 95%CI: 1.002-1.010, P = 0.004) and PI-RADS score (P < 0.001) again appeared independently associated with biopsy result. Although tumor location did not independently influence the biopsy result, tumors involving TZ and PZ were more likely to be positive for COG-TB alone than tumors within the TZ (OR: 10.429, CI 95%: 1.218-89.285, P = 0.032) (Table 3).
| With randomized biopsy | Without randomized biopsy | |||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age | 1.052 | 0.977-1.133 | 0.177 | 1.018 | 0.945-1.097 | 0.640 |
| PSAD | 1.008 | 1.003-1.012 | 0.001 | 1.006 | 1.002-1.010 | 0.004 |
| PI-RADS score | < 0.001 | < 0.001 | ||||
| 3 | Ref. | Ref. | Ref. | Ref. | ||
| 4 | 8.167 | 2.599-25.662 | < 0.001 | 4.394 | 1.431-13.491 | 0.010 |
| 5 | 35.474 | 5.655-222.507 | < 0.001 | 54.266 | 8.141-361.724 | < 0.001 |
| Tumor dimension | 1.060 | 0.304-3.690 | 0.928 | 0.960 | 0.281-3.277 | 0.948 |
| Tomor location | 0.198 | 0.063 | ||||
| TZ | Ref. | Ref. | Ref. | Ref. | ||
| PZ | 2.086 | 0.673-6.464 | 0.203 | 2.904 | 0.875-9.637 | 0.081 |
| Both | 6.144 | 0.687-54.935 | 0.104 | 10.429 | 1.218-89.285 | 0.032 |
| Total cores | 0.981 | 0.857-1.124 | 0.786 | |||
TRUS-guided biopsy (TRUS-Bx) has long been the standard of care for prostate biopsy and still represents the reference standard modality for diagnosing PCa[2]. However, multiple studies have demonstrated that TRUS-Bx may lead to the absence of csPCa while detecting more insignificant PCa than the new strategy of MRI-guided TBx[14], and the transrectal approach may result in more infection-related complications than the transperineal approach[15,16]. Thus, image-guided biopsy via a transperineal approach has become promising and has been the focus of research in recent years.
One of the attractive features of TBx is its high detection rate. Earlier studies mostly focused on comparing TBx with TRUS-Bx in patients with at least one previous negative result[17-23], and the overall PCa detection rate is the most commonly used primary endpoint. Hadashick[24] and Miyagawa et al[25] reported two series of studies comparing transperineal TBx and transperineal TRUS-Bx performed on the same patient simultaneously. Overall PCa detection rates of 59% and 61% have been reported, respectively. However, the detection rate of csPCa was unknown in either study. Veeru et al[26] reported a detection rate of 57% (103/182) using transperineal TBx. The overall detection rates of PCa and csPCa for COG-TB alone in this study were 46.5% and 40.9%, respectively. Regarding the combination of COG-TB and SBx, the detection rates increased to 52.8% and 44.1%, respectively. The possible reasons for the lower detection rate in our study may be as follows: First, the inclusion criteria were more rigorous given the requirements of PSA level < 20 ng/mL and biopsy naïve history. Second, the differences in the strategy of targeted biopsy and thresholds for declaring a suspicious lesion on mp-MRI may also contribute to the difference in detection rates.
Another promising feature of TBx is its potential to reduce unnecessary cores taken without compromising the detection rate of csPCa. Recently, several prospective multicenter studies have proved the superiority of TBx over TRUS-Bx in the detection of csPCa. The PROMIS study[27] assessed mp-MRI and SBx against template prostate mapping biopsy in biopsy-naïve men and showed that mp-MRI was significantly more sensitive than SBx in detecting cancer ISUP grade group 3 or higher tumors or tumors with cancer core lengths > 6 mm. Another multicenter study (PRICISION)[28] assigned biopsy-naïve men to either TBx or SBx, and the results showed a significantly higher detection rate in the TBx group. Owing to the superiority of TBx in detecting csPCa, several studies have concluded that additional SBx can be omitted[29]. However, it remains controversial whether SBx should be abandoned when performing TBx[30,31]. A recently published prospective study (MRI-FIRST)[32] found no differences between SBx and TBx in the detection rate of ISUP grade group 2 or higher PCa, but the combination of these techniques showed added value, concluding that systematic biopsy cannot be avoided. In the current study, we applied a combined approach with COG-TB followed by SBx. The results showed that COG-TB alone missed 4/56 csPCa, and SBx provided an added value of approximately 7.4%, which should not be neglected. A total of 4.5 (1-12) and 14 (3-33) cores were taken for the targeted and SBx regions, respectively. This variation is mainly due to differences in prostate volume and the number of targeted regions. Furthermore, we applied a customized model that is easier to follow than those reported in previous studies[33], wherein the gland was cut into eight regions in a single plane with the apex of the prostate as the extra ninth region. After TBx, two-four cores were collected within each region outside the targeted lesions. We proposed this model to further standardize the biopsy scheme and reduce the maximum number of cores taken while guaranteeing a systematic sampling method. In this study, a feasible and safe follow-up method was developed.
Tumor dimension, PI-RADS score, prostate volume, and PSAD may influence the detection rate[34]. The results of our study are consistent with previous results, and we conducted further analysis on predictors of TBx. Lesions with a higher PSAD and PI-RADS score may be more likely to be positive for COG-TB. Moreover, lesions involving both the PZ and TZ were more likely to be positive for COG-TB, probably due to the larger tumor size. However, the cores taken from per-targeted lesions were not independent risk factors. A sufficient number of cores taken for the targeted region represents a possible reason for this result[28].
This study has some limitations. First, this was a single-centered retrospective analysis, the conclusions of which needs to be further confirmed by prospective multicentered studies. Second, all men included in the study were preselected using mp-MRI, which probably resulted in a higher positive result. Finally, various definitions of csPCa were employed in previous studies, whereas we only applied one definition in our study, the results of which will definitely be heterogeneous with studies using different definitions. Despite these limitations, the current study proposed a novel template for prostate SBx after TBx and a feasible approach for COG-TB combined with SBx with a relatively high detection rate. Several potential influencing factors were found that could serve as a reference for the stratification of biopsy patients.
The current study proposed a feasible approach for COG-TB combined with randomized biopsy using a cognitive fusion technique with an encouraging detection rate of csPCa and decreasing risk of missing lesions negative on mp-MRI. Patients with higher PSAD and PI-RADS scores were more likely to be positive under this biopsy strategy.
Various strategies for targeted biopsy (TBx) based on multiparametric magnetic resonance imaging (mp-MRI) have emerged, which may improve the accuracy of detecting clinically significant PCa in recent years. Cognitive fusion targeted biopsy is a more ecnomical and accessible strategy but requires more experience.
As cognitive fusion targeted biopsy requires higher level of experience, a more easily followed template would be meaningful for the generalization of this technique and could help reduce operator variability.
To investigate the diagnostic efficiency of a template for cognitive MRI-ultrasound fusion transperineal targeted plus randomized biopsy in detecting PCa, and to evaluate the potential influencing factors for the detection rate.
Patients with elevated PSA levels but less than 20 ng/mL, and having at least on suspicious lesion on MRI were retrospectively studied. The detection rate of all cancer and clinically significant cancer were calculated. Multivariate logistic regression analysis was used to analyze the potential influencing factors.
Cognitive fusion targeted biopsy alone detected 59/127 cases of PCa, specifically 52/59 cases with clinically significant PCa (csPCa) . A randomized biopsy showed an approximate 7.1% added value for csPCa detection. PSA density and PI-RADS score were independently associated with the results of cognitive fusion targeted biopsy combined with randomized biopsy and targeted biopsy alone.
This single-centered study proposed a feasible template for cognitive MRI-ultrasound fusion transperineal targeted plus randomized biopsy. Patients with higher PSAD and PI-RADS scores were more likely to be diagnosed with PCa using this biopsy strategy.
Prospective multicentered studies are needed to further test our template and to confirm the influencing factors for the detection rate.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leowattana W S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2417] [Cited by in RCA: 3053] [Article Influence: 508.8] [Reference Citation Analysis (0)] |
| 2. | Ghabili K, Tosoian JJ, Schaeffer EM, Pavlovich CP, Golzari SE, Khajir G, Andreas D, Benzon B, Vuica-Ross M, Ross AE. The History of Prostate Cancer From Antiquity: Review of Paleopathological Studies. Urology. 2016;97:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002;167:2435-2439. [PubMed] |
| 4. | Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, Fakhari M, Seitz C, Susani M, Borkowski A, Boccon-Gibod L, Schulman CC, Marberger M. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001;166:1679-1683. [PubMed] |
| 5. | Roethke MC, Kuru TH, Schultze S, Tichy D, Kopp-Schneider A, Fenchel M, Schlemmer HP, Hadaschik BA. Evaluation of the ESUR PI-RADS scoring system for multiparametric MRI of the prostate with targeted MR/TRUS fusion-guided biopsy at 3.0 Tesla. Eur Radiol. 2014;24:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Pepe P, Garufi A, Priolo G, Candiano G, Pietropaolo F, Pennisi M, Fraggetta F, Aragona F. Prostate cancer detection at repeat biopsy: can pelvic phased-array multiparametric MRI replace saturation biopsy? Anticancer Res. 2013;33:1195-1199. [PubMed] |
| 7. | Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, Dorey FJ, Marks LS. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 8. | Pepe P, Garufi A, Priolo G, Pennisi M. Can 3-Tesla pelvic phased-array multiparametric MRI avoid unnecessary repeat prostate biopsy in patients with PSA < 10 ng/mL? Clin Genitourin Cancer. 2015;13:e27-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, Margolis D, Schnall MD, Shtern F, Tempany CM, Thoeny HC, Verma S. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69:16-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1765] [Cited by in RCA: 2232] [Article Influence: 223.2] [Reference Citation Analysis (0)] |
| 10. | Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, Tempany CM, Choyke PL, Cornud F, Margolis DJ, Thoeny HC, Verma S, Barentsz J, Weinreb JC. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol. 2019;76:340-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 1503] [Article Influence: 250.5] [Reference Citation Analysis (0)] |
| 11. | Epstein JI, Amin MB, Reuter VE, Humphrey PA. Contemporary Gleason Grading of Prostatic Carcinoma: An Update With Discussion on Practical Issues to Implement the 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2017;41:e1-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 12. | Valerio M, Anele C, Bott SRJ, Charman SC, van der Meulen J, El-Mahallawi H, Emara AM, Freeman A, Jameson C, Hindley RG, Montgomery BSI, Singh PB, Ahmed HU, Emberton M. The Prevalence of Clinically Significant Prostate Cancer According to Commonly Used Histological Thresholds in Men Undergoing Template Prostate Mapping Biopsies. J Urol. 2016;195:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24564] [Article Influence: 1169.7] [Reference Citation Analysis (0)] |
| 14. | Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68:438-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 507] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 15. | Roberts MJ, Bennett HY, Harris PN, Holmes M, Grummet J, Naber K, Wagenlehner FME. Prostate Biopsy-related Infection: A Systematic Review of Risk Factors, Prevention Strategies, and Management Approaches. Urology. 2017;104:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, Weidner W, Loeb S. Complications After Systematic, Random, and Image-guided Prostate Biopsy. Eur Urol. 2017;71:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 335] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 17. | Haffner J, Lemaitre L, Puech P, Haber GP, Leroy X, Jones JS, Villers A. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108:E171-E178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 18. | Pinto PA, Chung PH, Rastinehad AR, Baccala AA Jr, Kruecker J, Benjamin CJ, Xu S, Yan P, Kadoury S, Chua C, Locklin JK, Turkbey B, Shih JH, Gates SP, Buckner C, Bratslavsky G, Linehan WM, Glossop ND, Choyke PL, Wood BJ. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 19. | Ponholzer A, Madersbacher S. Re: Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. Eur Urol. 2011;60:178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Sciarra A, Panebianco V, Ciccariello M, Salciccia S, Cattarino S, Lisi D, Gentilucci A, Alfarone A, Bernardo S, Passariello R, Gentile V. Value of magnetic resonance spectroscopy imaging and dynamic contrast-enhanced imaging for detecting prostate cancer foci in men with prior negative biopsy. Clin Cancer Res. 2010;16:1875-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Labanaris AP, Engelhard K, Zugor V, Nützel R, Kühn R. Prostate cancer detection using an extended prostate biopsy schema in combination with additional targeted cores from suspicious images in conventional and functional endorectal magnetic resonance imaging of the prostate. Prostate Cancer Prostatic Dis. 2010;13:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Prando A, Kurhanewicz J, Borges AP, Oliveira EM Jr, Figueiredo E. Prostatic biopsy directed with endorectal MR spectroscopic imaging findings in patients with elevated prostate specific antigen levels and prior negative biopsy findings: early experience. Radiology. 2005;236:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Hambrock T, Somford DM, Hoeks C, Bouwense SA, Huisman H, Yakar D, van Oort IM, Witjes JA, Fütterer JJ, Barentsz JO. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 24. | Hadaschik BA, Kuru TH, Tulea C, Rieker P, Popeneciu IV, Simpfendörfer T, Huber J, Zogal P, Teber D, Pahernik S, Roethke M, Zamecnik P, Roth W, Sakas G, Schlemmer HP, Hohenfellner M. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. J Urol. 2011;186:2214-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Miyagawa T, Ishikawa S, Kimura T, Suetomi T, Tsutsumi M, Irie T, Kondoh M, Mitake T. Real-time Virtual Sonography for navigation during targeted prostate biopsy using magnetic resonance imaging data. Int J Urol. 2010;17:855-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Kanthabalan A, Abd-Alazeez M, Arya M, Allen C, Freeman A, Jameson C, Kirkham A, Mitra AV, Payne H, Punwani S, Ramachandran N, Walkden M, Emberton M, Ahmed HU. Transperineal Magnetic Resonance Imaging-targeted Biopsy vs Transperineal Template Prostate Mapping Biopsy in the Detection of Localised Radio-recurrent Prostate Cancer. Clin Oncol (R Coll Radiol). 2016;28:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A, Kirkham AP, Oldroyd R, Parker C, Emberton M; PROMIS study group. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2029] [Cited by in RCA: 2260] [Article Influence: 282.5] [Reference Citation Analysis (0)] |
| 28. | Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, Briganti A, Budäus L, Hellawell G, Hindley RG, Roobol MJ, Eggener S, Ghei M, Villers A, Bladou F, Villeirs GM, Virdi J, Boxler S, Robert G, Singh PB, Venderink W, Hadaschik BA, Ruffion A, Hu JC, Margolis D, Crouzet S, Klotz L, Taneja SS, Pinto P, Gill I, Allen C, Giganti F, Freeman A, Morris S, Punwani S, Williams NR, Brew-Graves C, Deeks J, Takwoingi Y, Emberton M, Moore CM; PRECISION Study Group Collaborators. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018;378:1767-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1568] [Cited by in RCA: 2138] [Article Influence: 305.4] [Reference Citation Analysis (0)] |
| 29. | Kaufmann S, Kruck S, Kramer U, Gatidis S, Stenzl A, Roethke M, Scharpf M, Schilling D. Direct comparison of targeted MRI-guided biopsy with systematic transrectal ultrasound-guided biopsy in patients with previous negative prostate biopsies. Urol Int. 2015;94:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Giannarini G, Briganti A, Crestani A, Rossanese M, Montorsi F, Ficarra V. Dismiss Systematic Transrectal Ultrasound-guided and Embrace Targeted Magnetic Resonance Imaging-informed Prostate Biopsy: Is the Paradigm Ready to Shift? Eur Urol. 2016;69:381-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Radtke JP, Kuru TH, Boxler S, Alt CD, Popeneciu IV, Huettenbrink C, Klein T, Steinemann S, Bergstraesser C, Roethke M, Roth W, Schlemmer HP, Hohenfellner M, Hadaschik BA. Comparative analysis of transperineal template saturation prostate biopsy vs magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol. 2015;193:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 32. | Rouvière O, Puech P, Renard-Penna R, Claudon M, Roy C, Mège-Lechevallier F, Decaussin-Petrucci M, Dubreuil-Chambardel M, Magaud L, Remontet L, Ruffion A, Colombel M, Crouzet S, Schott AM, Lemaitre L, Rabilloud M, Grenier N; MRI-FIRST Investigators. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019;20:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 742] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 33. | Gorin MA, Meyer AR, Zimmerman M, Harb R, Joice GA, Schwen ZR, Allaf ME. Transperineal prostate biopsy with cognitive magnetic resonance imaging/biplanar ultrasound fusion: description of technique and early results. World J Urol. 2020;38:1943-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Murphy IG, NiMhurchu E, Gibney RG, McMahon CJ. MRI-directed cognitive fusion-guided biopsy of the anterior prostate tumors. Diagn Interv Radiol. 2017;23:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |