Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.11108
Peer-review started: August 6, 2021
First decision: September 2, 2021
Revised: September 9, 2021
Accepted: October 29, 2021
Article in press: October 29, 2021
Published online: December 16, 2021
Processing time: 126 Days and 2.9 Hours
Rhizopus microsporus (R. microsporus) lung infection is an invasive fungal disease with high mortality that is increasingly common in immunocompromised patients. However, it is very rare in immunocompetent patients. Here, we present the case of a 19-year-old girl who developed R. microsporus lung infection without any known immunodeficiency.
The patient presented to our hospital because of hemoptysis and irritative cough without expectoration. She was first treated for community-acquired pneumonia until the detection of R. microsporus in bronchoalveolar lavage fluid by metagenomics next-generation sequencing (mNGS). After a combination therapy of intravenous inhalation and local airway perfusion of amphotericin B, she eventually recovered, with significant absorption of lung infections.
Early diagnosis and treatment are very important for pulmonary mucormycosis. Compared to fungal culture, mNGS is a relatively precise and convenient method to obtain pathogenic results. A combination therapy of intravenous inhalation and local airway perfusion of amphotericin B may be a promising strategy for the treatment of pulmonary mucormycosis in the future.
Core Tip: We present the case of a 19-year-old girl who developed Rhizopus microsporus (R. microsporus) lung infection without any known immunodeficiency. Due to the early detection of the R. microsporus in bronchoalveolar lavage fluid by metagenomics next generation sequencing, promptly anti-mucor therapy was started. A new attempt of a combination therapy of intravenous, inhalation, and local airway perfusion of amphotericin B was then performed, which showed a good therapeutic effect.
- Citation: Chen L, Su Y, Xiong XZ. Rhizopus microsporus lung infection in an immunocompetent patient successfully treated with amphotericin B: A case report. World J Clin Cases 2021; 9(35): 11108-11114
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/11108.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.11108
Due to advancements in the diagnosis and treatment of immunosuppressed diseases, an increasing number of clinicians are aware of Rhizopus microsporus (R. microsporus) lung infection in immunocompromised patients. Rhizopus belongs to the zygomycotina and is also the most common species that causes pulmonary mucormycosis[1,2]. R. microsporus lung infection is more common in people with immunodeficiency diseases, especially in diabetic patients with poor blood sugar control and patients with hematological malignancies. It is also common in patients using immunosuppressive agents after transplantation to prevent transplant rejection. If not treated in time, the mortality rate is as high as 70%-100%[3]. A retrospective study in 2019 revealed 851 patients with mucormycosis from 2000 to 2017[4]. In that study, diabetes was the most common underlying disease (340/851, 40%), and pulmonary mucormycosis (172/851, 20%) was the third most common clinical manifestation. A total of 447 (53%) cases of certain Mucorales organisms were identified by culture, and R. microsporus (213/447, 48%) was the most common pathogen.
Mucormycosis in immunocompetent patients is rare and is easily ignored by clinicians. In the current study, we report a rare case of pulmonary R. microsporus infection in an immunocompetent young patient. Depending on the early pathological results via percutaneous lung puncture and pathogenic conclusions from metagenomics next-generation sequencing (mNGS), we avoid misdiagnosis and buy much time for the subsequent successful treatment of the patient.
Hemoptysis for 15 d.
A 19-year-old girl was admitted on January 26, 2021 because of hemoptysis for 15 d. The girl started coughing blood for no apparent reason 15 d ago, and the maximum amount of hemoptysis was approximately 100 mL at one time. She also had irritative coughs without expectoration. The patient did not complain of other symptoms, including fever, chest pain, respiration difficulties, headache, or vomiting. Seven days before admission, she received intravenous cefotaxime (2 g, twice daily) and an unknown hemostatic medication for 7 d at a local hospital.
The patient’s history was unremarkable, without previous contact with infectious agents.
The patient was living on campus and had no contact with animals or fungus exposure, with no history of smoking or drinking, denying a history of drug abuse. There was no history of infectious disease in her family.
On admission, the patient’s temperature was 36.5 °C, pulse rate was 86/min, respiration rate was 20/min, and blood pressure was 92/68 mmHg. Except for diminished vocal fremitus, dulls on percussion, and moist rales being found in her lower right lung, no other remarkable abnormalities were observed.
Relevant laboratory examinations were as follows: White blood cell count: 12.74 × 109/L (reference interval: 4.0-10.0 × 109); hemoglobulin: 82 g/L (115-150 g/L); neutrophils%: 53% (50%-70%); lymphocytes%: 22% (20%-50%); hypersensitive C-reactive protein: 36.1 mg/L (< 4 mg/L); and D-dimer: 12.94 mg/L (< 0.5 µg/mL). No obvious abnormalities were found on an electrocardiogram or for the rheumatic immune system, routine urine and liver function, electrolytes, renal function, HbAIc, and HIV tests.
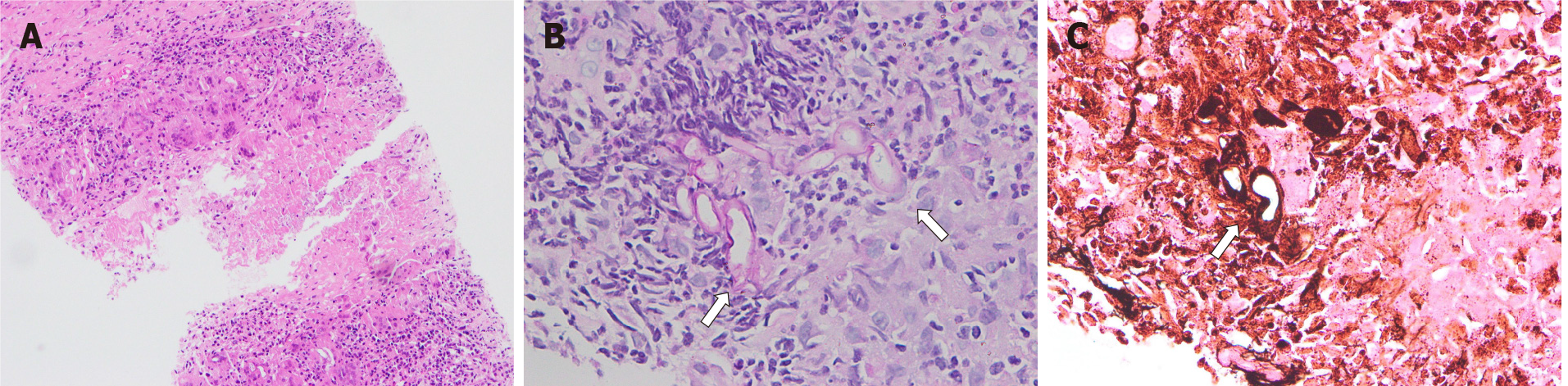
According to the China Community Acquired Pneumonia Treatment Guidelines (2016), the patient was given intravenous piperacillin-tazobactam (4.5 g, 3 times daily). No abnormalities were found in sputum culture or smear. The serum (1-3)-beta-D-glucan (G test) and galactomannan assays (GM test) were negative. Percutaneous lung biopsy with the right lower lobe was performed 2 d after admission, and positive staining of hexamine silver indicated pulmonary mycosis with granulomatous inflammation and necrosis. The histopathological features of thick and scattered hyphae were in line with mucormycosis (Figure 1). PCR test of tuberculosis was negative.
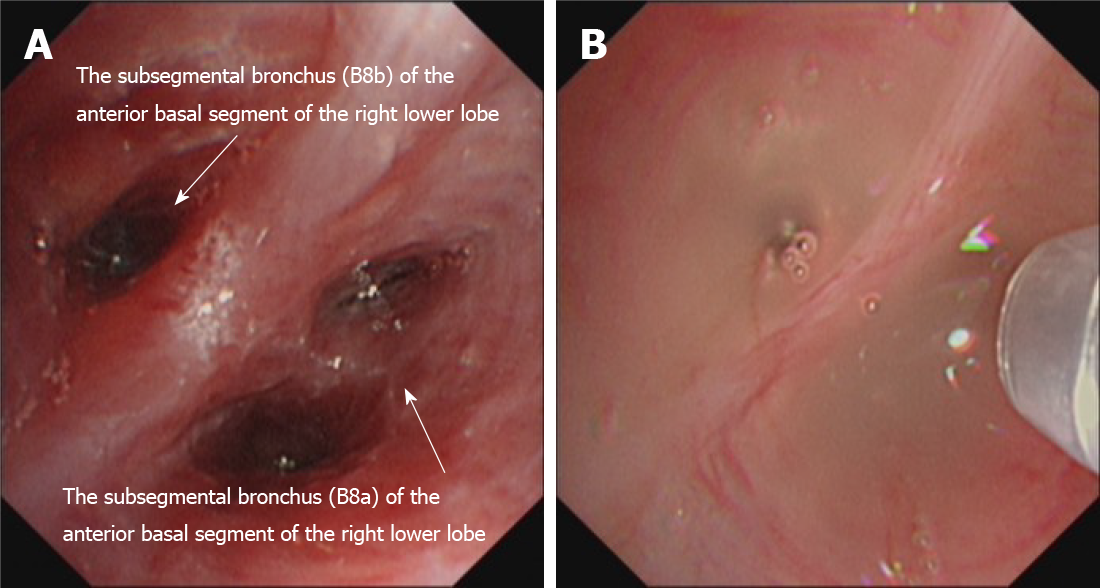
Three days after admission, the patient received electronic bronchoscopy. Purulent secretions and a swollen mucosa were observed in the basal segment of the right lower lobe (Figure 2A). An analysis of bronchoalveolar lavage fluid (BALF) was quickly performed by mNGS (VISION MEDICALS, Wuhan, Hubei Province, China). The mNGS results were obtained after 2 d, suggesting Rhizopus microspore infection. No bacteria, viruses, mycoplasma, chlamydia, Mycobacterium tuberculosis complex, or other pathogenic microorganisms were detected. The G test and GM test of BALF were negative. A diagnosis of Rhizopus microspore lung infection was made.
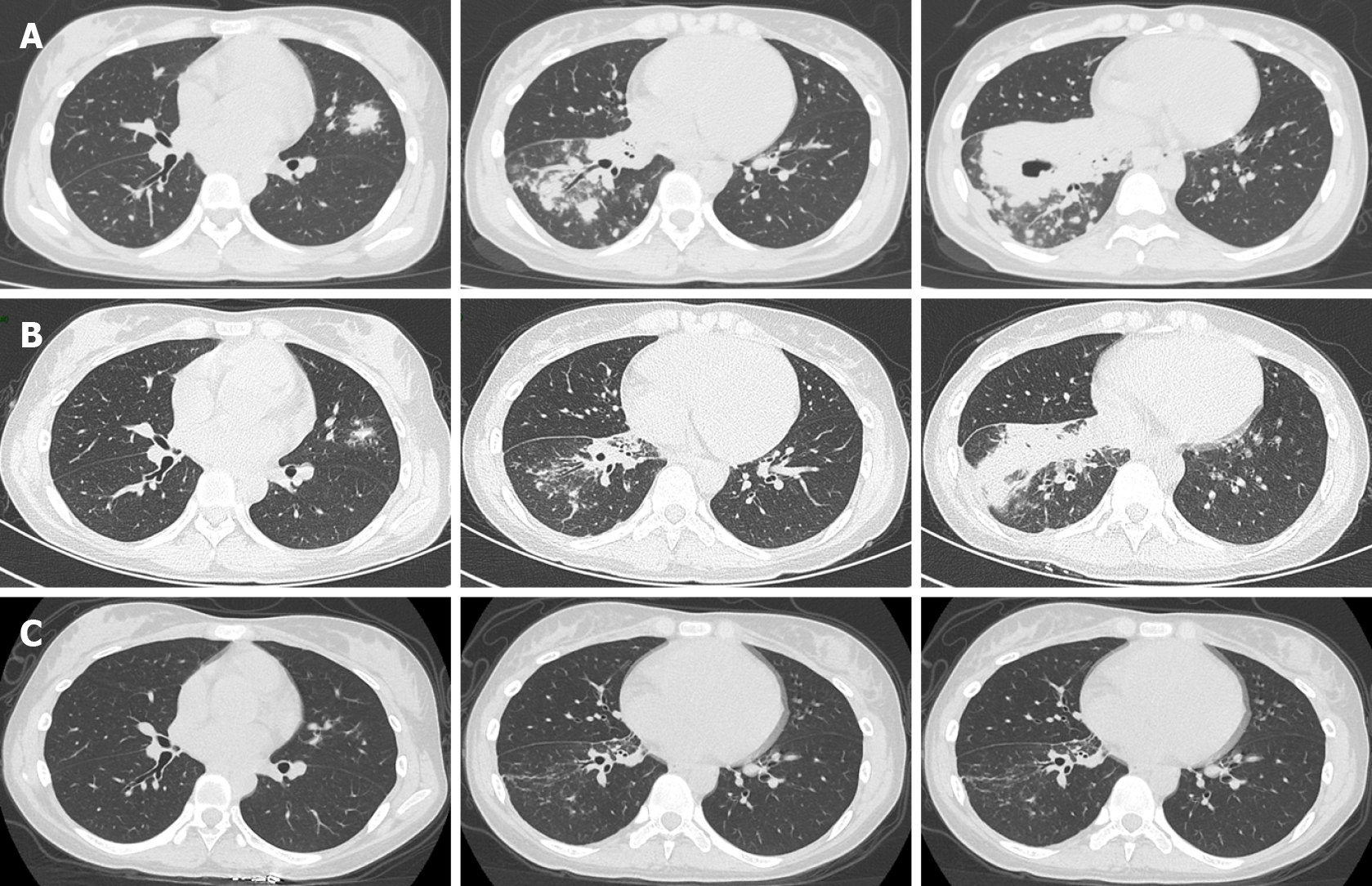
Chest computed tomography (CT) suggested bilateral pulmonary infection with cavitation involving the pulmonary right lower lobe (Figure 3A).
Rhizopus microspore lung infection.
Surgical resection is indeed one of the important therapies to deal with Rhizopus infection. We made consultation with the thoracic experts. Considering that the operation may be traumatic, and the patient’s parents disagreed with the lobectomy. Therefore, we started medical treatment. According to the Chinese guidelines for the diagnosis and treatment of invasive fungal disease in patients with hematological disorders and cancers (the 6th revision), amphotericin B (AmB) for injection (5 mg intravenous daily, then gradually increased to 120 mg daily) was administered as antifungal treatment 5 d after admission, and intravenous antibiotics were stopped. As a result, after 12 d of antifungal treatment (intravenous AmB for 120 mg daily), the hemoptysis and cough of the patient were suspended, and the white blood cell count decreased to 8.26 × 109/L (reference interval: 4.0-10.0 × 109). Although lung symptoms seemed improved, the patient began to experience severe nausea and vomiting with hypokalemia. Considering both the effectiveness and safety, a treatment combining intravenous and inhaled AmB was performed. The intravenous dose of AmB was adjusted to 60 mg daily, combined with inhalation of AmB 10 mg twice a day. After administering antiemetic therapy and potassium supplementation at the same time, the patient's digestive symptoms gradually improved.
On March 3, 2021, after 30 d of antifungal treatment, chest CT showed a decrease in lung inflammation and an absorption of cavitation in the right lower lobe (Figure 3B). Perfusion with AmB (10 mg dissolved in 10 mL saline) on the basal segment of the right lower lobe was then tried three times through a microtube in an electronic bronchoscope on March 4, March 11, and March 21 (Figure 2B). A slight improvement of the swollen mucosa of the right lower lobe was observed after the treatment, and no secretions were found in the local airway.
Prior to discharge, the patient received an alternative therapy to posaconazole oral solution (400 mg twice daily) according to a Chinese expert consensus on the clinical use of posaconazole.
Significant absorption of lung infections was observed at the chest CT follow-up on April 15, 2021 (+ 80 d) (Figure 3C).
R. microsporus lung infection is rare in immunocompetent patients and seldom reported[5-11]. In a study in 2009, 24 patients with proven or probable pulmonary mucormycosis at Peking Union Medical College Hospital from January 2005 to December 2018 were retrospectively analyzed, and seven patients (29.2%) had no obvious predisposing risk factors[12]. Although some of these patients had normal immune function, they shared an environmental exposure history with mucor spores such as decaying food, soil, and animal excrement[1], which might be the diagnostic basis for pulmonary mucormycosis. In contrast, in the case that we reported, the patient was a 19-year-old young female student who usually lived on campus without any underlying diseases, weakened immune function, or mucor-related environmental exposure history. The patient was treated at another hospital for a period of time, accompanied by a prolonged course and nonabsorbable lung lesions. Invasive examinations, such as percutaneous lung puncture and electronic bronchoscopy, were tried promptly. The pathologic findings of mucor hyphae and the mNGS results both resulted in the conclusion of R. microsporus pulmonary infection. Commonly, pulmonary mucor infection lacks specificity in imaging and is variable. Techniques to determine infection by Aspergillus or Mucor based on the histopathological features of hyphae are not completely reliable[13]. Other clinical clues that suggest pulmonary mucormycosis rather than aspergillosis include concurrent sinus, previous voriconazole therapy, the presence of more than ten lesions, and the presence of pleural effusion for imaging findings[14]. None of the above abnormal manifestations were observed in this patient. On chest radiography, lobar and segmental consolidation is the most common imaging finding, and imaging in some patients shows a multilobar distribution[15], which is consistent with our case.
Prompt initiation of appropriate therapy is critical for patients with pulmonary mucomycosis[16]. A study also found that early intervention may lead to better outcomes[17]. Although histopathology will probably remain the gold standard for the diagnosis of mucormycosis[18,19], obtaining a biopsy specimen is not always feasible in most vulnerable populations. Moreover, the distinction between aspergillosis and scedosporiosis and between aspergillosis and fusariosis and certain mucormycosis from tissue sections may be difficult or impossible[20]. However, the clinical distinction between aspergillosis and mucormycosis is crucial since there is an increased incidence of mucormycosis in patients treated with voriconazole for suspected aspergillosis[21]. In recent years, mNGS methods have been used to try to improve the detection and identification of pathogens and have become a topic of concern as routine pathogen identification tools[22]. In our case, the identification of Rhizopus microspores in BALF by mNGS was achieved in the early stage. Considering that antibiotic treatment was ineffective in this patient and mNGS did not indicate other bacterial or viral infections, we made a final diagnosis of lung infection of Rhizopus microspores for the young immunocompetent patient. Unfortunately, we failed to obtain positive culture results from lung puncture specimens, which might be related to insufficient amounts or redundant necrotic contents of the specimens. However, early anti-mucor treatment was started immediately, which eventually led to an overall good therapeutic effect.
AmB is commonly used for the treatment of mucormycosis of the lung, and other optional drugs include posaconazole and isaconazole[17,23]. In addition to intravenous use of AmB, inhalation of AmB has been used in the clinic[24] and has the advantages of high local concentration and low systemic side effects. For the application of AmB in the local airway through bronchoscopy, there is still a lack of clinical reports. We initiated treatment with AmB in the early stage, with a combination therapeutic strategy of intravenous inhalation and local airway perfusion, followed by sequential posaconazole oral administration, which eventually brought about a satisfactory therapeutic effect with the absorption of lung lesions. However, there is not enough evidence for perfusion by AmB through the local airway. The advantage of this method is that it can be used for the precise treatment of local mucormycosis lesions. At the same time, the local drug concentration is higher compared with systemic use and less irritating to other nonfocal areas. The disadvantage is that it is limited by the patient's conditions and technical conditions of the medical team, and the patient is required to treat through bronchoscopy repeatedly, which means the requirement of a certain degree of compliance. To date, perfusion with AmB in the local airway might be safe and feasible, accompanied by small adverse effects. It can be used in combination, alternately or sequentially, with intravenous and inhalation therapy, although the prognostic efficacy needs further follow-up.
R. microsporus lung infection in immunocompetent patients is rare, which reminds clinicians to be alert to the potential risk of Rhizopus infection in these patients. Considering the high mortality rate of the disease, early diagnosis and treatment are very important for the prognosis of patients. In our clinical practice, a combination strategy of intravenous inhalation and local airway perfusion of AmB may be a new strategy for the treatment of pulmonary mucormycosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shijubou N S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
| 1. | Agrawal R, Yeldandi A, Savas H, Parekh ND, Lombardi PJ, Hart EM. Pulmonary Mucormycosis: Risk Factors, Radiologic Findings, and Pathologic Correlation. Radiographics. 2020;40:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Reid G, Lynch JP 3rd, Fishbein MC, Clark NM. Mucormycosis. Semin Respir Crit Care Med. 2020;41:99-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Larché J, Machouart M, Burton K, Collomb J, Biava MF, Gérard A, Fortier B. Diagnosis of cutaneous mucormycosis due to Rhizopus microsporus by an innovative PCR-restriction fragment-length polymorphism method. Clin Infect Dis. 2005;41:1362-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM, Chen SC. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 551] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 5. | Butala A, Shah B, Cho YT, Schmidt MF. Isolated pulmonary mucormycosis in an apparently normal host: a case report. J Natl Med Assoc. 1995;87:572-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | He J, Sheng G, Yue H, Zhang F, Zhang HL. Isolated pulmonary mucormycosis in an immunocompetent patient: a case report and systematic review of the literature. BMC Pulm Med. 2021;21:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Tsyrkunou AV, Ellison RT 3rd, Akalin A, Wiederhold N, Sutton DA, Lindner J, Fan H, Levitz SM, Zivna I. Multifocal Rhizopus microsporus lung infection following brush clearing. Med Mycol Case Rep. 2014;6:14-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Yang J, Zhang J, Feng Y, Peng F, Fu F. A case of pulmonary mucormycosis presented as Pancoast syndrome and bone destruction in an immunocompetent adult mimicking lung carcinoma. J Mycol Med. 2019;29:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Santos Silva J, Torres C, Clemente S, Calvinho P. Isolated Pulmonary Mucormycosis in an immunocompetent patient. Rev Port Cir Cardiotorac Vasc. 2019;26:151-153. [PubMed] |
| 10. | Lee JS, Kim HC, Park SW, So HS, Woo CY, Choi JH, Kim SH, Kim SJ, Oh YM. A case of isolated pulmonary mucormycosis in an immunocompetent host. Tuberc Respir Dis (Seoul). 2013;74:269-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Sarkar S, Jash D, Maji A, Maikap MK. Solitary pulmonary nodule: A rare presentation of pulmonary mucormycosis in an immunocompetent adult. Lung India. 2014;31:70-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Peng M, Meng H, Sun Y, Xiao Y, Zhang H, Lv K, Cai B. Clinical features of pulmonary mucormycosis in patients with different immune status. J Thorac Dis. 2019;11:5042-5052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 678] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 14. | Chamilos G, Marom EM, Lewis RE, Lionakis MS, Kontoyiannis DP. Predictors of pulmonary zygomycosis vs invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis. 2005;41:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | McAdams HP, Rosado de Christenson M, Strollo DC, Patz EF Jr. Pulmonary mucormycosis: radiologic findings in 32 cases. AJR Am J Roentgenol. 1997;168:1541-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Pyrgos V, Shoham S, Walsh TJ. Pulmonary zygomycosis. Semin Respir Crit Care Med. 2008;29:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42:e61-e65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 434] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 18. | Dadwal SS, Kontoyiannis DP. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev Mol Diagn. 2018;18:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Lackner M, Caramalho R, Lass-Flörl C. Laboratory diagnosis of mucormycosis: current status and future perspectives. Future Microbiol. 2014;9:683-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Jensen HE, Salonen J, Ekfors TO. The use of immunohistochemistry to improve sensitivity and specificity in the diagnosis of systemic mycoses in patients with haematological malignancies. J Pathol. 1997;181:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Pongas GN, Lewis RE, Samonis G, Kontoyiannis DP. Voriconazole-associated zygomycosis: a significant consequence of evolving antifungal prophylaxis and immunosuppression practices? Clin Microbiol Infect. 2009;15 Suppl 5:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Han D, Li Z, Li R, Tan P, Zhang R, Li J. mNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol. 2019;45:668-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 237] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 23. | Ruiz Camps I, Salavert Lletí M. [The treatment of mucormycosis (zygomycosis) in the 21st century]. Rev Iberoam Micol. 2018;35:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Kuiper L, Ruijgrok EJ. A review on the clinical use of inhaled amphotericin B. J Aerosol Med Pulm Drug Deliv. 2009;22:213-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |