Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.11078
Peer-review started: June 24, 2021
First decision: July 26, 2021
Revised: July 27, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: December 16, 2021
Processing time: 168 Days and 22.1 Hours
Invasive mucinous adenocarcinoma of the lung, formerly known as mucinous bronchioloalveolar carcinoma, is a rare category of lung tumors and radiologically characterized by dense pneumonic consolidation, ground-glass opacity, crazy paving, and nodules. However, early pleural effusion is uncommon in this malignancy.
The case of a 32-year-old male patient who visited our facility with symptoms of cough and gradually aggravated shortness of breath was reported. X-ray examination revealed a massive left hydrothorax. The patient underwent thoracocentesis, and pleural fluid tumor markers, including carcinoembryonic antigen, carbohydrate antigen 19-9, neuron-specific enolase, and cytokeratin 21-1 fragment, were significantly elevated. A similar tendency was observed among the serum tumor markers. After draining the pleural effusion, the patient underwent chest computed tomography, and no obvious mass was found in the lung. Thoracoscopy revealed that the left visceral pleura was covered with nodular, cauliflower-like protrusions of various sizes. These histopathological results suggested cancerous cells, and the immunohistochemical findings were consistent with mucinous adenocarcinoma of pulmonary origin. It tested positive for cytokeratin, cytokeratin 5/6, carcinoembryonic antigen, and thyroid transcription factor-1.
The patient was diagnosed with a rare case of lung mucinous adenocarcinoma. Subsequent genetic testing was positive for epidermal growth factor receptor-21 mutations and echinoderm microtubule-associated protein-like 4-lymphoma anaplastic kinase fusion. This prompted treatment with alfatinib and crizotinib.
Core tip: Invasive mucinous adenocarcinoma of the lung is a rare category of lung tumors, radiologically characterized by dense pneumonic consolidation, ground-glass opacity, crazy paving, and nodules. Early pleural effusion is uncommon in this malignancy. Molecularly, invasive mucinous adenocarcinoma has frequent Kirsten rat sarcoma viral oncogene mutations and a lower prevalence of epidermal growth factor receptor and anaplastic lymphoma kinase rearrangements. Here, a rare case of double-mutant invasive mucinous lung adenocarcinoma presenting as a massive malignant pleural effusion in a 32-year-old male patient was reported.
- Citation: Wang T. Double-mutant invasive mucinous adenocarcinoma of the lung in a 32-year-old male patient: A case report. World J Clin Cases 2021; 9(35): 11078-11084
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/11078.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.11078
According to the global cancer statistics in 2018, lung cancer is the leading cause of cancer-related mortality, killing approximately 1761000 people worldwide[1]. Histologically, lung cancer is divided into non-small cell lung cancer and small cell lung cancer, with the former pathological type accounting for 80% of cases[2]. Invasive mucinous adenocarcinoma (IMA) of the lung was introduced as a new category in the 2015 World Health Organization classification of lung tumors. It accounts for 2%-5% of lung adenocarcinomas, the most common histologic subtype of non-small cell lung cancer[3,4]. IMA is diagnosed based on tall columnar cell morphology with abundant intracellular/extracellular mucus and invasive adenocarcinoma patterns, such as lepidic, acinar, papillary, and predominant patterns. Due to its pathological and growth characteristics, the typical computed tomography (CT) findings of IMA include consolidation, ground-glass opacity, and nodules.
In contrast, pleural IMAs are rare[5-7]. A case of IMA presenting as a massive pleural effusion in the left thoracic cavity in a 32-year-old male patient was reported. After drainage, multiple nodules on the left visceral pleura, instead of apparent pulmonary masses, were found on a chest CT scan. Gene testing showed that the biopsy tissue had a double mutation, namely positive epidermal growth factor receptor-21 (EGFR-21) mutations and echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (ALK) fusion. To the best of my knowledge, this is the first published report on dual-mutation IMA with early pleural invasion in a young patient.
A 32-year-old male chronic smoker (ten cigarettes a day for more than 10 years) was referred to our hospital’s respiratory department on June 25, 2020. He complained of shortness of breath and a cough.
The patient had a history of progressive shortness of breath and a cough for 20 d before consult.
He had no prior chronic diseases but recently lost 4 kg of his body weight.
The patient denied having a family history of lung cancer, hypertension, and coronary heart disease.
Physical examination showed that respiratory sounds were absent in the patient’s left lung field.
He was admitted to our department where he underwent thoracocentesis that resulted in the extraction of 700 mL of pleural effusion. Related laboratory results of the yellow turbid pleural fluid revealed an exudate with dramatically high tumor biomarkers and normal adenosine deaminase, which was unexpected. Detailed data are as follows: total cell count = 8015 × 106/L, leukocyte count = 440 × 106/L, positive Rivalta test, lactate dehydrogenase = 676 U/L, total protein 51 g/L, adenosine deaminase = 13 U/L, carcinoembryonic antigen = 34.6 ng/mL (normal range: 0-5), carbohydrate antigen 19-9 > 1000 U/mL (normal range: 0-37), neuron-specific enolase = 22.65 ng/mL (normal range: 0-18), and cytokeratin 21-1 fragment > 500 ng/mL (normal range: 0-3.3). The patient’s serum tumor biomarkers exhibited a less obvious upward trend than their counterparts in the pleural effusion, with carcinoembryonic antigen 7.96 ng/mL, carbohydrate antigen 19-9 204.2 U/mL, and cytokeratin 21-1 fragment 6.33 ng/mL. Cytological tests revealed allotype tumor cells in the hydrothorax. We then performed closed thoracic drainage because of the massive pleural effusion and apparent symptoms of this patient.
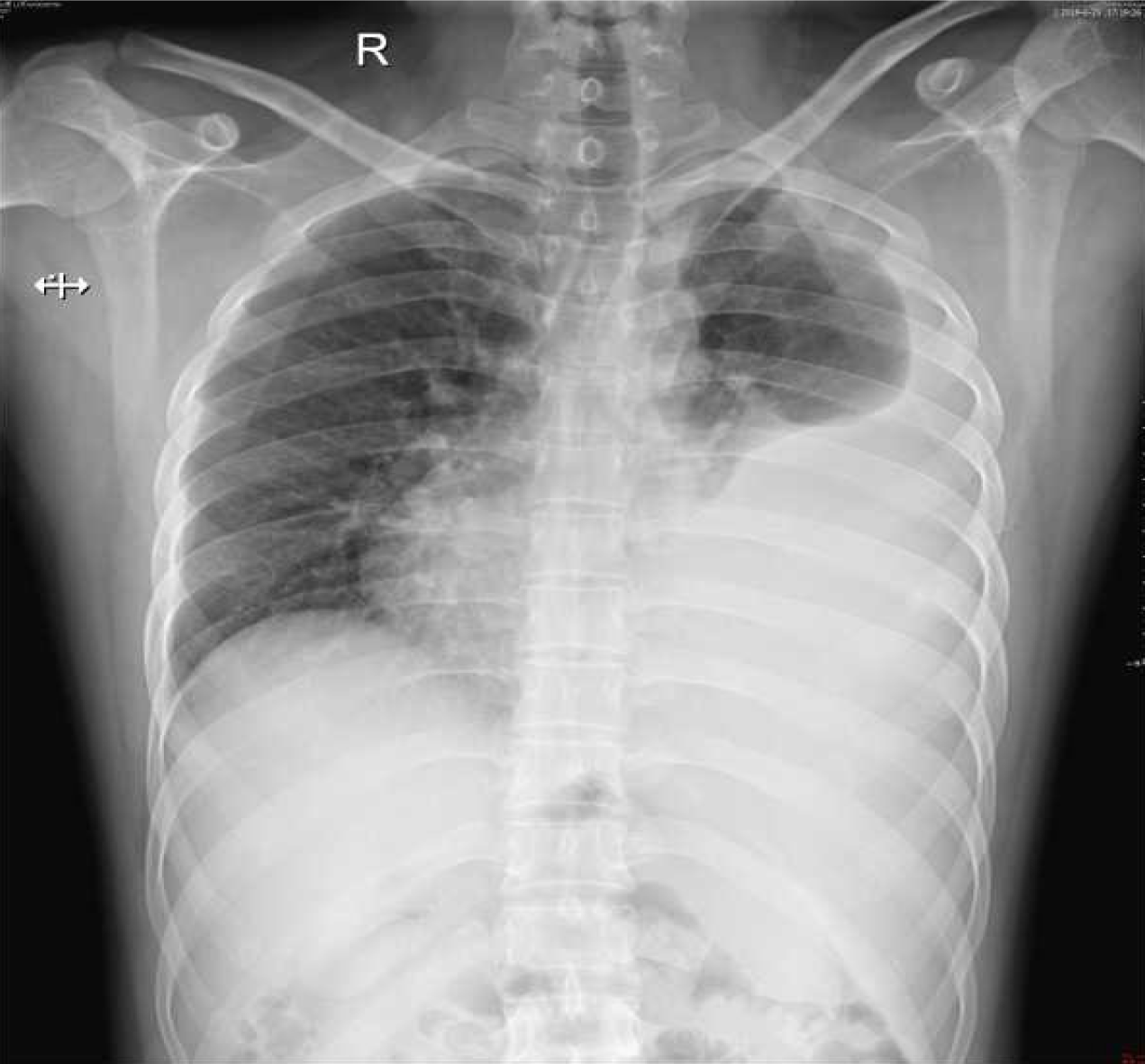
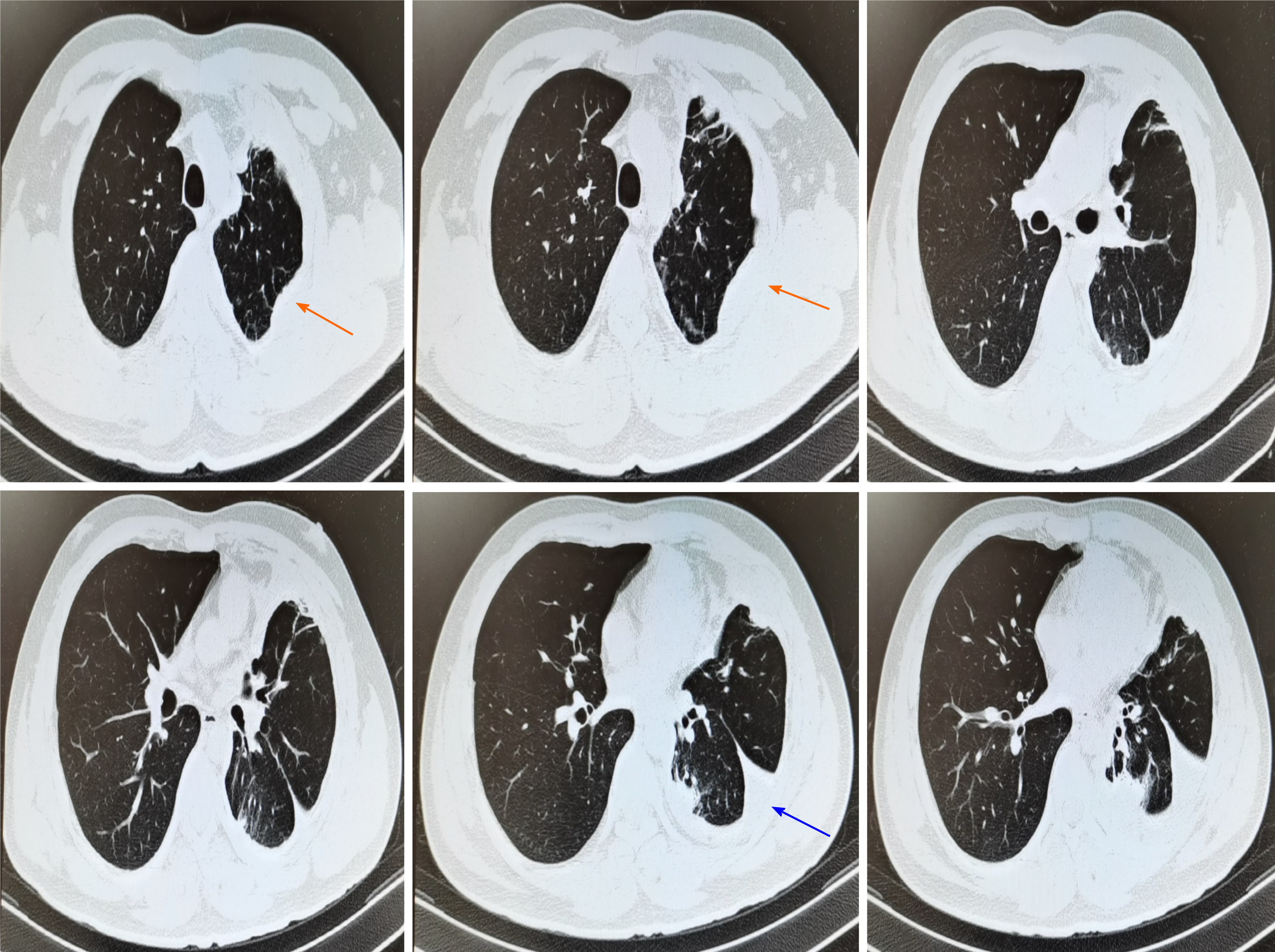
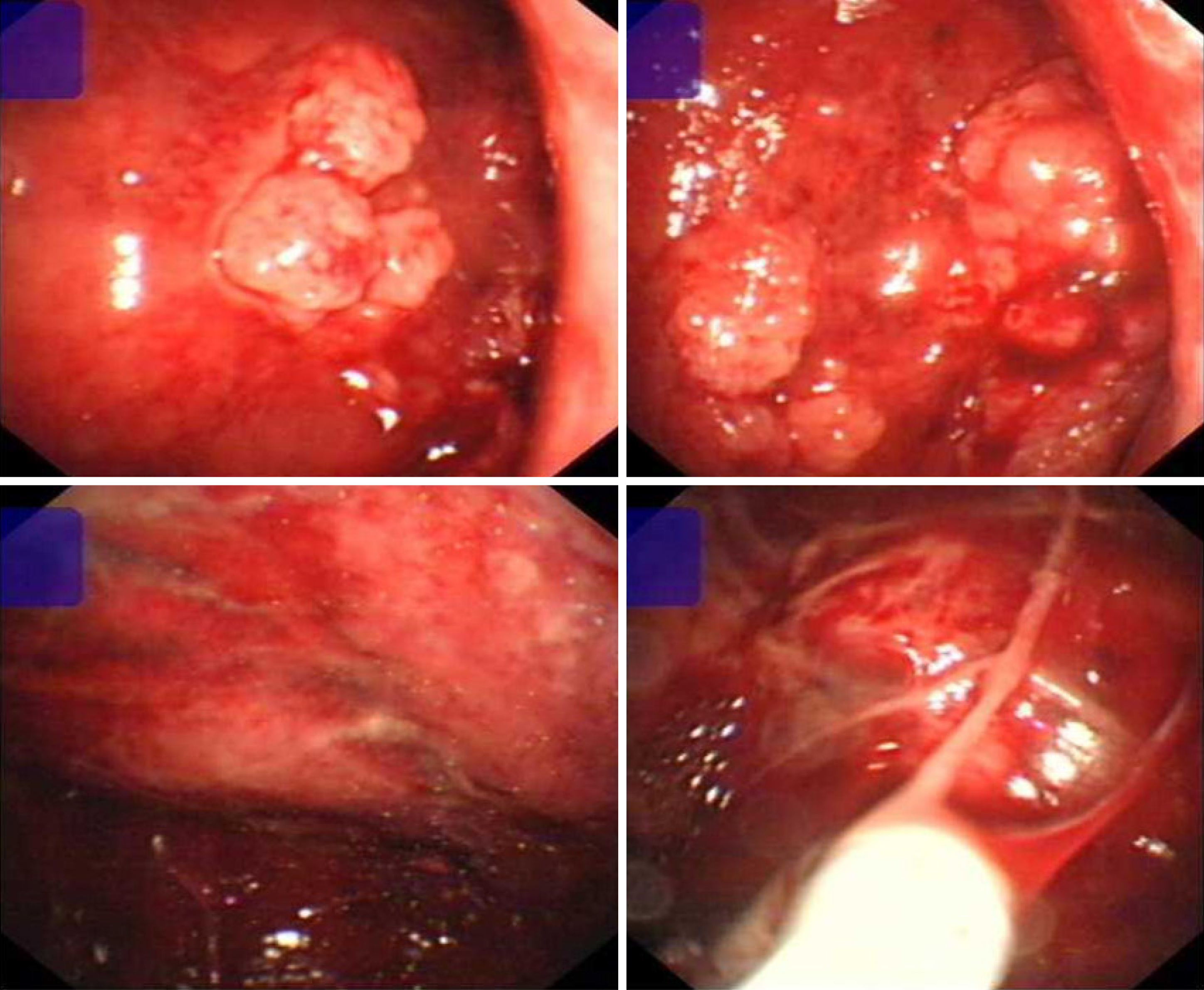
Chest X-ray showed a severe left hydrothorax (Figure 1), and the patient was hospitalized with a suspected diagnosis of tuberculous exudative pleurisy. After pleural effusion drainage, the patient underwent chest CT, demonstrating no evident pulmonary masses or nodules; however, multiple nodules were found in the left visceral pleura (Figure 2). Subsequent thoracoscopy revealed that the visceral pleura was filled with nodular, cauliflower-like protrusions of various sizes (Figure 3).
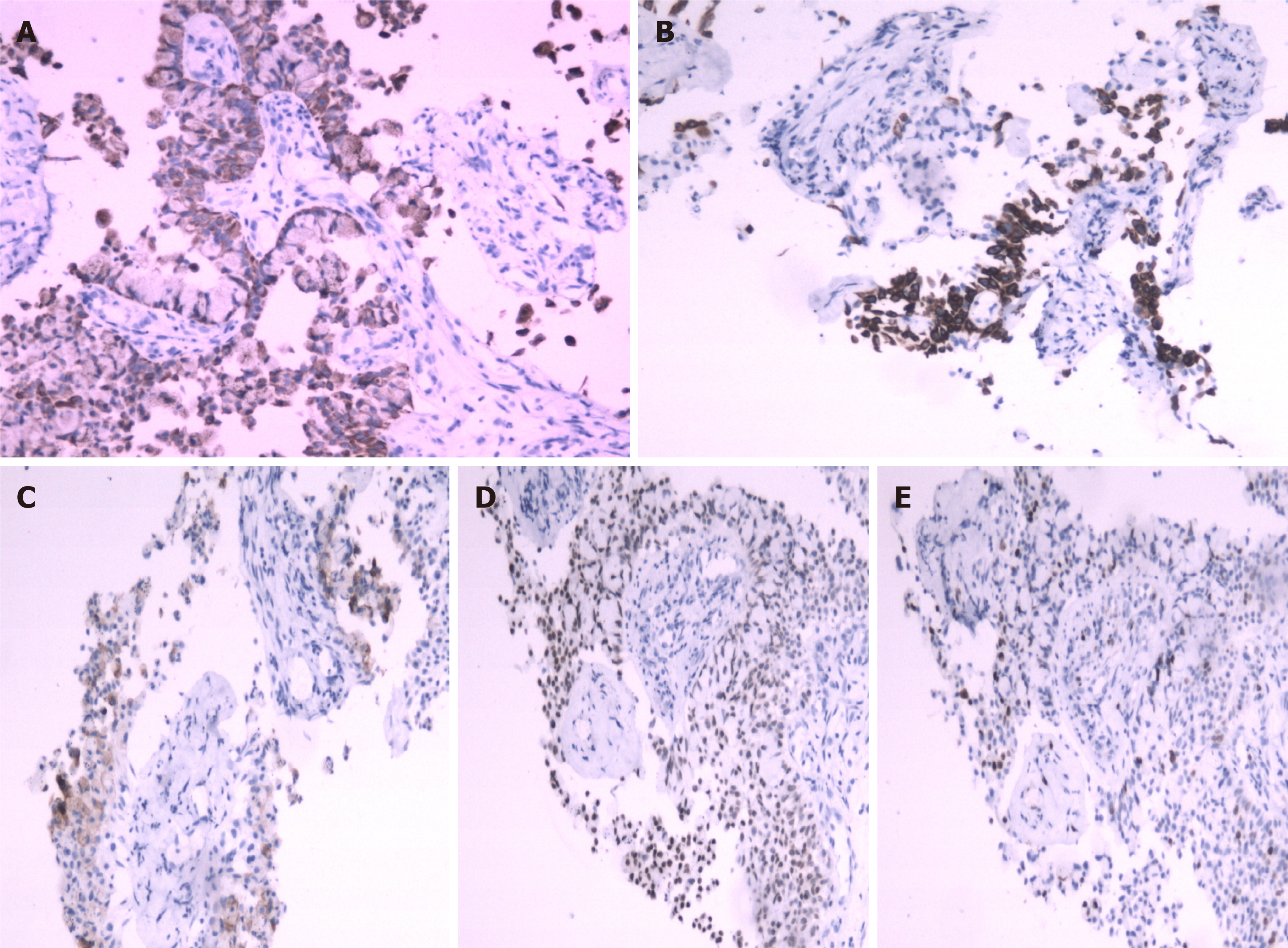
The initial pathological diagnosis was allotypic epithelioid cells, and immunohistochemical analysis showed tumor cell positivity for cytokeratin, cytokeratin 5/6, carcinoembryonic antigen, thyroid transcription factor-1, and Ki-67 (20%). The tumor tested negative for calretinin, cytokeratin 20, and p53, which were compatible with lung IMA (Figure 4). Genetic testing revealed positive EGFR-21 mutations and echinoderm microtubule-associated protein-like 4-ALK fusion. This prompted treatment with alfatinib and crizotinib.
Considering the side effects of the two targeted drugs, we initially prescribed alfatinib alone with a daily dose of 40 mg. After 1 mo, re-examination showed decreased pleural nodules, no pleural effusion recurrence, and significantly reduced serum lung cancer biomarkers. Meanwhile, the patient had a long-standing unresolved left lateral chest pain. After 2 mo, the second follow-up showed no changes in serum lung cancer biomarkers. However, the number of pleural nodules increased, and small metastases were observed in the contralateral lung. We adjusted the therapeutic plan based on these findings. Both alfatinib and crizotinib were administered.
The patient’s left lateral chest pain resolved within 1 wk. Thus far, the patient has taken alfatinib 40 mg per day and crizotinib 250 mg twice per day for more than 10 mo, and side effects, such as mild diarrhea and skin rashes, have occurred. After 12 mo, chest CT scan re-examination showed apparent reductions in the pleural nodules and no recurrence of pleural effusion.
Lung cancer, which is associated with high morbidity and mortality among all types of tumors, frequently occurs in the sixth to eighth decades of life. It is uncommon among people around 30 years of age[8]. A new and rare type of adenocarcinoma, IMA, was reclassified as a variant of invasive adenocarcinoma by the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification system because of its unique clinical, pathological, and radiological features as well as its unique genetic characteristics (lower prevalence of EGFR mutations)[9,10].
IMA has a tall columnar morphology with abundant mucus inside or outside the tumor cells. It exhibits adenocarcinoma patterns, including acinar, papillary, micropapillary, and solid predominant patterns. Specifically, IMA is divided into two groups based on the mucinous percentage: pure mucinous (invasive mucinous component > 90%) and mixed mucinous/non-mucinous (non-mucinous invasive pattern > 10%)[11]. IMA, a classification with poor survival outcomes compared with other adenocarcinoma subtypes, frequently spreads aerogenously, forming satellite tumors. Lymph node involvement and distant metastasis are less common[3,12].
On CT, IMA presents as consolidation, nodules, or ground-glass opacity. In a study by Nie et al[13], 54 of 68 patients with IMA had solitary-type tumors on their chest CT scan. Nearly 80% of the imaging signs of IMA are solitary nodules or masses. The other 20% of CT findings are the pneumonic type, which is defined as consolidation without a defined shape, distributed along the lung lobe or lung segment, and sometimes with air bronchogram. In contrast, pleural effusion, as an early sign of IMA, is rare. In our case, immunohistochemical analysis showed that the tumor cells were of pulmonary origin. No clear pulmonary signs were observed on chest imaging after pleural effusion drainage. Instead, multiple nodules were observed in the left visceral pleura, which was more suggestive of pleural mesothelioma. Atelectasis was also observed in the left lower lung, which may have negatively influenced assessment and diagnosis.
Concerning the molecular features of IMA, several studies have linked IMA with frequent Kirsten rat sarcoma viral oncogene mutations and a lower prevalence of EGFR and ALK rearrangements, indicating a poor prognosis for target-specific drug treatment[14,15]. In our case, the EGFR-21 mutations and echinoderm microtubule-associated protein-like 4-ALK fusion were both positive, which is rare for IMA. This case emphasizes the significance of applying medicine to different targets in lung cancer. We will conduct a continuous follow-up of this young patient in the future.
In conclusion, atypical IMA is challenging to diagnose, especially in young patients. It is necessary to consider IMA in patients with unusual laboratory test results and radiological presentations.
We are thankful to Dr. Mingwei Chen for his advice on writing this rare case as a report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferreira GSA S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55853] [Article Influence: 7979.0] [Reference Citation Analysis (132)] |
| 2. | Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31:992-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 402] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 3. | Mansuet-Lupo A, Bobbio A, Blons H, Becht E, Ouakrim H, Didelot A, Charpentier MC, Bain S, Marmey B, Bonjour P, Biton J, Cremer I, Dieu-Nosjean MC, Sautès-Fridman C, Régnard JF, Laurent-Puig P, Alifano M, Damotte D. The new histologic classification of lung primary adenocarcinoma subtypes is a reliable prognostic marker and identifies tumors with different mutation status: the experience of a French cohort. Chest. 2014;146:633-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Masuzawa K, Minematsu N, Sasaki M, Ohsawa K, Yamamoto T, Iwamaru A, Ogata K, Betsuyaku T, Murakami M. Invasive mucinous adenocarcinoma of the lung presenting as a large, thin-walled cyst: A case report and literature review. Mol Clin Oncol. 2017;6:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Akira M, Atagi S, Kawahara M, Iuchi K, Johkoh T. High-resolution CT findings of diffuse bronchioloalveolar carcinoma in 38 patients. AJR Am J Roentgenol. 1999;173:1623-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Aquino SL, Chiles C, Halford P. Distinction of consolidative bronchioloalveolar carcinoma from pneumonia: do CT criteria work? AJR Am J Roentgenol. 1998;171:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Kim TH, Kim SJ, Ryu YH, Chung SY, Seo JS, Kim YJ, Choi BW, Lee SH, Cho SH. Differential CT features of infectious pneumonia vs bronchioloalveolar carcinoma (BAC) mimicking pneumonia. Eur Radiol. 2006;16:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Narahari NK, Uppin SG, Kapoor A, Stalin BJ, Paramjyothi GK. Invasive mucinous adenocarcinoma of the lung in a 19-year-old female. Asian Cardiovasc Thorac Ann. 2018;26:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Shim HS, Kenudson M, Zheng Z, Liebers M, Cha YJ, Hoang Ho Q, Onozato M, Phi Le L, Heist RS, Iafrate AJ. Unique Genetic and Survival Characteristics of Invasive Mucinous Adenocarcinoma of the Lung. J Thorac Oncol. 2015;10:1156-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Shao K, Wang Y, Xue Q, Mu J, Gao Y, Wang B, Zhou L, Gao S. Clinicopathological features and prognosis of ciliated muconodular papillary tumor. J Cardiothorac Surg. 2019;14:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3651] [Cited by in RCA: 3635] [Article Influence: 259.6] [Reference Citation Analysis (0)] |
| 12. | Cadranel J, Quoix E, Baudrin L, Mourlanette P, Moro-Sibilot D, Morere JF, Souquet PJ, Soria JC, Morin F, Milleron B; IFCT-0401 Trial Group. IFCT-0401 Trial: a phase II study of gefitinib administered as first-line treatment in advanced adenocarcinoma with bronchioloalveolar carcinoma subtype. J Thorac Oncol. 2009;4:1126-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Nie K, Nie W, Zhang YX, Yu H. Comparing clinicopathological features and prognosis of primary pulmonary invasive mucinous adenocarcinoma based on computed tomography findings. Cancer Imaging. 2019;19:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Fujimoto M, Kawakami F, Tsuruyama T, Travis WD, Date H, Haga H. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 356] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 15. | Tian Y, Zheng W, Ge H, Wang Y, Zha N, Huang S, Guo Z. Molecular characteristics of multifocal invasive mucinous adenocarcinoma of the lung: Report of a rare case. Thorac Cancer. 2017;8:710-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |