Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10979
Peer-review started: June 24, 2021
First decision: July 27, 2021
Revised: August 3, 2021
Accepted: November 2, 2021
Article in press: November 2, 2021
Published online: December 16, 2021
Processing time: 168 Days and 15.4 Hours
Malignant obstructive jaundice (MOJ) is a common pathologic manifestation of malignant biliary obstruction. Recently, several clinical trials have explored the clinical effectiveness of intraluminal 125I seed-based brachytherapy for MOJ patients, and various outcomes have been reported.
To assess the efficacy and safety of percutaneous biliary stents with 125I seeds compared to conventional metal stents in patients with unresectable MOJ.
A systematic search of English-language databases (PubMed, Embase, Cochrane Library, and Web of Science) was performed to identify studies published prior to June 2020 that compared stents with or without 125I seeds in the treatment of unresectable MOJ. The outcomes analyzed included primary outcomes (stent patency and overall survival) and secondary outcomes (complications and liver function parameters).
Six randomized controlled trials and four retrospective studies involving 875 patients were eligible for the analysis. Of the 875 included patients, 404 were treated with 125I seed stents, while 471 were treated with conventional stents. Unadjusted pooled analysis demonstrated that compared to conventional stents,
Placement of stents combined with brachytherapy using 125I seeds contributes to a longer stent patency and higher overall survival than placement of conventional stents without extra complications or severe liver damage. Thus, it can be considered an effective and safe treatment for unresectable MOJ.
Core Tip: In recent years, the incidence of malignant obstructive jaundice (MOJ) in Asia has been 40 times higher than that in the Western world, which is a vital issue that requires significant attention. Irradiation stents using 125I seeds have been widely applied in the treatment of unresectable MOJ. However, more convincing evidence-based reviews of the efficacy and safety of 125I seed stents are needed. We used the latest data to further validate the superiority of 125I seed stents, providing strong evidence for clinicians to make correct decisions in clinical practice. Furthermore, we found that 125I seed stents resulted in equivalent complication and serum index outcomes as conventional stents, indicating that 125I seed stents are safe and well tolerated.
- Citation: Chen WY, Kong CL, Meng MM, Chen WQ, Zheng LY, Mao JT, Fang SJ, Chen L, Shu GF, Yang Y, Weng QY, Chen MJ, Xu M, Ji JS. Percutaneous biliary stent combined with brachytherapy using 125I seeds for treatment of unresectable malignant obstructive jaundice: A meta-analysis. World J Clin Cases 2021; 9(35): 10979-10993
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10979.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10979
Malignant obstructive jaundice (MOJ) is a common pathologic manifestation of malignant biliary obstruction caused by various adenocarcinomas[1]. Since the disease process is insidious but develops rapidly, only a minority of MOJ patients (< 20%) are suitable for radical operation, leading to a poor overall prognosis[2]. For patients with unresectable MOJ or those who are unwilling to undergo surgery, biliary stent implantation is a mainstay to relieve the biliary obstruction and clinical symptoms caused by progressive neoplasms[3]. Nevertheless, the stent itself has no effect on tumor suppression. The ingrowth or overgrowth of tumors, biliary epithelial cell proliferation, and biliary sludge formation often cause restenosis[4,5]. Thus, extra antitumor therapies are needed to improve the prognosis of patients with unresectable MOJ[6-8].
Intraluminal iodine-125 (125I) seed brachytherapy, due to its antitumor growth function and specificity for destroying target tumors, has been widely applied in local tumor treatment[9-12]. Several studies have demonstrated that intraluminal 125I seed-based brachytherapy has excellent therapeutic effects in the treatment of unresectable MOJ[13-15]. However, most studies are single-center or retrospective with relatively small sample sizes, and the number of randomized controlled trials (RCTs) is still limited.
To provide more convincing clinical evidence, we conducted a meta-analysis to accurately assess the efficacy and safety of percutaneous biliary stents with 125I seeds compared with conventional stents in patients with unresectable MOJ.
This meta-analysis was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines[16]. Institutional review board approval was not required for this analysis.
The following electronic databases were searched: PubMed, Embase, Cochrane Library, and Web of Science (before June 2020). The ClinicalTrials.gov website was also searched for randomized trials that were registered as completed but not yet published. Search terms such as “125I seed,” “brachytherapy,” “biliary stent,” “malignant obstructive jaundice,” and “malignant biliary obstruction” were included. The detailed search strategy is listed in Supplementary Table 1. In addition, the reference lists of identified studies were screened manually to include other potentially eligible trials. The following inclusion criteria were applied: (1) Studies involving adult patients (aged 18-90 years) with a confirmed diagnosis of MOJ; (2) studies comparing percutaneous biliary stent placement with the placement of 125I seeds and conventional metal stents; and (3) studies published in English. The following exclusion criteria were applied: (1) Abstracts without full texts; (2) studies registered but not completed; (3) studies that included patients whose data was published in multiple papers; and (4) studies with a sample size smaller than 20.
Each study (title and abstract) identified through the search strategy was screened for potential relevance by two authors (Chen WY and Kong CL). The full articles of studies chosen as being relevant were reviewed by the same authors for final inclusion. Differences of opinions were resolved by consensus.
The following data were independently extracted by two authors: Trial information (first author, year of publication, country, design, period of enrollment, intervention, number of included patients, and stent manufacturer and type), baseline patient characteristics (age, sex, causes of MOJ, and obstruction level), and outcomes (clinical effectiveness and complications). The extracted data were documented into a standardized Excel (Microsoft Corp, Redmond, WA, United States) file and were checked by another author. Any disagreement was resolved through discussion and a reassessment and recheck of the data and/or involvement of a senior author.
All randomized controlled trials were analyzed using Cochrane Collaboration’s tool. The risk of bias assessment in trials was based on random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessment, incomplete outcome data, selective reporting, and other factors. Each category was assessed as “yes” (low risk of bias), “no” (high risk of bias), or “unclear”. Cohort studies were assessed using the Newcastle–Ottawa Scale (NOS) with three main domains: Study group selection, comparability of cohorts, and ascertainment of outcomes. A study with an NOS score of 7 or higher was considered high quality.
This meta-analysis analyzed primary outcomes (stent patency and overall survival) and secondary outcomes (complications and liver function parameters). Stent patency was calculated from the date of stent placement to the first episode of stent restenosis. Stent restenosis was defined as the presentation of clinical signs of recurrent jaundice with elevated bilirubin levels along with biliary dilation on imaging study. Overall survival was defined as the interval between initial stenting and patient death or the last follow-up. Classification of complications was performed according to the Common Terminology Criteria for Adverse Events (CTCAE 4.02) or the guidelines of the Society of Interventional Radiology Standards of Practice Committee[17]. Postoperative procedure-related complications mainly included hemobilia, pancreatitis, cholangitis, and pain. Liver function parameters were evaluated by assessing the change in serum indices before and 1 wk after treatment, including total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST).
Three types of 125I seed stents were mentioned in the included studies. Type I stents refer to self-expanded stents with 125I seed strand fixation in a drainage catheter. Type II stents refer to 125I seed-loaded stents. Type III stents refer to self-expanded stents with 125I seed strand fixation between the stent and the bile duct wall. The 125I seed strand is a combination of a 4F catheter and multiple 125I seeds.
Statistical analyses were performed using Review Manager (version 5.0) and Stata 15.1. For time-to-event data, the aggregated hazard ratio (HR) and its 95% confidence interval (95%CI) were applied to report the final pooled estimate. HRs and the corresponding 95%CIs were directly obtained if mentioned in the manuscript; however, if not, the HR and lnHR were estimated by the method of Tierney et al[18] from Kaplan–Meier curves or the calculated value of the O-E and V. The outcomes of dichotomous and continuous variables are expressed as odds ratios (ORs) and weighted mean differences, respectively. Statistical heterogeneity across the included studies was quantified by the I2 statistic. When heterogeneity was significant (I2> 50%), a random-effects model was applied to calculate the pooled effect sizes; otherwise, a fixed-effects model was used. Sensitivity analysis was performed by excluding one trial in each turn to explore the potential causes of heterogeneity. Subgroup analysis was conducted according to the type of 125I seed stent and study design (RCT and retrospective study). Potential publication bias was appraised using Egger’s and Begg’s tests. Publication bias was adjusted using the trim and fill method[19]. Two-sided P < 0.05 was considered significant.
The PRISMA flow diagram for the selection process is presented in Figure 1. The initial database search yielded 244 potentially relevant studies, ten of which were included in this meta-analysis[13-15,20-26]. Of these studies, four were retrospective cohort studies and six were RCTs published between 2012 and 2018[13,14,20,21,25,26]. Five of these RCTs were single-center studies, while one was a multicenter study performed at 20 centers in China[21]. All of them were conducted in China and written in English. These studies included a total of 875 patients, among whom 404 (46.17%) underwent biliary stent placement combined with brachytherapy using 125I seeds, and 471 (53.83%) received conventional metal stents only for treatment. Percutaneous transhepatic biliary drainage was performed before stent placement in all cases. Three studies used type I stents[14,24,25], three used type II[20,21,26], and four used type III[13,15,22,23]. The target population was patients with unresectable MOJ, and the majority of them had cholangiocarcinoma (n = 331, 37.83%) and pancreatic carcinoma (n = 177, 20.23%). The trial information and patients’ baseline characteristics are shown in Table 1, while the intervention details for the deployment of 125I seeds and the main outcomes are listed in Table 2, with detailed data shown in Supplementary Tables 2 and 3.
| Ref. | Design | Country | Period of enrolment | Groups | Number of patients | Age (yr) | Sex (male/female) | Obstruction levels | Causes of MOJ |
| Chen et al[25], 2012 | Single-centre, RCT | China | Mar. 2009-Jan. 2010 | 125I + stent | 17 | 61.2 ± 14.5 | 12/5 | Hilar and distal | Cholangiocarcinoma (n = 7), hepatocellular carcinoma (n = 2), pancreatic cancer (n = 3), hepatic metastases from the stomach or colorectum (n = 5) |
| Stent | 17 | 63.9 ± 9.3 | 10/7 | Cholangiocarcinoma (n = 6), hepatocellular carcinoma (n = 4), pancreatic cancer (n = 3), hepatic metastases from the stomach or colorectum (n = 4) | |||||
| Hasimu et al[13], 2017 | Single-centre, RCT | China | July 2011-June 2014 | 125I + stent | 28 | 70.93 ± 8.58 | 11/17 | Hilar and distal | Cholangiocarcinoma (n = 48), gallbladder cancer (n = 7) |
| Stent | 27 | 70.26 ± 9.71 | 14/13 | ||||||
| Chen et al[26], 2018 | Single-centre, RCT | China | Sep. 2014-Nov. 2016 | 125I + stent | 13 | 66 (49, 88) | 8/5 | Lower | Pancreatic head carcinoma (n = 7), gallbladder carcinoma (n = 4), bile duct carcinoma (n = 2) |
| Stent | 19 | 68 (48, 86) | 12/7 | Pancreatic head carcinoma (n = 11), bile duct carcinoma (n = 5), gallbladder carcinoma (n = 2), ampullary carcinoma (n = 1) | |||||
| Jiao et al[14], 2017 | Single-centre, RCT | China | Jan. 2013- Jan. 2015 | 125I + stent | 31 | 60.4 ± 8.8 | 12/17 | Hilar and distal | Primary adenocarcinoma (n = 19), metastatic adenocarcinoma (n = 12) |
| Stent | 30 | 60.2 ± 8.1 | 16/14 | Primary adenocarcinoma (n = 21), metastatic adenocarcinoma (n = 9) | |||||
| Zhu et al[20], 2012 | Single-centre, RCT | China | Nov. 2008-Oct. 2010 | 125I + stent | 12 | 62.5 ± 21.0 | 7/5 | Hilar and distal | Primary adenocarcinoma (n = 8), metastatic adenocarcinoma (n = 4) |
| Stent | 11 | 71.0 ± 22.0 | 9/2 | Primary adenocarcinoma (n = 5), metastatic adenocarcinoma (n = 6) | |||||
| Zhu et al[21], 2018 | Multicentre, RCT | China | Oct. 2013-Mar. 2016 | 125I + stent | 164 | 65.0 (56.0, 75.0) | 103/61 | Hilar and distal | Biliary tract cancer (n = 80), pancreatic carcinoma (n = 46), lymph node metastases (n = 38) |
| Stent | 164 | 64.0 (57.0, 75.0) | 109/55 | Biliary tract cancer (n = 74), pancreatic carcinoma (n = 53), lymph node metastases (n = 37) | |||||
| Pan et al[15], 2020 | Retrospective cohort study | China | Mar. 2014-Dec. 2017 | 125I + stent | 30 | 56.53 ± 12.24 | 23/7 | Hilar and distal | NR |
| Stent | 54 | 60.44 ± 11.83 | 35/19 | ||||||
| Wang et al[24], 2017 | Retrospective cohort study | China | Sep. 2010-Feb. 2013 | 125I + stent | 24 | 57.3 (41, 80) | 29/21 | Hilar and distal | Holangiocarcinoma (n = 18), pancreatic head carcinoma (n = 14), hilar lymph node metastasis (n = 12), ampullary carcinoma (n = 6) |
| Stent | 26 | ||||||||
| Zhou et al[22], 2019 | Retrospective cohort study | China | Nov. 2015-Oct. 2017 | 125I + stent | 45 | 61.7 (32, 87) | 31/14 | Hilar, middle and distal | Cholangiocarcinoma (n = 18), gallbladder carcinoma (n = 6), pancreatic carcinoma (n = 4), hepatocellular carcinoma (n = 7), gastric cancer (n = 7), ampullary cancer (n = 0), hilar node metastases from other solid malignancies (n = 3) |
| Stent | 87 | 64.4 (35, 92) | 59/28 | Cholangiocarcinoma (n = 32), gallbladder carcinoma (n = 9), pancreatic carcinoma (n = 17), hepatocellular carcinoma (n = 9), gastric cancer (n = 11), ampullary cancer (n = 1), hilar node metastases from other solid malignancies (n = 8) | |||||
| Zhou et al[23], 2020 | Retrospectively cohort study | China | Jan. 2017-June 2018 | 125I + stent | 40 | 70.2 ± 13.8 | 21/19 | Hilar | Cholangiocarcinoma (n = 22), pancreatic cancer (n = 10), gallbladder cancer (n = 2), duodenal cancer (n = 2), metastatic cancer (n = 4) |
| stent | 36 | 68.1 ± 12.2 | 21/15 | Cholangiocarcinoma (n = 19), pancreatic cancer (n = 8), gallbladder cancer (n = 3), duodenal cancer (n = 1), metastatic cancer (n = 5) |
| Ref. | Intervention | Stent manufacturer and typea | Outcomes |
| Chen et al[25], 2012 | 125I seed strands performed after stent insertion | Nitinol self-expendable stent (Luminexx III; BARD); Type I | Laboratory values before and after stent placement, complications, stent patency |
| Conventional stent | |||
| Hasimu et al[13], 2017 | Biliary stent with 125I seed strands | Nitinol self-expandable stent (S.M.A.R.T.; Cordis Corporation, Miami Lakes, FL, United States); Type III | Stent patency, survival, relief of symptoms, technical and clinical success, complications, laboratory values before and after stent placement, radiation safety |
| Conventional stent | |||
| Chen et al[26], 2018 | 125I seeds-loaded-biliary stent | Self-expendable stent (produced by Mirco-tech, Nanjing, China); Type II | Laboratory values before and after stent placement, complications, stent patency, survival, CR, PR, SD, PD |
| Conventional stent | |||
| Jiao et al[14], 2017 | SEMS with 125I seed strands | A Nitinol self-expendable stent (Niti-S Biliary stent, Taewoong, Seoul, Korea); Type I | Technical success, laboratory values before and after stent placement, stent patency, overall survival, early or late complications |
| Conventional stent | |||
| Zhu et al[20], 2012 | 125I seeds-loaded-biliary stent | Outer self-expandable 125I radioactive seeds-loaded stent and inner conventional self-expanding biliary nitinol alloy stent (Nanjing MicroInvasive Medical Inc., Nanjing, China); Type II | Technical success, jaundice relief, radiation safety, complications (subjective and objective), survival, stent patency, laboratory values before and after stent placement |
| Conventional stent | |||
| Zhu et al[21], 2018 | 125I seeds-loaded-biliary stent | Inner conventional uncovered SEMS (Nanjing Micro-Tech Co. Ltd., Nanjing, China) and outer 125I seed-Xloaded stent; Type II | Stent restenosis, patency time, technical success, relief of jaundice, survival, complications |
| Conventional stent | |||
| Pan et al[15], 2020 | Biliary stent with 125I seed strands | Biliary stent (E-Luminexx Biliary Stent; Wachhausstrasse 6D76227, BARD Corporation, Karlsruhe, Germany); Type III | Stent patency, overall survival, complications, laboratory values before and after stent placement, independent factors associated with survival |
| Conventional stent | |||
| Wang et al[24], 2017 | Biliary stent with 125I seed strands | Biliary internal stent (Micro-Tech Co., Ltd. Nanjing, China); Type I | Success rate, laboratory values before and after stent placement, stent patency, survival |
| Conventional stent | |||
| Zhou et al[22], 2019 | UCSEMS with 125I seed strands | Three types of SEMS [E-Luminexx (Bard Peripheral Vascular, Tempe, AZ, United States), S.M.A.R.T (Cordis, Milpitas, CA, United States), and Zilver (Cook Medical, Bloomington, IN, United States)];Type III | Technical success, clinical success, complications, follow-up time, stent patency, survival, laboratory values before and after stent placement |
| UCSEMS | |||
| Zhou et al[23], 2020 | SEMS with 125I seed strands | Self-expandable metallic stent (Cook Medical, Bloomington, IN, United States); Type III | Technical success, clinical success, laboratory values before and after stent placement, complications, overall survival, and stent patency |
| Conventional stent |
According to the Cochrane Collaboration’s tool, the risk of bias varied among the six RCT studies included in this meta-analysis, ranging from low to high levels (Supplementary Figures 1 and 2). All studies had a high risk of performance bias because it was difficult to conceal the grouping and interventional procedures from the participants, researchers, and outcome measurers. Three RCTs (50%) did not describe the method of allocation concealment in detail. According to the NOS, the retrospective cohort studies had high quality scores, which were measured to be between 7 and 9 (Supplementary Table 4).
Egger’s and Begg’s tests were carried out to evaluate the potential publication bias for primary endpoints. There was no evidence that publication bias occurred in the outcome of stent patency (Egger’s test P = 0.705), whereas it was observed in the outcome of overall survival (Egger’s test P = 0.027). The conclusions were not changed after adjustment for publication bias by using the trim-and-fill method.
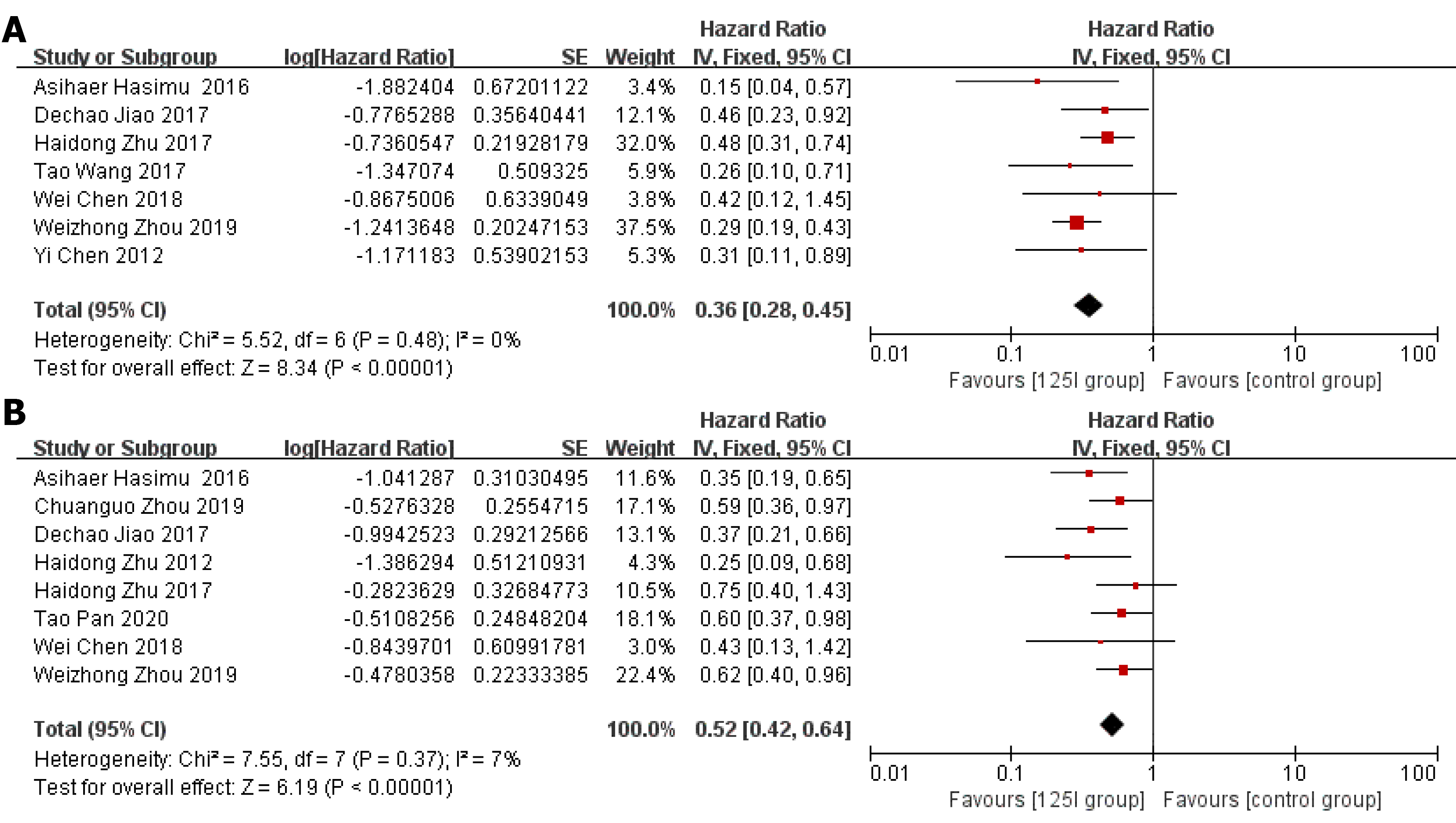
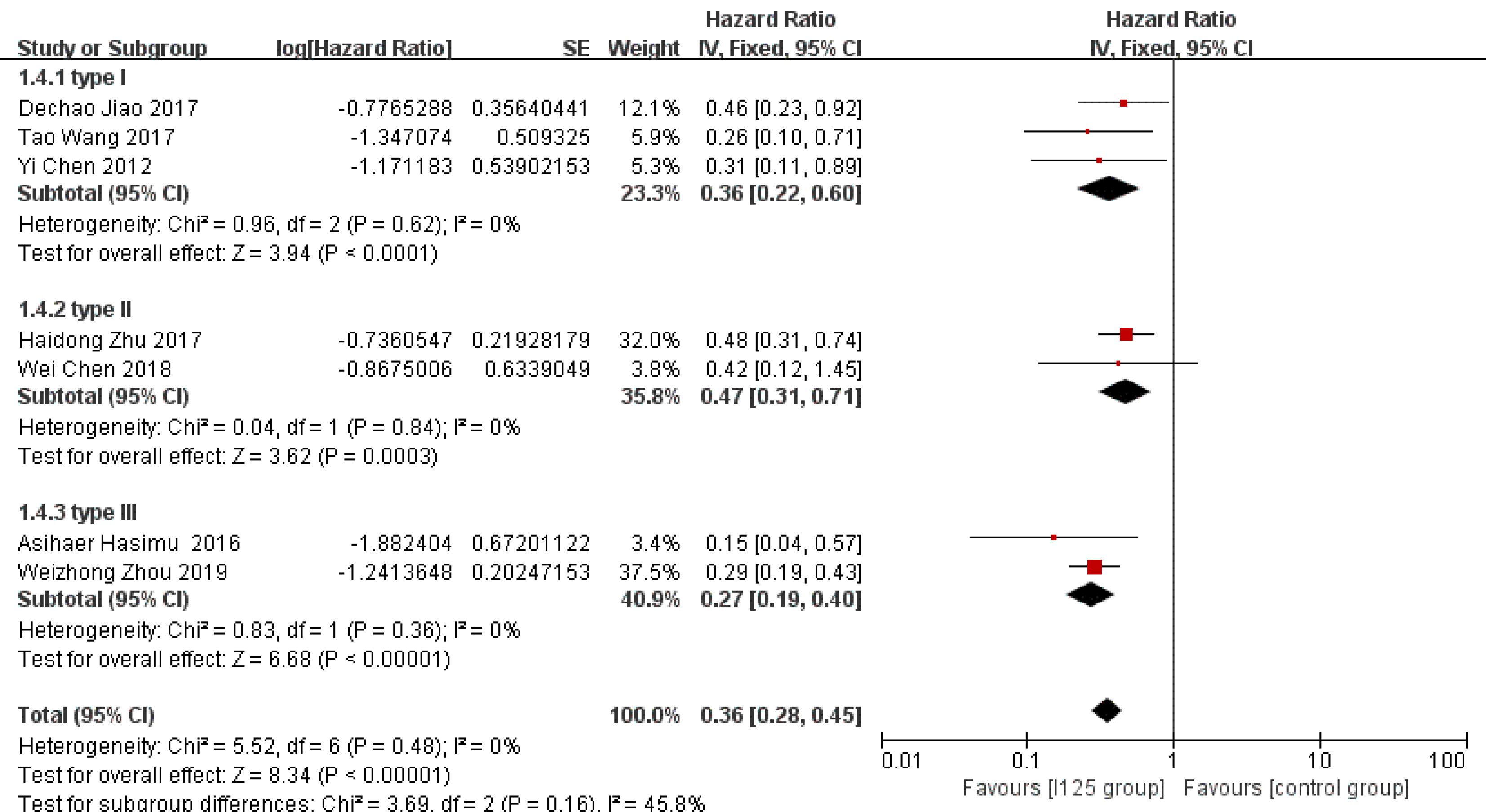
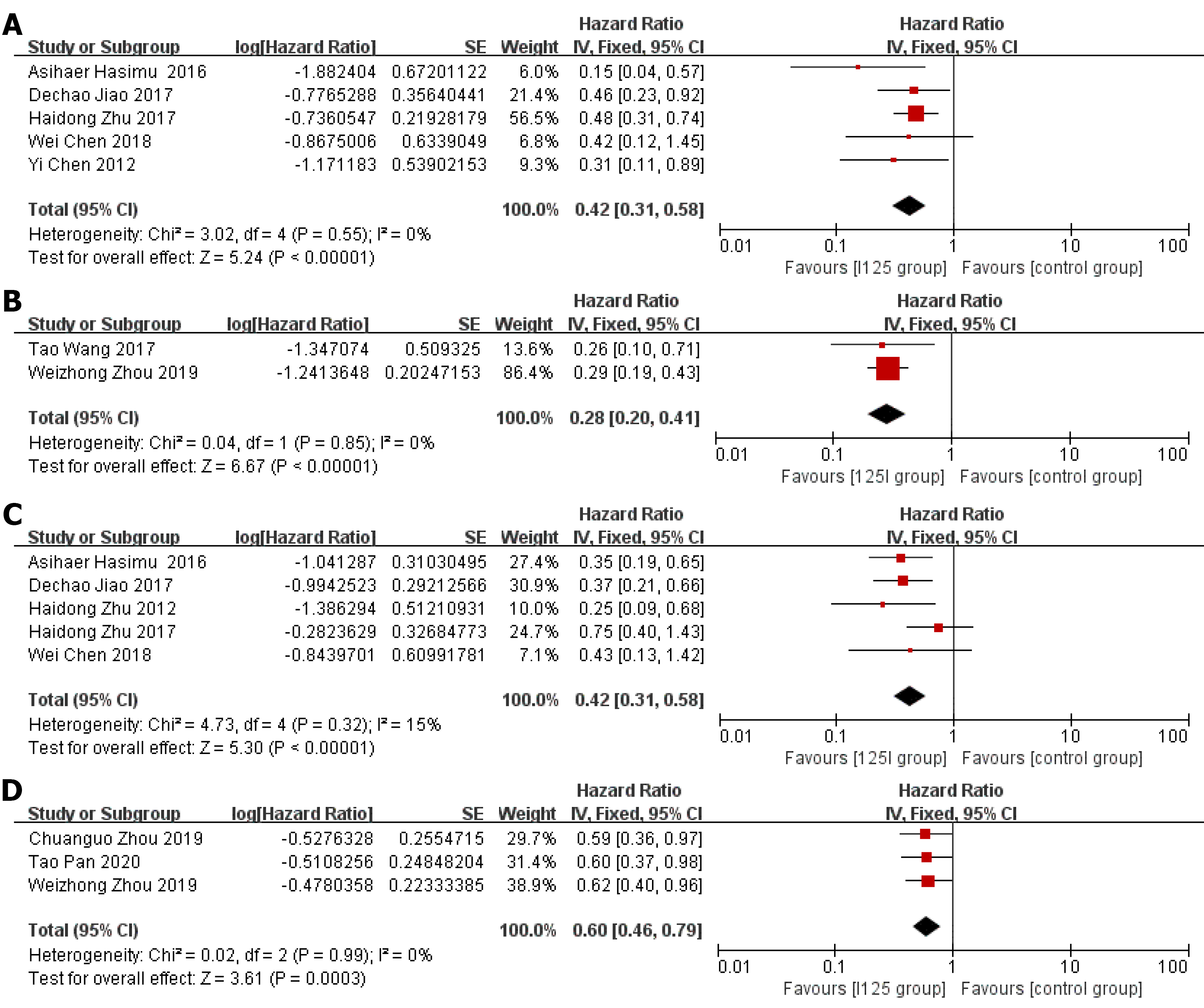
Stent patency: HR data for stent patency were extracted from seven studies[13,14,21,22,24-26]. The utilization of 125I seed stents resulted in a better stent patency than the use of conventional stents (HR = 0.36, 95%CI = 0.28-0.45, P < 0.0001; Figure 2A). There was no significant heterogeneity among these studies (I2= 0%, P = 0.48). The test for subgroup analyses revealed no significant difference in heterogeneity based on the type of 125I seed stent and type of study design. The results showed that compared with conventional stents, three 125I seed stent types were all associated with a significantly prolonged stent patency (Figure 3). Both RCTs and retrospective studies demonstrated that the 125I seed stent group was superior to the conventional stent group in patency (RCTs: HR = 0.42, 95%CI = 0.31-0.58, P < 0.00001; retrospective studies: HR = 0.28, 95%CI = 0.20-0.41, P < 0.00001; Figure 4A and B).
Overall survival: HR data for overall survival were extracted from eight studies[13-15,20-23,26]. In comparison with the use of conventional stents, the application of 125I seed stents resulted in a better overall survival (HR = 0.52, 95%CI = 0.42-0.64, P < 0.00001, Figure 2B). Heterogeneity among these studies was not significant (I2= 7%, P = 0.37). The test for subgroup analysis demonstrated no significant difference in heterogeneity according to the type of study design (P = 0.904). The results of a stratified analysis of RCTs and retrospective studies showed that the 125I seed stent group had a better overall survival than the conventional stent group (RCTs: HR = 0.42, 95%CI = 0.31-0.58, P < 0.00001; retrospective studies: HR = 0.60, 95%CI = 0.46-0.79, P = 0.0003; Figure 4C and D).
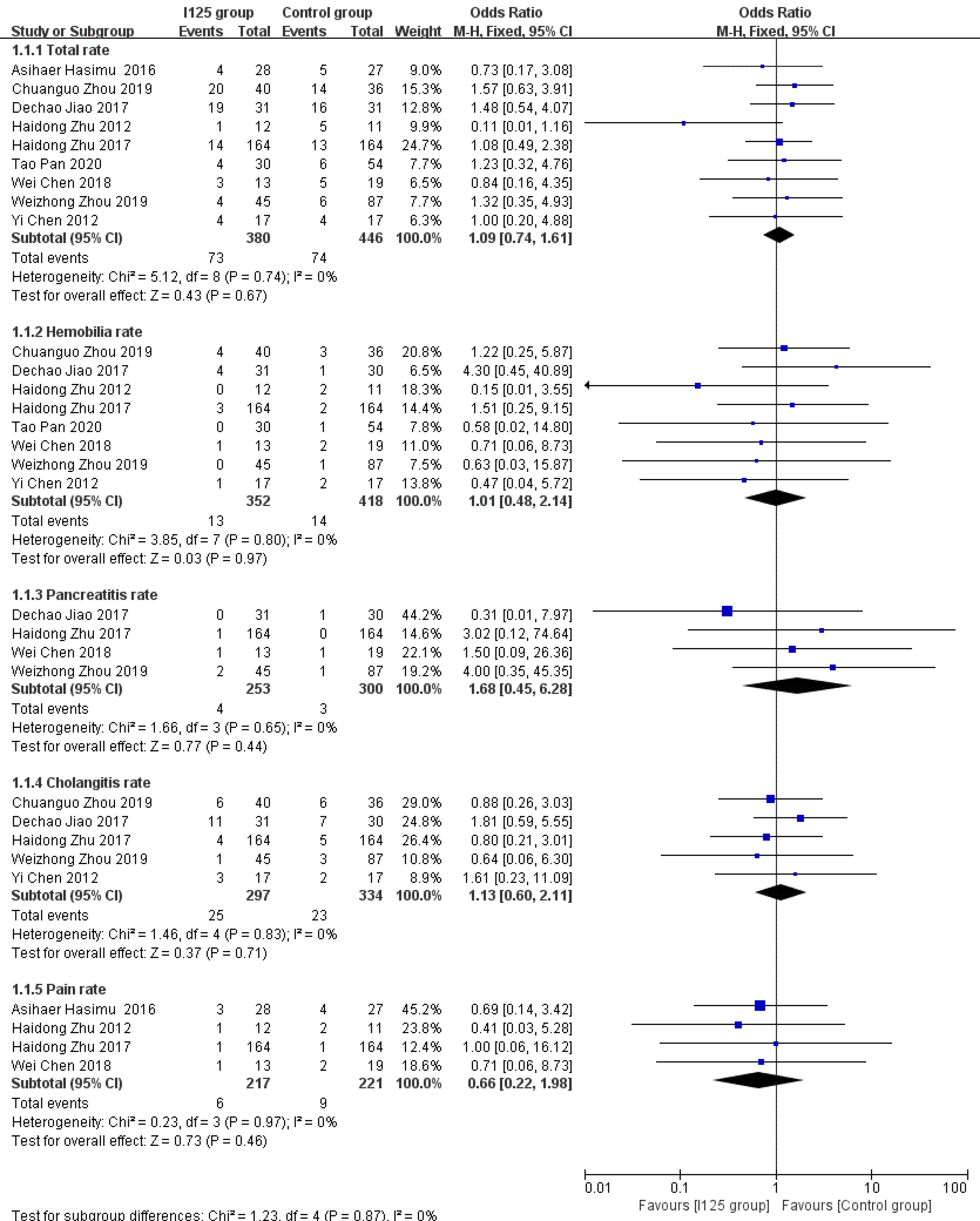
Complications: A total of nine studies provided incidence data for all complications or at least one kind of complication. Overall, both groups had low overall complication rates, with slightly worse results being observed in the 125I seed stent group than in the conventional stent group (19.2% vs 16.5%). However, this difference was not statistically significant (OR = 1.12, 95%CI = 0.75-1.67, P = 0.57; Figure 5), and there was a low level of heterogeneity among these studies (I2= 0%, P = 0.74). There were also no significant differences between the 125I seed stent group and the conventional stent group in the incidence of hemobilia (OR = 1.02, 95%CI = 0.45–2.3, P = 0.96; I2 = 0%, P = 0.8), pancreatitis (OR = 1.79, 95%CI = 0.42-7.53, P = 0.43; I2 = 0%, P = 0.65), cholangitis (OR = 1.13, 95%CI = 0.60-2.13, P = 0.71; I2= 0%, P = 0.83), or pain (OR = 0.67, 95%CI = 0.22-2, P = 0.47; I2 = 0%, P = 0.97) (Figure 5).
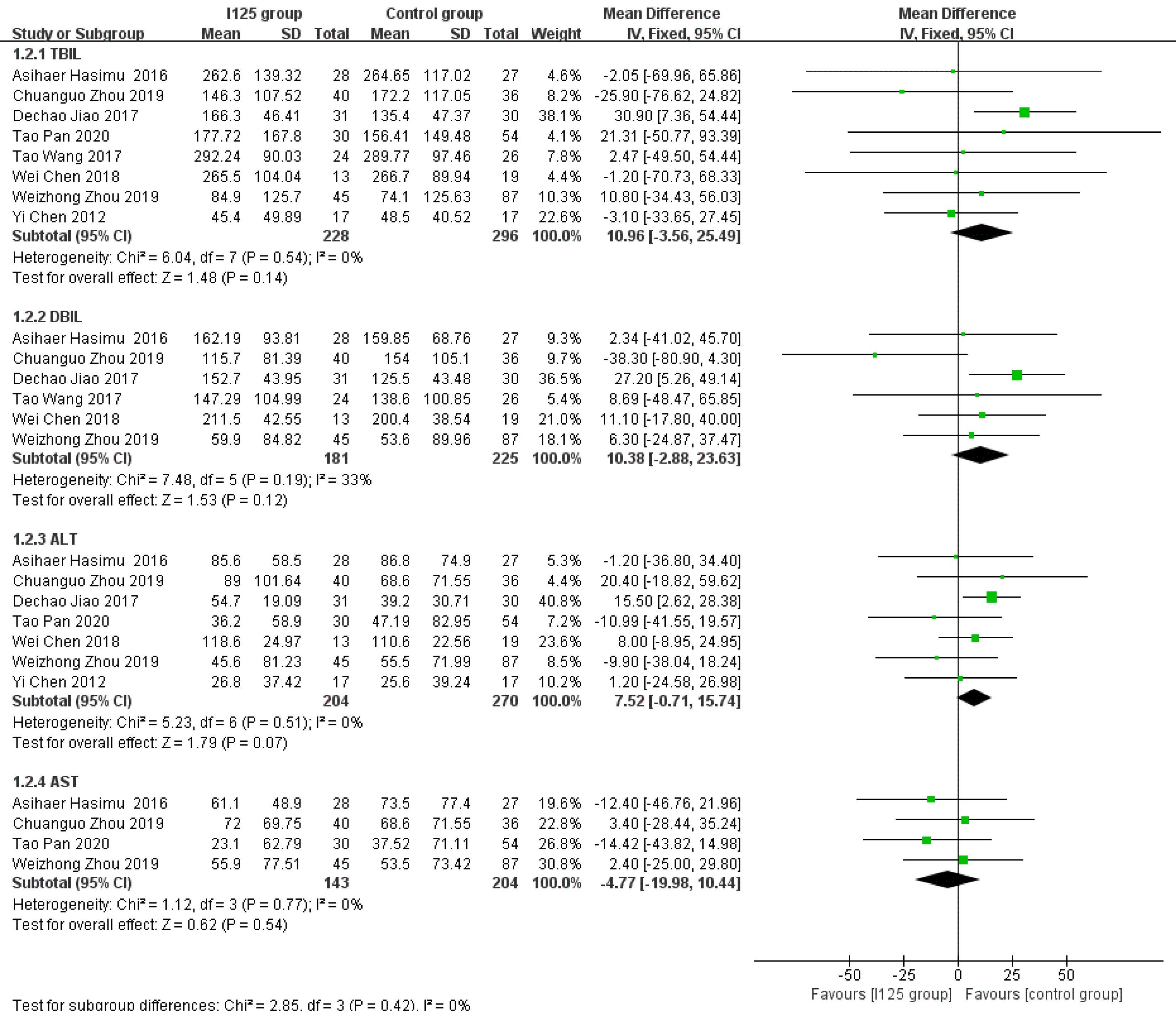
Posttreatment reductions in the levels of serum indices: After the procedure, there was a significant decrease in liver function indices, including TBIL, DBIL, ALT, and AST. The numbers of studies that reported pretreatment and posttreatment TBIL, DBIL, ALT, and AST data were 8, 6, 7, and 4, respectively. We calculated the degree of reduction in each index, and found that there were no significant differences in the posttreatment reductions in the levels of TBIL (MD = 10.96, 95%CI = -3.56-25.49, P = 0.14; I2= 0%, P = 0.54), DBIL (MD = 7.37, 95%CI = -9.76-24.5, P = 0.4; I2= 33%, P = 0.19), ALT (MD = 7.52, 95%CI = -0.71-15.74, P = 0.07; I2= 0%, P = 0.51), and AST (MD = -4.77, 95%CI = -19.98-10.44, P = 0.54; I2= 0%, P = 0.77) between the 125I seed stent group and the conventional stent group (Figure 6).
This meta-analysis showed that biliary stents irradiated using 125I seeds resulted in a longer stent patency and higher overall survival than conventional stents in the treatment of unresectable MOJ. The same results were observed for the median or mean time of stent patency and overall survival in the included studies. However, due to the different presentations of the main results, this study transformed these original results into HR values rather than conducting a pooled analysis. The risk of restenosis was associated with patient death[21]. The longer stent patency was attributed to the short-distance irradiation effect of radioactive seeds embedded in the stents. Brachytherapy using 125I seed stents was developed to inhibit tumor ingrowth, relieve the obstruction, and finally prolong the survival time of patients with unresectable MOJ. This result further confirms the superior effect of irradiation stents using 125I seeds, which provides strong evidence for clinicians to make correct decisions in clinical practice.
The implantation of stents irradiated using 125I is safe and well tolerated. All particle stents have some radiation hazards, and they also increase the complexity of the operation, which may cause certain damage to the intima and radiation damage to the gastrointestinal or bile duct during the treatment procedure[27]. However, our analysis showed that the treatment with 125I seed stents did not result in a higher incidence rate of complications than conventional stents. Additionally, none of the studies reported fatal complications, such as biliary or intestinal perforation or massive hemorrhage, and there was no device- or procedure-related mortality. Cholangitis is a more frequent complication associated with irradiated stents, with an incidence of 8.4% in the 125I seed stent group and 6.9% in the conventional stent group in this meta-analysis. However, this difference was not statistically significant. The incidence rates of other complications, such as hemobilia, pancreatitis, and pain, between the two groups were also comparable. Therefore, we concluded that no additional biliary complications occurred due to the use of 125I seed stents in patients with unresectable MOJ.
After implantation of the stents, the reductions in the serum indices of patients indicated improved therapeutic efficacy. Part of the biliary system is intrahepatic. Theoretically, irradiation biliary stent implantation may induce damage to the liver parenchyma. However, the reductions in the levels of serum indices (TBIL, DBIL, ALT, and AST) after treatment were not significantly different between the two groups, which demonstrated that the 125I seed stents were as effective as the conventional stents in improving liver function in patients with unresectable MOJ. Nevertheless, 125I seed stents have obvious advantages in inhibiting tumor growth. An investigation by Wang et al[24] showed that the levels of tumor markers (CA-199 and CA-242) in the 125I seed stent group were significantly reduced after stent implantation, while no significant change was observed in the conventional stent group. This might be the reason why patients with unresectable MOJ treated with irradiated stents show amelioration of obstructive jaundice and a delayed disease process.
In terms of radiation safety, irradiation dose is the focus of brachytherapy[28]. The amount of 125I embedded in the stents is based on the tumor size and relevant recommendations of the Treatment Planning System (TPS, FTT Technology Ltd. Co., Beijing, China). The radiation doses used in all studies met the minimum threshold for effective brachytherapy treatment of adenocarcinoma (7.87 cGy and 30 Gy), while some of the studies used a higher dose (80–990 Gy) within the safety limits established through animal experiments and clinical trials[25]. A suitable dose has the optimal capacity to kill the primary tumor effectively. Although several previous reports indicated that a decrease in white blood cell count and immunoglobulin (IgA, IgG, and IgM) levels is associated with long-term and low-dose radiotherapy with 125I-based particles[29,30], the results of two included studies showed no significant differences between the pre- and post-procedure irradiated stent groups[20,26]. This again proved the safety of the radiation dose and 125I seed stents.
The curative effect of irradiated stents varies in patients with unresectable MOJ with different tumor etiologies and obstruction levels. However, due to the small sample size of enrolled patients, most studies did not explore the differences in the efficacy of irradiated stents for different pathological tumors, except for the study by Zhu et al[21]. In Zhu’s multicenter study, subgroup analysis of tumor etiology was performed, and the researchers first proposed that patients with biliary tract cancer could benefit more from irradiated stents using 125I seeds than those with pancreatic carcinoma and lymph node metastases. These results suggest that 125I seed stents provide better tumor control for localized malignant obstruction from the biliary tract. Nevertheless, obstruction can occur at any level within the biliary tract, most often in hilar and distal bile ducts. Zhou et al[23] and Chen et al[26] focused on the role of 125I seed stents in malignant hilar and lower biliary tract obstruction, respectively. The conclusions of these two studies are consistent with those of other studies, suggesting that 125I seed stents can serve as a safe, feasible, and effective method with minimal invasiveness for the treatment of obstruction at different levels within the biliary tract.
As mentioned in this analysis, there are three main types of 125I seed stents currently applied in the bile duct. Subgroup analysis based on the type of stent demonstrated that all three types of 125I seed stents were equally effective in prolonging stent patency. 125I seed strands have the advantages of replaceability and sustained radiation[31]. However, the use of a bile duct drainage tube as a carrier has certain limitations for invasive tumor growth along the bile duct wall. The radiation dose can be evenly distributed by using seed-loaded stents, but this type of stent is composed of two-layer stents and a large diameter sheath, which is not suitable for patients with hilar strictures. A self-expanded stent with 125I seed strand fixation between the stent and the bile duct wall is widely adopted in current studies due to its simple process and broader applicability. However, nonintegrated radiation stents still have many internal radiation stent-related issues that need to be solved.
This meta-analysis included RCTs and retrospective studies. In the subgroup analysis, a disparity between the results of RCTs and retrospective studies was not observed in stent patency and overall survival. Although RCTs provide a higher level of clinical evidence, retrospective studies have their own strengths as well, such as a potentially wider range of patients and therefore probably more real-world data. There was no significant heterogeneity in the test for subgroup differences, which indicated that the potential bias caused by the type of study design was small.
This meta-analysis still has several limitations: (1) There was a lack of stratified randomization and strict control of blinding in some research centers, which could influence the quality of this study to some extent; (2) the analysis had publication bias, which could be the result of the inclusion of studies concerning small sample sizes and only those that were written in English; (3) no studies involved in-depth comparative investigations of the applicable conditions and cost-effectiveness of three types of irradiated stents, which could limit the application of the results to some extent; and (4) all the studies were conducted in China, which could have had a potential impact on the generalizability of the results.
In conclusion, percutaneous biliary stents combined with brachytherapy using 125I seeds offers a longer stent patency and higher overall survival than conventional stents for patients with unresectable MOJ, resulting in equivalent complication and serum index outcomes. High-quality multicenter prospective randomized studies are needed to further assess the long-term therapeutic outcomes and safety of irradiated stents using 125I seeds and to define the selection criteria for stent type.
Malignant obstructive jaundice (MOJ) is a common condition caused by various adenocarcinomas. Less than 20% of patients are suitable for radical surgery, leading to a poor overall prognosis. Recently, several clinical studies have raised concern regarding the clinical effectiveness of intraluminal 125I seed-based brachytherapy for patients with unresectable MOJ; hence, we analyzed evidence from randomized controlled trials (RCTs) and cohort studies comparing 25I seed stents and conventional stents.
Recently, there has been growing concern regarding the efficacy and safety of intraluminal 125I seed-based brachytherapy in the treatment of unresectable MOJ. However, most studies are single-center or retrospective with relatively small sample sizes and thus provide less convincing clinical evidence. The purpose of our study was to conduct a rigorous meta-analysis of RCTs and cohort studies on irradiated stents.
To investigate the clinical efficacy and safety of percutaneous biliary stents with 125I seeds compared with conventional metal stents in patients with unresectable MOJ.
We performed a meta-analysis of RCTs and cohort studies. Four English-language databases (PubMed, Embase, Cochrane Library, and Web of Science) were searched up to June 2020 for studies comparing stents with and without 125I seeds in the treatment of unresectable MOJ.
A total of ten studies were included (6 RCTs and 4 cohort studies), involving a total of 875 patients. Our study revealed that compared with conventional stents, 125I seed stents extended the stent patency time and overall survival period. No extra complications or severe liver damage was caused by 125I seed stents. This topic remains to be studied, and more research is needed to further assess the long-term therapeutic outcomes and safety of stents irradiated using 125I seeds.
Percutaneous biliary stents combined with brachytherapy using 125I seeds offers a longer stent patency and higher overall survival than conventional stents for patients with unresectable MOJ, resulting in equivalent complications and serum index outcomes.
To promote the clinical application of 125I seed stents for the treatment of MOJ, future studies are needed to conduct in-depth comparative studies on the applicable conditions and cost-effectiveness of the three types of irradiated stents. In addition, it is necessary to compare the efficacy of irradiation stents using 125I seeds for MOJ caused by different adenocarcinomas.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chrcanovic BR S-Editor: Chang KL L-Editor: Wang TQ P-Editor: Chang KL
| 1. | de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 682] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 2. | Wang L, Yu WF. Obstructive jaundice and perioperative management. Acta Anaesthesiol Taiwan. 2014;52:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Zhang GY, Li WT, Peng WJ, Li GD, He XH, Xu LC. Clinical outcomes and prediction of survival following percutaneous biliary drainage for malignant obstructive jaundice. Oncol Lett. 2014;7:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 5. | Sun C, Yan G, Li Z, Tzeng CM. A meta-analysis of the effect of preoperative biliary stenting on patients with obstructive jaundice. Medicine (Baltimore). 2014;93:e189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Sofi AA, Khan MA, Das A, Sachdev M, Khuder S, Nawras A, Lee W. Radiofrequency ablation combined with biliary stent placement vs stent placement alone for malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2018;87:944-951.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Brunner TB, Eccles CL. Radiotherapy and chemotherapy as therapeutic strategies in extrahepatic biliary duct carcinoma. Strahlenther Onkol. 2010;186:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Boulay BR, Birg A. Malignant biliary obstruction: From palliation to treatment. World J Gastrointest Oncol. 2016;8:498-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Lu J, Guo JH, Zhu HD, Zhu GY, Chen L, Teng GJ. Safety and Efficacy of Irradiation Stent Placement for Malignant Portal Vein Thrombus Combined with Transarterial Chemoembolization for Hepatocellular Carcinoma: A Single-Center Experience. J Vasc Interv Radiol. 2017;28:786-794.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Zhang W, Li J, Li R, Zhang Y, Han M, Ma W. Efficacy and safety of iodine-125 radioactive seeds brachytherapy for advanced non-small cell lung cancer-A meta-analysis. Brachytherapy. 2018;17:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Venkat PS, Hoffe SE, Frakes JM. Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24:1073274817729259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Li JR, Sun Y, Liu L. Radioactive seed implantation and lobaplatin chemotherapy are safe and effective in treating patients with advanced lung cancer. Asian Pac J Cancer Prev. 2015;16:4003-4006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Hasimu A, Gu JP, Ji WZ, Zhang HX, Zhu DW, Ren WX. Comparative Study of Percutaneous Transhepatic Biliary Stent Placement with or without Iodine-125 Seeds for Treating Patients with Malignant Biliary Obstruction. J Vasc Interv Radiol. 2017;28:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Jiao D, Wu G, Ren J, Han X. Study of self-expandable metallic stent placement intraluminal 125I seed strands brachytherapy of malignant biliary obstruction. Surg Endosc. 2017;31:4996-5005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Pan T, Li MA, Mu LW, Zhu D, Qian JS, Li ZR. Stent placement with iodine-125 seeds strand effectively extends the duration of stent patency and survival in patients with unresectable malignant obstructive jaundice. Scand J Gastroenterol. 2020;55:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7463] [Cited by in RCA: 8256] [Article Influence: 825.6] [Reference Citation Analysis (0)] |
| 17. | Cardella JF, Kundu S, Miller DL, Millward SF, Sacks D; Society of Interventional Radiology. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189-S191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4955] [Article Influence: 275.3] [Reference Citation Analysis (0)] |
| 19. | Rothstein HR, Sutton AJ, Borenstein M. Publication Bias and Meta-Analysis: Prevention, Assessments and Adjustments. Chichester: Wiley, 2005: 356. [DOI] [Full Text] |
| 20. | Zhu HD, Guo JH, Zhu GY, He SC, Fang W, Deng G, Qin YL, Li GZ, Coldwell DM, Teng GJ. A novel biliary stent loaded with (125)I seeds in patients with malignant biliary obstruction: preliminary results vs a conventional biliary stent. J Hepatol. 2012;56:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Zhu HD, Guo JH, Huang M, Ji JS, Xu H, Lu J, Li HL, Wang WH, Li YL, Ni CF, Shi HB, Xiao EH, Lv WF, Sun JH, Xu K, Han GH, Du LA, Ren WX, Li MQ, Mao AW, Xiang H, Zhang KX, Min J, Zhu GY, Su C, Chen L, Teng GJ. Irradiation stents vs. conventional metal stents for unresectable malignant biliary obstruction: A multicenter trial. J Hepatol. 2018;68:970-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Zhou WZ, Fu YM, Yang ZQ, Shi HB, Liu S, Xia JG, Zhou CG. Study of Percutaneous Stent Placement with Iodine-125 Seed Strand for Malignant Biliary Obstruction. Cardiovasc Intervent Radiol. 2019;42:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Zhou C, Li H, Huang Q, Wang J, Gao K. Biliary self-expandable metallic stent combined with Iodine-125 seeds strand in the treatment of hilar malignant biliary obstruction. J Int Med Res 2020. 48:300060519887843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Wang T, Liu S, Zheng YB, Song XP, Sun BL, Jiang WJ, Wang LG. Clinical Study on Using 125I Seeds Articles Combined with Biliary Stent Implantation in the Treatment of Malignant Obstructive Jaundice. Anticancer Res. 2017;37:4649-4653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Chen Y, Wang XL, Yan ZP, Wang JH, Cheng JM, Gong GQ, Luo JJ. The use of ¹²⁵I seed strands for intraluminal brachytherapy of malignant obstructive jaundice. Cancer Biother Radiopharm. 2012;27:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Chen W, Fang XM, Wang X, Sudarshan SKP, Hu XY, Chen HW. Preliminary clinical application of integrated 125I seeds stents in the therapy of malignant lower biliary tract obstruction. J Xray Sci Technol. 2018;26:865-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Almadi MA, Barkun AN, Martel M. No benefit of covered vs uncovered self-expandable metal stents in patients with malignant distal biliary obstruction: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:27-37.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Luo JJ, Zhang ZH, Liu QX, Zhang W, Wang JH, Yan ZP. Endovascular brachytherapy combined with stent placement and TACE for treatment of HCC with main portal vein tumor thrombus. Hepatol Int. 2016;10:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Serhatlioğlu S, Oğur E, Ozan AT, Gürsu F, Gödekmerdan A, Ayar A. [Biochemical and immunological effects of ionizing radiation in radiology staff members]. Tani Girisim Radyol. 2004;10:97-102. [PubMed] |
| 30. | Wakeford R. Radiation in the workplace-a review of studies of the risks of occupational exposure to ionising radiation. J Radiol Prot. 2009;29:A61-A79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Monk BJ, Tewari KS, Puthawala AA, Syed AM, Haugen JA, Burger RA. Treatment of recurrent gynecologic malignancies with iodine-125 permanent interstitial irradiation. Int J Radiat Oncol Biol Phys. 2002;52:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |