Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10969
Peer-review started: June 18, 2021
First decision: July 26, 2021
Revised: July 29, 2021
Accepted: October 25, 2021
Article in press: October 25, 2021
Published online: December 16, 2021
Processing time: 175 Days and 4.4 Hours
The clinical significance of signet ring cells (SRCs) in surgical esophageal and esophagogastric junction adenocarcinoma (EEGJA) remains unclear now.
To explore the association between the presence of SRCs and the clinicopathological and prognostic characteristics in surgical EEGJA patients by combining and analyzing relevant studies.
The PubMed, Web of Science, and EMBASE electronic databases were searched for the relevant literature up to March 28, 2021. The relative risk (RR) with 95% confidence interval (CI) was calculated to assess the relationship between SRCs and clinicopathological parameters of surgical EEGJA patients, and the hazard ratio (HR) with 95%CI was calculated to explore the impact of SRC on the prognosis. All statistical analyses were conducted with STATA 12.0 software.
A total of ten articles were included, involving 30322 EEGJA patients. The pooled results indicated that the presence of SRCs was significantly associated with tumor location (RR: 0.76, 95%CI: 0.61-0.96, P = 0.022; I2 = 49.4%, P = 0.160) and tumor-node-metastasis stage (RR: 1.30, 95%CI: 1.02-1.65, P = 0.031; I2 = 73.1%, P = 0.002). Meanwhile, the presence of SRCs in surgical EEGJA patients predicted a poor overall survival (HR: 1.36, 95%CI: 1.12-1.65, P = 0.002; I2 = 85.7%, P < 0.001) and disease-specific survival (HR: 1.86, 95%CI: 1.55-2.25, P < 0.001; I2 = 63.1%, P = 0.043).
The presence of SRCs is related with advanced tumor stage and poor prognosis and could serve as a reliable and effective parameter for the prediction of postoperative survival and formulation of therapy strategy in EEGJA patients. However, more high-quality studies are still needed to verify the above findings.
Core Tip: Our manuscript indicated that the presence of signet ring cells (SRCs) was significantly associated with the tumor location (P = 0.022) and tumor-node-metastasis stage (P = 0.031). Meanwhile, the presence of SRCs in surgical esophageal and esophagogastric junction adenocarcinoma (EEGJA) patients predicted a poor overall survival (P = 0.002) and disease-specific survival (P < 0.001). The presence of SRC was related with advanced tumor stage and poor prognosis and could serve as a reliable and effective parameter for the prediction of postoperative survival and formulation of therapy strategy in EEGJA patients. However, more high-quality studies are still needed to verify the above findings.
- Citation: Wang YF, Xu SY, Wang Y, Che GW, Ma HT. Clinical significance of signet ring cells in surgical esophageal and esophagogastric junction adenocarcinoma: A systematic review and meta-analysis. World J Clin Cases 2021; 9(35): 10969-10978
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10969.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10969
Esophageal carcinoma is the eighth most common tumor worldwide with an estimated 456000 new cases in 2012 and an increasing incidence has been observed in recent decades (7.9/100000 in males and 1.4/100000 in females)[1-3]. Although great progress has been made in the surgical and adjuvant therapy of esophageal cancer in recent years, the survival of esophageal cancer patients remains poor due to the advanced stage at the time of diagnosis[4]. In Western countries, adenocarcinoma is the most common pathological subtype of esophageal cancer, although squamous cell carcinoma accounts for the highest proportion in Asian countries[5]. Signet ring cell (SRC) carcinoma is a rare mucinous subtype of adenocarcinoma that has been reported to be related with aggressive biology in gastrointestinal cancer[6,7].
Actually, the clinical significance of SRCs in gastric and colorectal carcinomas has been widely verified. Nie et al[8] included 19 studies involving 35947 cases and demonstrated that gastric carcinoma patients with SRCs tended to be younger (weighted mean difference = -3.88, P = 0.001) and predominantly female [odds ratio (OR): 1.60, P < 0.001]. Besides, early-stage gastric cancer patients with SRCs were related with a better overall survival (OS) [hazard ratio (HR): 0.57, P = 0.002], but advanced stage patients with SRCs were related with a worse prognosis (HR: 1.17, P < 0.001); however, in the total population, no significant difference in OS between non-SRC and SRC patients was observed (HR: 1.02, P = 0.830). Besides, after analyzing 2454 colorectal cancer patients, Tan et al[9] demonstrated that the presence of SRCs was an independent prognostic risk factor in colorectal cancer patients (SRC ratio < 50%: 2.182, P = 0.005; SRC ratio > 50%: 1.699, P = 0.016). Meanwhile, it has been reported that patients with an SRC ratio > 50% are more likely to experience an advanced stage of disease and worse survival than patients with an SRC ratio < 50%[10]. However, these meta-analyses focused on the gastric and colorectal carcinomas which are more likely to be combined with SRCs and their results were inconsistent with the studies about esophageal and esophagogastric junction adenocarcinoma (EEGJA). Meanwhile, for EEGJA, the clinical significance of SRCs remains unclear because of the inconsistent reports[11-20].
Therefore, we conducted this systematic review and meta-analysis to explore the clinical significance of the presence of SRCs in EEGJA patients and the impact of SRCs on the clinicopathological and prognostic characteristics, which might contribute to the prediction of prognosis and formulation of treatment strategy for EEGJA patients.
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[21].
The inclusion criteria were as follows: (1) Patients were pathologically diagnosed with EEGJA; (2) All patients received surgical therapy; (3) Patients were divided into two groups according to the presence or absence of SRCs, and prospective randomized controlled trials (RCTs) or retrospective cohort studies were both available; (4) Clinicopathological parameters or prognosis between the two groups were compared and relevant data were provided; (5) Although the HRs with corresponding 95% confidence intervals (CIs) were not directly reported in articles, the Kaplan-Meier survival curves were provided to calculate them; and (6) The articles were published in English.
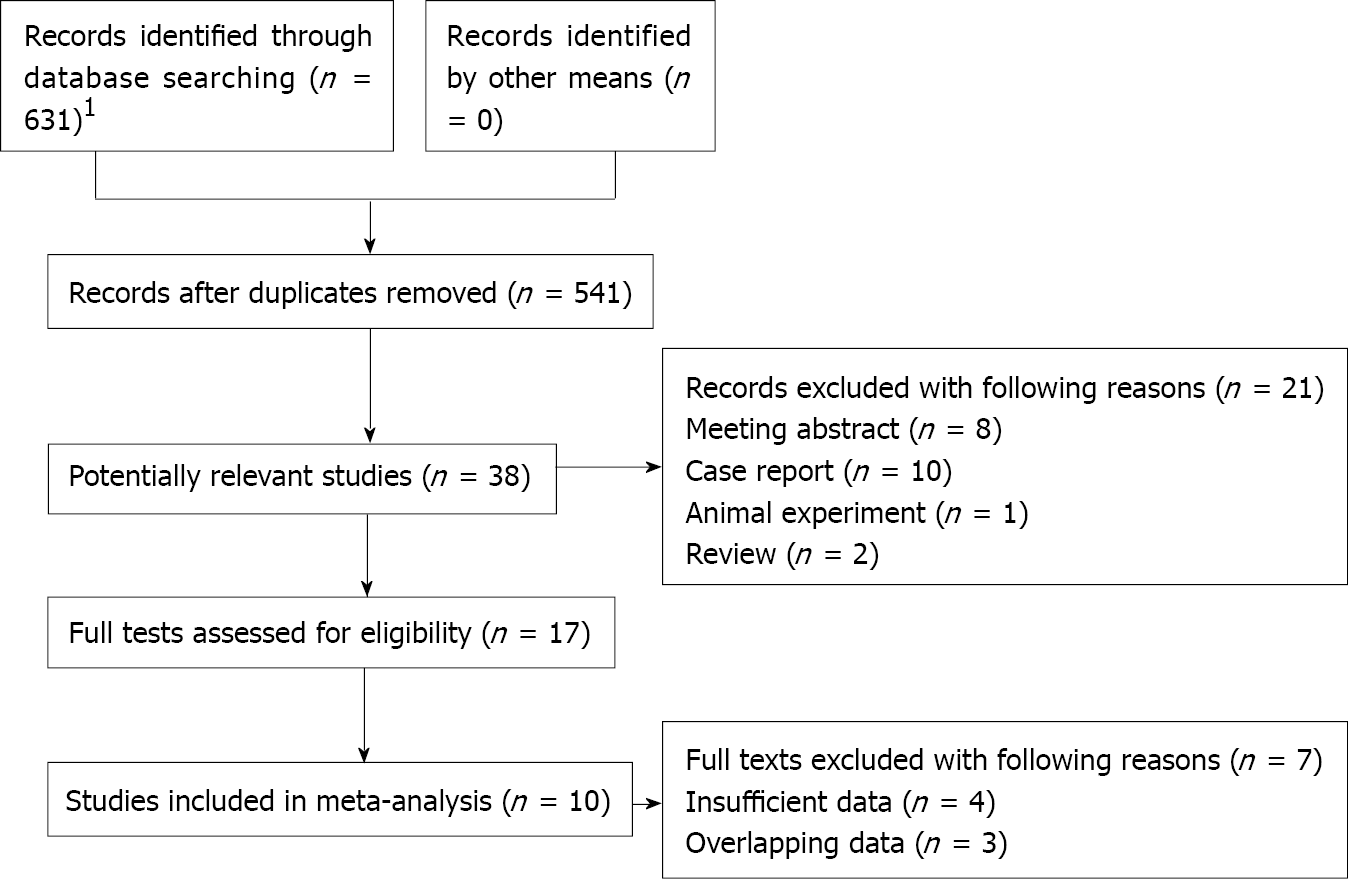
The PubMed, Web of Science, and EMBASE electronic databases were searched from the establishment date to March 28, 2021. The following terms were used: “esophageal”, “esophagus”, “esophagogastric”, “gastroesophageal”, “adenocarcinoma”, and “significance of signet ring cell”. A combination of subject terms and free words was applied. Besides, the references cited in included studies were also reviewed for availability (Figure 1).
The exclusion criteria were as follows: (1) Duplicated studies or studies with severely overlapped data; (2) Case reports, reviews, meeting abstracts, and animal trials; and (3) Adenocarcinomas located in other sites like the stomach were also enrolled without subgroup analysis for EEGJA patients.
The titles and abstracts were screened first and irrelevant publications were excluded. Then full texts of potentially related studies were further reviewed for availability.
The literature retrieval and selection were conducted by two investigators (Wang YF and Xu SY).
The data extraction was conducted by two authors (Wang YF and Xu SY) through the Microsoft Excel sheet independently. The following information was extracted from each included studies: The author, publication year, country where the study was conducted, sample size, tumor location (esophageal vs esophagogastric), tumor-node-metastasis (TNM), SRC ratio, sex, smoking, family history, lymph node metastasis status, relative risk (RR), and HR with 95%CI or corresponding data for their calculation.
The association of SRCs with sex, smoking, family history, tumor location, lymph node metastasis, and TNM stage were measured in this study. For the prognostic role of SRCs in EEGJA, the primary outcome was the OS and the second outcomes included the disease-free survival (DFS) and disease-specific survival (DSS).
The Newcastle-Ottawa Scale (NOS) was applied to evaluate the quality of included studies[22]. This scale includes object selection, comparability, and exposure assessment and objectively evaluates the risk of bias with the maximum score of 9, and studies with a score ≥ 6 were considered to have high quality. The quality assessment was performed by two authors (Wang YF and Xu SY) independently. Any disagreement was solved by team discussion.
All statistical analyses were conducted with STATA 12.0 software. The RR and HR with corresponding 95%CI were calculated to assess the association between the presence of SRCs and clinicopathological characteristics and prognosis of EEGJA patients, respectively. If the HR with 95%CI was not reported in articles directly, they would be calculated from Kaplan-Meier curves[23]. The heterogeneity among the included studies was evaluated by I2 statistics and Q test. When significant heterogeneity was observed [I2 > 50% and (or) P < 0.1], the random effects model was applied; otherwise, the fix effects model was used. The sensitivity analysis and meta-regression analysis were performed to detect the source of heterogeneity and evaluate the stability of pooled results. Besides, the Begg’s funnel plot and Egger’s test were conducted to detect publication bias[24,25]. Significant publication bias was defined as P < 0.05.
Initially, 631 records were searched and then 90 duplicated records were excluded. After reviewing the titles and abstracts, 503 records without any relativity were excluded. Then, 17 full tests were assessed for eligibility after eliminating 21 publications according to our exclusion criteria. Finally, ten retrospective articles were included in this systematic review and meta-analysis[11-20].
Among the ten included articles, Chirieac et al[11] and van Hootegem et al[17] enrolled two different subgroups of patients, which were regarded as two studies separately. Thus, a total of 12 retrospective cohort studies from ten publications were included, involving 30322 EEGJA patients. Most cases were from America and the sample size ranged from 163 to 14224. Three studies only enrolled esophageal adenocarcinoma patients. All studies were high-quality studies with an NOS score of 6 or higher. Detailed information is presented in Table 1.
| Author | Year | Country | Sample size | Location | TNM | SRC ratio | Endpoint | NOS |
| Chirieac et al[11] | 2005 | United States (ACC: 1985-2003) | 193 | Esophageal + EGJ | I-IV | > 0% | OS | 7 |
| Chirieac et al[11] | 2005 | United States (ACC: 1985-2003) | 219 | Esophageal + EGJ | I-IV | > 0% | OS | 7 |
| Yoon et al[12] | 2010 | United States (MCTR) | 796 | Esophageal + EGJ | NR | > 0% | OS, DSS, DFS | 6 |
| Yendamuri et al[13] | 2013 | United States (SEER) | 11825 | Esophageal | I-IV | > 0% | OS, | 7 |
| Nafteux et al[14] | 2014 | Belgium | 779 | Esophageal + EGJ | I-IV | 1%-50%, > 50% | DSS | 7 |
| Patel et al[15] | 2014 | United States (ACC: 2000-2012) | 723 | Esophageal + EGJ | II-IV | > 0% | OS, DFS | 7 |
| Chen et al [16] | 2017 | China | 671 | Esophageal | I-III | 1%-50%, > 50% | OS | 8 |
| Van Hootegem et al[17] | 2019 | Australia | 298 | Esophageal + EGJ | NR | > 0% | OS, DFS | 7 |
| Van Hootegem et al[17] | 2019 | Australia | 391 | Esophageal + EGJ | NR | > 0% | OS, DFS | 7 |
| Corsini et al[18] | 2020 | United States (ACC: 2006-2018) | 819 | Esophageal | NR | 1%-10%, 11%-49%, ≥ 50% | OS | 8 |
| Sathe et al[19] | 2020 | United States (NCD: 2004-2015) | 14224 | Esophageal + EGJ | I-III | NR | OS | 7 |
| Solomon et al[20] | 2021 | Israel | 163 | Esophageal + EGJ | I-III | > 0% | OS | 6 |
The pooled results indicated that SRCs were more likely to occur in esophageal adenocarcinoma rather than esophagogastric junction adenocarcinoma (RR: 0.76, 95%CI: 0.61-0.96, P = 0.022; I2 = 49.4%, P = 0.160) and TNM stage III/IV EEGJA patients (RR: 1.30, 95%CI: 1.02-1.65, P = 0.031; I2 = 73.1%, P = 0.002). However, no significant relation was observed between SRCs and the sex (RR: 0.99, 95%CI: 0.87-1.13, P = 0.917; I2 = 39.4%, P = 0.105), smoking (RR: 0.86, 95%CI: 0.55-1.35, P = 0.507; I2 = 86.9%, P = 0.006), family history (RR: 1.157, 95%CI: 0.762-1.757), and lymph node metastasis (RR: 1.05, 95%CI: 0.92-1.20, P = 0.488; I2= 7.2%, P = 0.340) (Table 2).
| Study | Sex (male) | Smoking | Family history | Tumor location (esophageal) | Lymph node metastasis | TNM stage (III/IV) |
| Chirieac et al[11] 2005 | 0.722 (0.254-2.053) | - | - | - | - | 0.895 (0.522-1.532) |
| Chirieac et al[11] 2005 | 0.730 (0.341-1.564) | - | - | - | - | 1.855 (0.997-3.454) |
| Yoon et al[12] 2010 | - | - | - | - | - | - |
| Yendamuri et al[13] 2013 | 0.975 (0.783-1.214) | - | - | - | - | 1.267 (1.060-1.513) |
| Nafteux et al[14] 2014 | 0.882 (0.558-1.395) | - | - | 0.636 (0.451-0.896) | - | 1.048 (0.702-1.563) |
| Patel et al[15] 2014 | 0.518 (0.309-0.867) | - | - | - | 1.357 (0.907-2.028) | - |
| Chen et al[16] 2017 | 1.461 (0.945-2.258) | 0.667 (0.495-0.899) | 1.157 (0.762-1.757) | - | - | 1.095 (0.811-1.480) |
| Van Hootegem et al[17] 2019 | 1.138 (0.662-1.956) | - | - | 0.888 (0.649-1.216) | 0.929 (0.677-1.275) | - |
| Corsini et al[18] 2020 | 0.771 (0.460-1.292) | 1.061 (0.923-1.219) | - | - | 1.038 (0.888-1.215) | - |
| Sathe et al[19] 2020 | 1.164 (0.912-1.485) | - | - | - | - | 1.832 (1.542-2.177) |
| Solomon et al[20] 2021 | - | - | - | - | - | - |
| Overall | 0.99, 0.87-1.13, P = 0.917 | 0.86, 0.55-1.35, P = 0.507 | 1.157 (0.762-1.757) | 0.76, 0.61-0.96, P = 0.022 | 1.05, 0.92-1.20, P = 0.488 | 1.30, 1.02-1.65, P = 0.031 |
A total of 11 studies explored the relationship of SRCs with the OS of EEGJA patients (16-18, and 20-25), and the pooled results demonstrated that the presence of SRCs predicted a much poor OS (HR: 1.36, 95%CI: 1.12-1.65, P = 0.002; I2 = 85.7%, P < 0.001) (Figure 2). Meanwhile, four studies assessed the impact of SRCs on the DFS of EEGJA patients[12,15,17] and the pooled results did not predict a significant association between SRCs and DFS (HR: 1.21, 95%CI: 0.94-1.57, P = 0.145; I2 = 63.1%, P = 0.043) (Figure 3). Besides, after combining two studies[17,19], a significant relationship was observed between the presence of SRCs and poor DSS (HR: 1.86, 95%CI: 1.55-2.25, P < 0.001; I2 = 0.0%, P = 0.323) (Table 3).
According to Figure 4, the sensitivity analysis revealed that the pooled results were stable and reliable.
Besides, the Begg’s funnel plot was symmetrical (Figure 5) and the P value of Egger’s test was 0.572, which indicated nonsignificant publication bias.
Due to the significant heterogeneity of the OS, meta-regression analysis was conducted based on some variables including the publication year, country, sample size, location, SRC ratio, TNM stage, and NOS score. Unfortunately, none of these parameters were the causes of heterogeneity.
The current systematic review and meta-analysis demonstrated that the presence of SRCs was significantly associated with tumor location (RR: 0.76, P = 0.022) and TNM stage (RR: 1.30, P = 0.031). Meanwhile, the presence of SRCs in surgical EEGJA patients predicted a poor OS (HR: 1.36, P = 0.002) and DSS (HR: 1.86, P < 0.001). The presence of SRCs might be an independent prognostic factor in EEGJA patients and contribute to the evaluation of prognosis and decision making.
Interestingly, Chirieac et al[11] demonstrated that the presence of SRCs predicted a much poor prognosis in patients who received surgery alone (P = 0.05), but for patients who received neoadjuvant chemotherapy and surgery, the presence of SRCs predicted a better prognosis (P = 0.02). This phenomenon indicated that SRCs might play an essential role in the response to chemotherapy. In the study by Corsini et al[18], EEGJA patients with usual type had a much higher pathologic complete response than patients with SRCs (25% vs 10%, P = 0.006). Meanwhile, Solomon et al[20] also revealed that patients with SRCs were less sensitive to neoadjuvant chemoradiotherapy (OR: 6.118, 95%CI: 1.299-28.821, P = 0.022) and less likely to experience downstaging after neoadjuvant chemotherapy (OR: 0.306, 95%CI: 0.099-0.946, P = 0.040). The presence of SRCs may predicted a poor response to chemotherapy, which is opposite to the results reported by Chirieac et al[11]. Thus, more relevant studies are still needed to identify the clinical role of SRC in the chemotherapy and radiotherapy of EEGJA patients.
Although the pooled results indicated that there was no significant association between the presence of SRCs and DFS of EEGJA patients (HR: 1.21, 95%CI: 0.94-1.57, P = 0.145), Yoon et al[12] and Patel et al[15] both reported that patients with SRCs were more likely to experience a worse DFS (HR: 1.49, 95%CI: 1.15-1.93, P = 0.001; HR: 1.34, 95%CI: 1.02-1.80, P = 0.048). Therefore, we believed that SRCs might also predict a poor DFS in EEGJA patients, which needs more studies to further verify.
Besides, Nafteux et al[14] demonstrated that the presence of SRCs was significantly associated with a higher recurrence rate (56% vs 42% for usual type adenocarcinoma, P = 0.003). In their multivariate analysis, SRC ratio > 50% was an independent risk factor for recurrence (OR: 2.070, 95%CI: 1.159-3.696, P = 0.014), which also indicated that the presence of SRCs was related with a poor prognosis in EEGJA patients.
Actually, there are still several valuable fields about the clinical significance of SRCs in EEGJA patients that are worth further investigation. First, as mentioned above, the role that SRCs play in the chemotherapy and radiotherapy is unclear, and more studies comparing the clinical outcomes between EEGJA patients with and without neoadjuvant chemoradiotherapy or postoperative chemoradiotherapy are needed. Second, it is necessary to identify whether SRCs could serve as a reliable predictor for the selection of therapeutic strategies. Third, to explore the role of the proportion change of SRC during chemotherapy in predicting the prognosis of EEGJA patients might be significative. Fourth, according to previous reports, the proportion of SRCs may also be related with the prognosis of EEGJA patients and patients with different ratios of SRCs might have different survival rates.
There were some limitations in this study. First, all included studies were retrospective, which may cause some bias. Second, the association of SRCs with other parameters such as alcohol drinking, differentiation status, Helicobacter pylori infection, and age was not explored due to the lack of relevant data. Third, we failed to conduct subgroup analysis based on the clinicopathological parameters such as age, sex, and TNM stage because the detailed data were not available even if we contacted the authors of included studies.
The presence of SRCs is related with advanced tumor stage and poor prognosis and could serve as a reliable and effective parameter for the prediction of postoperative survival and formulation of therapy strategy in EEGJA patients. However, more prospective high-quality studies are still needed to verify our findings.
The clinical role of signet ring cells (SRCs) in surgical esophageal and esophagogastric junction adenocarcinoma (EEGJA) remains unclear now.
To explore the clinical role of the presence of SRCs in surgical EEGJA patients.
To explore the association between the presence of SRCs and the clinicopathological and prognostic characteristics in surgical EEGJA patients.
Several electronic databases were searched to identify the relevant articles. The relative risks and hazard ratios with their corresponding 95% confidence intervals were estimated, respectively.
The presence of SRCs was significantly associated with the tumor location (P = 0.022) and tumor-node-metastasis stage (P = 0.031). Meanwhile, the presence of SRCs in surgical EEGJA patients predicted a poor overall survival (P = 0.002) and disease-specific survival (P < 0.001).
SRC was significantly related with advanced tumor stage and poor prognosis in EEGJA patients.
The presence of SRCs could serve as a reliable and effective parameter for the prediction of postoperative survival and formulation of therapy strategy in EEGJA patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ayenew AA, Patil S, Sánchez JIA S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Wheeler JB, Reed CE. Epidemiology of esophageal cancer. Surg Clin North Am. 2012;92:1077-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 2. | Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatol. 2016;31:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 3. | Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1027] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 4. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64684] [Article Influence: 16171.0] [Reference Citation Analysis (177)] |
| 5. | Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 209] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 6. | Song IH, Hong SM, Yu E, Yoon YS, Park IJ, Lim SB, Kim JC, Yu CS, Kim J. Signet ring cell component predicts aggressive behaviour in colorectal mucinous adenocarcinoma. Pathology. 2019;51:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Piessen G, Messager M, Lefevre JH, Goéré D, Mabrut JY, Meunier B, Brigand C, Hamy A, Glehen O, Mariette C; FREGAT Working Group – FRENCH. Signet ring cell adenocarcinomas: different clinical-pathological characteristics of oesophageal and gastric locations. Eur J Surg Oncol. 2014;40:1746-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Nie RC, Yuan SQ, Li YF, Chen YM, Chen XJ, Zhu BY, Xu LP, Zhou ZW, Chen S, Chen YB. Clinicopathological Characteristics and Prognostic Value of Signet Ring Cells in Gastric Carcinoma: A Meta-Analysis. J Cancer. 2017;8:3396-3404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Tan Y, Fu J, Li X, Yang J, Jiang M, Ding K, Xu J, Li J, Yuan Y. A minor (<50%) signet-ring cell component associated with poor prognosis in colorectal cancer patients: a 26-year retrospective study in China. PLoS One. 2015;10:e0121944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | An Y, Zhou J, Lin G, Wu H, Cong L, Li Y, Qiu X, Shi W. Clinicopathological and Molecular Characteristics of Colorectal Signet Ring Cell Carcinoma: A Review. Pathol Oncol Res. 2021;27:1609859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Chirieac LR, Swisher SG, Correa AM, Ajani JA, Komaki RR, Rashid A, Hamilton SR, Wu TT. Signet-ring cell or mucinous histology after preoperative chemoradiation and survival in patients with esophageal or esophagogastric junction adenocarcinoma. Clin Cancer Res. 2005;11:2229-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Yoon HH, Khan M, Shi Q, Cassivi SD, Wu TT, Quevedo JF, Burch PA, Sinicrope FA, Diasio RB. The prognostic value of clinical and pathologic factors in esophageal adenocarcinoma: a mayo cohort of 796 patients with extended follow-up after surgical resection. Mayo Clin Proc. 2010;85:1080-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Yendamuri S, Huang M, Malhotra U, Warren GW, Bogner PN, Nwogu CE, Groman A, Demmy TL. Prognostic implications of signet ring cell histology in esophageal adenocarcinoma. Cancer. 2013;119:3156-3161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Nafteux PR, Lerut TE, Villeneuve PJ, Dhaenens JM, De Hertogh G, Moons J, Coosemans WJ, Van Veer HG, De Leyn PR. Signet ring cells in esophageal and gastroesophageal junction carcinomas have a more aggressive biological behavior. Ann Surg. 2014;260:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Patel VR, Hofstetter WL, Correa AM, Agarwal A, Rashid A, Bhutani MS, Lin SH, Ajani JA, Swisher SG, Maru DM. Signet ring cells in esophageal adenocarcinoma predict poor response to preoperative chemoradiation. Ann Thorac Surg. 2014;98:1064-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Chen L, Liu X, Gao L, Wang R, Gao D, Bai D. The clinicopathological features and prognosis of signet ring cell carcinoma of the esophagus: A 10-year retrospective study in China. PLoS One. 2017;12:e0176637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | van Hootegem SJM, Smithers BM, Gotley DC, Brosda S, Thomson IG, Thomas JM, Gartside M, van Lanschot JJB, Lagarde SM, Wijnhoven BPL, Barbour AP. The Impact of Signet Ring Cell Differentiation on Outcome in Patients with Esophageal and Gastroesophageal Junction Adenocarcinoma. Ann Surg Oncol. 2019;26:2375-2384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Corsini EM, Foo WC, Mitchell KG, Zhou N, Maru DM, Ajani JA, Hofstetter WL; Esophageal Adenocarcinoma Working Group, Correa AM, Antonoff MB, Lin SH, Mehran RJ, Rajaram R, Rice DC, Roth JA, Sepesi B, Swisher SG, Vaporciyan AA, Walsh GL. Esophageal adenocarcinoma with any component of signet ring cells portends poor prognosis and response to neoadjuvant therapy. J Thorac Cardiovasc Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Sathe TS, Resio BJ, Hoag JR, Monsalve AF, Pathak R, Blasberg JD, Mase V Jr, Dhanasopon A, Boffa DJ. Surgically Managed Signet Ring Cell Esophageal Carcinomas in the National Cancer Database. Ann Thorac Surg. 2020;109:1656-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Solomon D, Abbas M, Feferman Y, Haddad R, Perl G, Kundel Y, Morgenstern S, Menasherov N, Kashtan H. Signet Ring Cell Features are Associated with Poor Response to Neoadjuvant Treatment and Dismal Survival in Patients with High-Grade Esophageal Adenocarcinoma. Ann Surg Oncol. 2021;28:4929-4940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | McLeroy KR, Northridge ME, Balcazar H, Greenberg MR, Landers SJ. Reporting guidelines and the American Journal of Public Health's adoption of Preferred Reporting Items for Systematic reviews and Meta-Analyses. Am J Public Health. 2012;102:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12667] [Article Influence: 844.5] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Wu Y, Li J, Lai Y, Zhou K, Che G. Prognostic and clinicopathological significance of FGFR1 gene amplification in resected esophageal squamous cell carcinoma: a meta-analysis. Ann Transl Med. 2019;7:669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] |
| 25. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40566] [Article Influence: 1448.8] [Reference Citation Analysis (2)] |