Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10899
Peer-review started: June 30, 2021
First decision: August 19, 2021
Revised: September 1, 2021
Accepted: October 31, 2021
Article in press: October 31, 2021
Published online: December 16, 2021
Processing time: 163 Days and 2.8 Hours
Decreased serum magnesium (Mg2+) is commonly seen in critically ill patients. Hypomagnesemia is significantly more frequent in patients with severe acute pancreatitis. Acute kidney injury (AKI) in patients with acute pancreatitis (AP) is associated with an extremely high mortality. The association underlying serum Mg2+ and AKI in AP has not been elucidated.
To explore the association between serum Mg2+ on admission and AKI in patients with AP.
A retrospective observational study was conducted in a cohort of patients (n = 233) with AP without any renal injury before admission to our center from August 2015 to February 2019. Demographic characteristics on admission, severity score, laboratory values and in-hospital mortality were compared between patients with and without AKI.
A total of 233 patients were included for analysis, including 85 with AKI. Compared to patients without AKI, serum Mg2+ level was significantly lower in patients with AKI at admission [OR = 6.070, 95%CI: 3.374-10.921, P < 0.001]. Multivariate logistic analysis showed that lower serum Mg2+ was an independent risk factor for AKI [OR = 8.47, 95%CI: 3.02-23.72, P < 0.001].
Our analysis indicates that serum Mg2+ level at admission is independently associated with the development of AKI in patients with AP and may be a potential prognostic factor.
Core Tip: Acute kidney injury (AKI) is a serious complication of acute pancreatitis (AP) and is often difficult to predict at an early stage. However, our clinical analysis found that serum Mg2+ on admission is a good predictor of the occurrence of AKI in AP patients. Therefore, this may provide a new method for the early prediction of AKI after AP.
- Citation: Yu XQ, Deng HB, Liu Y, Qu C, Duan ZH, Tong ZH, Liu YX, Li WQ. Serum magnesium level as a predictor of acute kidney injury in patients with acute pancreatitis. World J Clin Cases 2021; 9(35): 10899-10908
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10899.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10899
Acute pancreatitis (AP) is an autodigestive disease triggered by acinar cells, and about 20% of the patients progress to fatal severe acute pancreatitis (SAP)[1-4]. Acinar cell injury accompanied by intracellular electrolyte imbalance, further aggravating cell damage and even death is the recognized pathogenesis of AP[5,6]. In particular, organelle damage caused by intracellular calcium (Ca2+) influx into mitochondria is the main risk factor for AP[7]. An in vitro AP model showed that Ca2+ channel antagonists could effectively reduce Ca2+ influx and increase mitochondrial membrane potential, thereby protecting acinar cells[8,9]. As an important cation in cells, magnesium (Mg2+) is a coenzyme involved in a variety of enzymatic reactions and plays a role in maintaining membrane potential and physiological function[10-12]. In addition, Mg2+ plays a protective role in AP acinar cells by antagonizing Ca2+ signals[13]. On the contrary, abnormal regulation of Mg2+ acts as a pivotal trigger in the pathogenesis of AP[14].
Acute kidney injury (AKI) is a common complication of SAP with poor prognosis, especially when patients require renal replacement therapy, the mortality rate is > 75%[15,16]. SAP-associated AKI is related to systemic inflammatory response syndrome (SIRS), hypoxemia, renal microcirculation injury after trypsin release, renal perfusion pressure reduction caused by intraperitoneal high pressure or low blood volume, endotoxins and reactive oxides[17]. Therefore, early prediction of AKI in AP is very important to improve the course and prognosis of the disease.
AKI is often accompanied by complex electrolyte disturbances[18]. However, the relationship between Mg2+ and the occurrence of AP-associated AKI in AP pathophysiology has not been fully elucidated. Based on the beneficial role of Mg2+ in acinar cells of AP, we therefore sought to assess the value of serum Mg2+ on admission in correlation with the incidence of AKI in AP.
We conducted a retrospective study of patients with AP admitted to the Center of Severe Acute Pancreatitis of Jinling Hospital between August 2015 and February 2019. All the data were extracted from an electronic database, which stored prospectively collected clinical data of all AP patients admitted to our center. We obtained the approval of the Acute Pancreatitis Database Management Committee (2018 JLAPDMC-009), and all the analyses were performed in accordance with the committee's regulations. Informed consent involving data storage and academic use of data was obtained from each patient during their hospitalization. Patients who met the following criteria were included: (1) Diagnosis of AP (ICD-10, K85) under the 2012 revision of the Atlanta classification; and (2) Admission to our department within one week after the disease onset. The exclusion criteria included any of the following: (1) The time from abdominal pain onset to hospital admission ≥ 7 d; (2) Age younger than 18 years; and (3) Suspected chronic pancreatitis, cancer, and chronic liver diseases such as cirrhosis or viral hepatitis, chronic kidney diseases such as nephritis, or renal failure. AKI (ICD-10: N17) was diagnosed according to the kidney disease: Improving Global Outcomes criteria based on serum/plasma creatinine and urine output. Patients meeting the diagnostic criteria for AP during hospitalization were included in the AKI group. The diagnosis of low serum Mg2+ was made by laboratory mea
Demographic and baseline characteristics on admission included the following: Age, gender, body mass index (BMI), disease severity score (APACHE II), sequential organ failure assessment (SOFA), computed tomography severity index (CTSI), the Atlanta classification, comorbidities (diabetes, hypertension, hyperlipidemia), white blood cells, lymphocytes%, interleukin-6 (IL-6), procalcitonin (PCT), platelets, blood urea nitrogen (BUN), creatinine, HCO3-, and Cl-.
Statistical analysis was performed using R software, version 3.6.2 (R Foundation for Statistical Computing). The Kolmogorov-Smirnov test was used to test the normality. Continuous variables are presented as means and standard derivations or medians and interquartile ranges. Categorical variables are presented as number (frequency). The Mann-Whitney U test was used to evaluate the differences in baseline characteristics between the two groups. The Chi-square test or Fisher's exact test was used to analyze categorical variables for group comparisons. All variables with statistically significant prognostic value in univariate analysis were selected for further multivariate analysis. Odds ratio (OR) and 95% confidence intervals (CIs) are presented. Receiver operating characteristic curves were constructed to evaluate the sensitivity and specificity of serum Mg2+ in predicting AKI. P value < 0.05 was considered statistically significant.
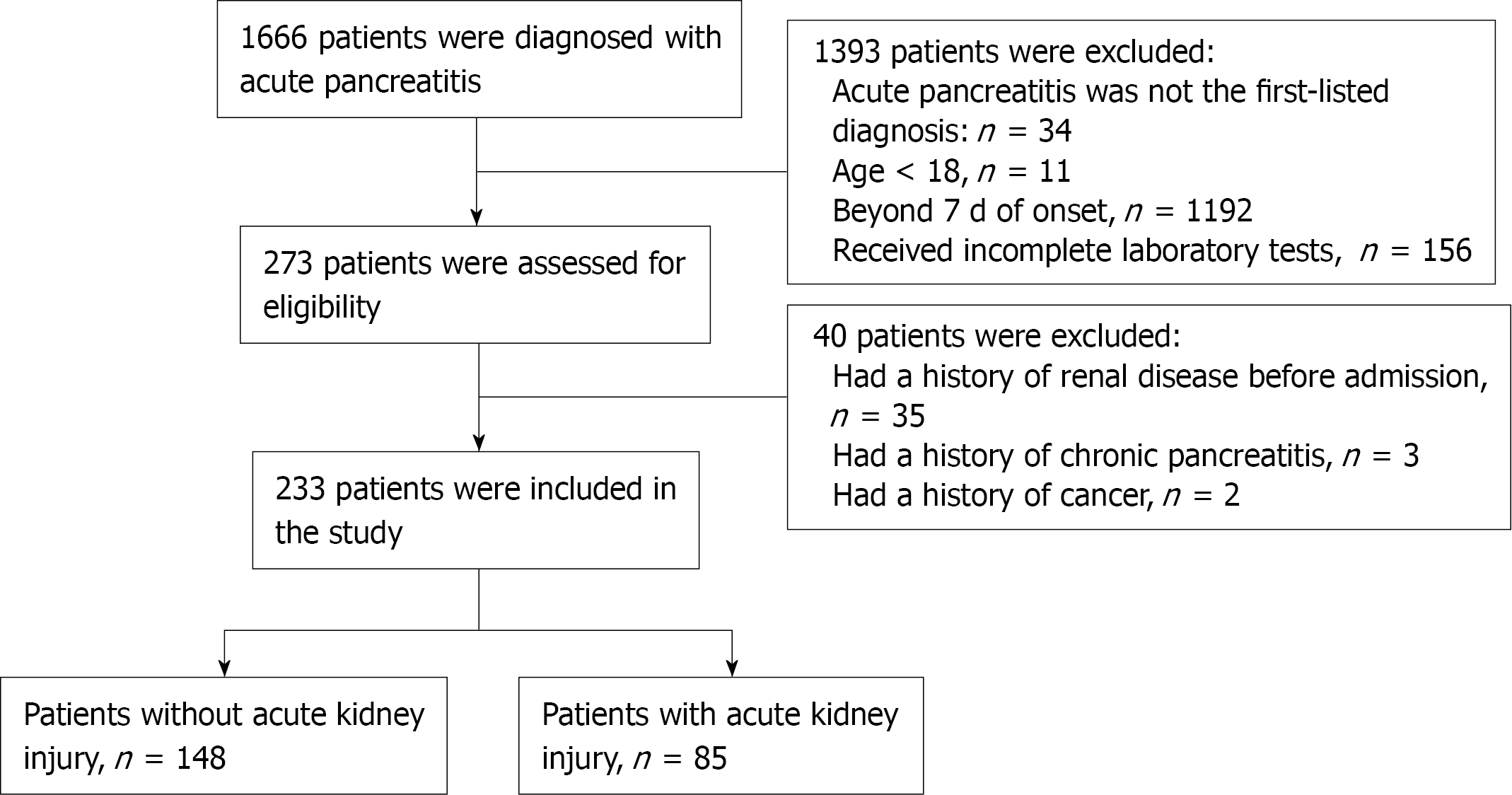
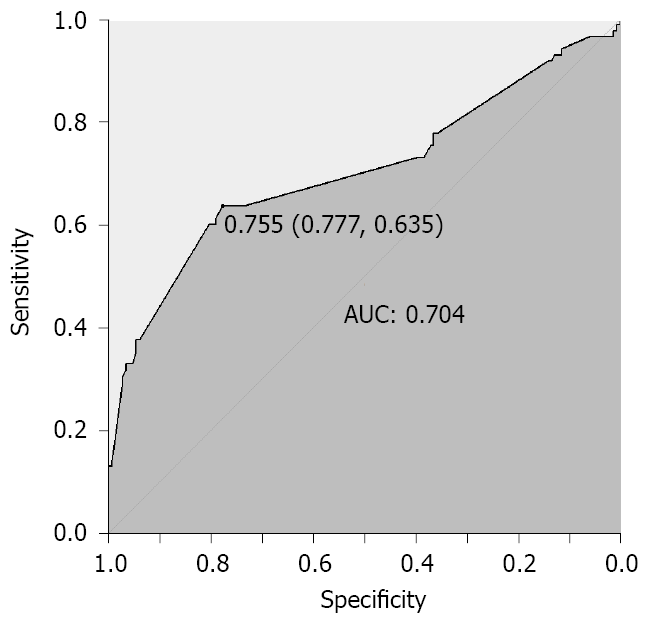
A total of 233 patients were included for analysis. The participant selection process is shown in Figure 1. The serum Mg2+ level of 0.755 mg/dL was identified as an effective cut-off point for in-hospital AKI occurrence (area under curve = 0.704; 95%CI: 0.640-0.775, P < 0.001), with a sensitivity of 77.7%, and specificity of 63.5% (Figure 2). Baseline characteristics of these patients are shown in Table 1. Compared with the non-low serum Mg2+ group, the group with low serum Mg2+ had higher BMI (P = 0.028) and APACHE II (P = 0.002). With regard to laboratory parameters, patients in the low serum Mg2+ group had higher admission IL-6 (P < 0.001), PCT (P < 0.001), and lower HCO3- (P < 0.001).
| Mg2+ (mg/dL) | P value | AKI, n = 85 | Non-AKI, n = 148 | P value | ||
| < 0.755 mg/dL, n = 87 | ≥ 0.755 mg/dL, n = 146 | |||||
| Age, yr | 39 (32, 52) | 44 (34, 58) | 0.063 | 38 (30, 50) | 44.5 (35.5, 54.5) | 0.011 |
| Gender, male, n (%) | 59 (67.8) | 98 (67.1) | 0.913 | 59 (69.4) | 98 (66.2) | 0.913 |
| BMI | 27.1 (24.7, 30.1) | 25.6 (23.9, 28.1) | 0.028 | 27.6 (24.8, 30.7) | 25.4 (23.4, 27.7) | < 0.001 |
| APACHE II | 9 (7, 12) | 7 (5, 9) | 0.002 | 11 (8, 14) | 7 (4, 9) | < 0.001 |
| SOFA | 3 (3, 4) | 3 (2, 4) | 0.075 | 4 (3, 5) | 3 (2, 4) | < 0.001 |
| CTSI | 6 (3, 6) | 5 (3, 6) | 0.122 | 6 (6, 6) | 4 (2, 6) | < 0.001 |
| Severity classification, n (%) | 0.064 | < 0.001 | ||||
| MAP | 21 (24.1) | 50 (34.2) | 7 (8.2) | 64 (43.2) | ||
| MSAP | 47 (54.0) | 79 (54.1) | 45 (53.0) | 81 (54.7) | ||
| SAP | 19 (21.8) | 17 (11.6) | 33 (38.8) | 3 (2.1) | ||
| Comorbidities | ||||||
| Diabetes | 23 (26.4) | 24 (16.4) | 0.066 | 20 (23.5) | 27 (18.2) | 0.066 |
| Hypertension | 22 (25.3) | 36 (24.7) | 0.914 | 22 (25.9) | 36 (24.3) | 0.914 |
| Hyperlipidemia | 25 (28.7) | 36 (24.7) | 0.493 | 21 (24.7) | 40 (27.0) | 0.493 |
| Laboratory data | ||||||
| WBC | 13.4 (10.6, 16.6) | 12.8 (10.1, 15.7) | 0.336 | 12.9 (10.9, 16.6) | 12.9 (10.3, 16.1) | 0.685 |
| Ly% | 8.1 (5.1, 11.2) | 6.7 (4.7, 10.6) | 0.297 | 6.9 (4.9, 10.5) | 7.2 (5, 11.2) | 0.769 |
| IL-6 | 199.6 (104.8, 366.4) | 115.4 (45.4, 201.5) | < 0.001 | 222.8 (130.4, 370) | 104.8 (45.4, 178.4) | < 0.001 |
| PCT | 1.2 (0.4, 3.3) | 0.4 (0.1, 1.6) | < 0.001 | 2.1 (1.1, 7.7) | 0.3 (0.1, 0.8) | < 0.001 |
| Platelets | 193 (142, 238) | 174 (134, 224) | 0.215 | 199 (132, 236) | 176.5 (142, 218) | 0.248 |
| BUN | 5.4 (3.7, 6.3) | 5.1 (4, 6.9) | 0.576 | 6 (4, 8.3) | 4.8 (3.8, 5.9) | < 0.001 |
| Creatinine | 61 (49, 8) | 63 (53, 8) | 0.924 | 50 (41, 57.3) | 51 (46, 59) | 0.184 |
| HCO3- | 18.9 (15.1, 23.5) | 22 (18.7, 24.2) | < 0.001 | 17.8 (13.7, 21.3) | 22.6 (19.8, 24.7) | < 0.001 |
| Cl- | 103 (99, 105) | 102 (100, 105) | 0.825 | 103.7 (101, 107) | 102 (99, 104) | < 0.001 |
| Mg2- | 0.7 (0.6, 0.7) | 0.885 (0.8, 0.9) | < 0.001 | |||
The in-hospital clinical outcomes are shown in Table 2, divided according to admission serum Mg2+ level. The serum Mg2+ < 0.755 mg/dL group consisted of 87 patients (54 cases in the AKI group and 33 cases in the non-AKI group), and the serum Mg2+ ≥ 0.755 mg/dL group consisted of 146 patients (31 cases in the AKI group and 115 cases in the non-AKI group). Lower serum Mg2+ was correlated with the occurrence of AKI (62.1% vs 21.2%, P < 0.001). The length of intensive care unit (ICU) stay (P < 0.001) and hospital stay (P < 0.001) of patients with low serum Mg2+ level was longer.
| Mg2+ (mg/dL) | P value | |||
| < 0.755 mg/dL, n = 87 | ≥ 0.755 mg/dL, n = 146 | |||
| Primary outcome, n (%) | ||||
| AKI | 54 (62.1) | 31 (21.2) | < 0.001 | |
| Clinical course, days median | ||||
| ICU days | 3 (2, 6) | 2 (1, 4) | < 0.001 | |
| Hospital days | 6 (4, 10) | 4 (3, 7) | < 0.001 | |
| Severe outcome, n (%) | ||||
| ICU mortality | 1 (1.15) | 3 (2.05) | 0.999 | |
| 30 d mortality | 1 (1.15) | 3 (2.05) | 0.999 | |
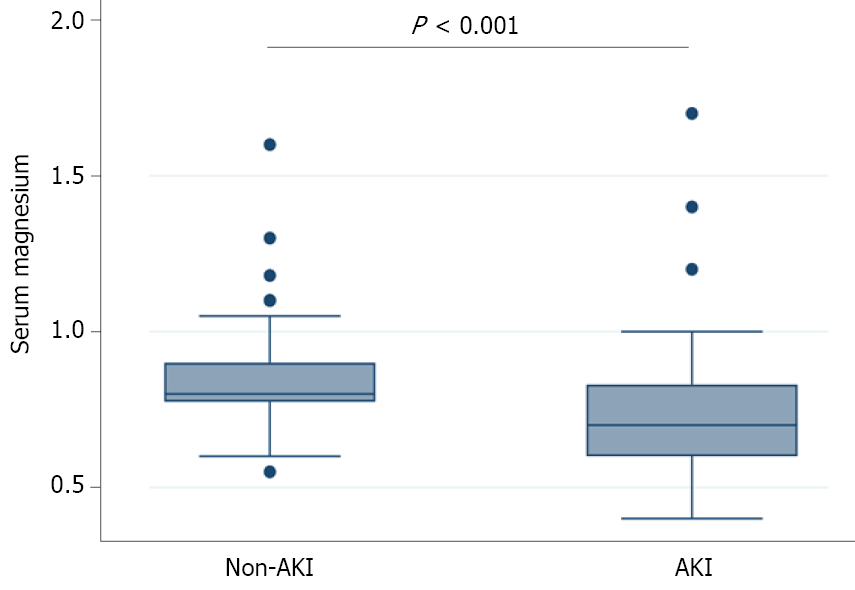
As shown in Figure 3, compared with the non-AKI group, the AKI group had significantly lower serum Mg2+ level (P < 0.001). Following univariate logistic regression analysis, BMI (OR = 1.155, P < 0.001), APACHE II (OR=1.385, P < 0.001), SOFA (OR = 1.589, P < 0.001), CTSI (OR = 1.479, P < 0.001), severity classification (P < 0.001), IL-6 (OR = 1.006, P < 0.001), PCT (OR = 1.350, P < 0.001), BUN (OR = 1.368, P < 0.001), creatinine (OR = 1.051, P < 0.001), HCO3- (OR = 0.843, P < 0.001), and Cl- (OR = 1.100, P = 0.003) were important indicators of AKI in AP patients (Table 3). Multivariate logistic analysis showed that lower serum Mg2+ (OR = 5.525, P < 0.001) was an independent risk factor for AKI (Table 3).
| Univariate analysis | OR | 95%CI | P value | Multivariate model | OR | 95%CI | P value |
| Mg2+ < 0.755, mg/dL | 6.070 | (3.374, 10.921) | < 0.001 | Mg2+ < 0.755, mg/dL | 5.525 | (2.074, 14.718) | < 0.001 |
| Age | 0.981 | (0.963, 1.000) | 0.052 | Age | 0.966 | (0.926, 1.007) | 0.104 |
| Gender | 1.158 | (0.652, 2.054) | 0.617 | ||||
| BMI | 1.155 | (1.073, 1.244) | < 0.001 | BMI | 1.081 | (0.946, 1.236) | 0.251 |
| APACHE II | 1.385 | (1.256, 1.527) | < 0.001 | APACHE II | 1.130 | (0.976, 1.310) | 0.103 |
| SOFA | 1.589 | (1.307, 1.931) | < 0.001 | SOFA | 0.896 | (0.604, 1.330) | 0.585 |
| CTSI | 1.479 | (1.279, 1.711) | < 0.001 | CTSI | 1.107 | (0.815, 1.505) | 0.516 |
| Severity classification (MAP as reference) | Severity classification (MAP as reference) | ||||||
| MSAP | 4.870 | (2.071, 11.450) | < 0.001 | MSAP | 1.126 | (0.240, 5.289) | 0.880 |
| SAP | 84.857 | (23.269, 309.458) | < 0.001 | SAP | 15.260 | (1.817, 128.189) | 0.012 |
| Diabetes | 1.379 | (0.718, 2.647) | 0.334 | ||||
| Hypertension | 1.086 | (0.588, 2.007) | 0.791 | ||||
| Hyperlipidemia | 0.886 | (0.480, 1.634) | 0.698 | ||||
| WBC | 1.026 | (0.970, 1.086) | 0.910 | ||||
| Ly | 0.734 | (0.938, 1.046) | 0.734 | ||||
| IL-6 | 1.006 | (1.004, 1.009) | < 0.001 | IL-6 | 1.003 | (0.999, 1.006) | 0.113 |
| PCT | 1.350 | (1.166, 1.562) | < 0.001 | PCT | 1.109 | (0.959, 1.283) | 0.163 |
| Platelets | 1.003 | (0.999, 1.007) | 0.139 | ||||
| BUN | 1.368 | (1.196, 1.565) | < 0.001 | BUN | 1.102 | (0.826, 1.470) | 0.508 |
| Creatinine | 1.051 | (1.034, 1.069) | < 0.001 | Creatinine | 1.052 | (1.014, 1.091) | 0.006 |
| HCO3- | 0.843 | (0.794, 0.896) | < 0.001 | HCO3- | 0.993 | (0.894, 1.103) | 0.892 |
| Cl- | 1.100 | (1.032, 1.172) | 0.003 | Cl- | 1.042 | (0.936, 1.160) | 0.453 |
In this research, we examined the involvement of serum Mg2+ and AKI in AP patients. Our results suggest that serum Mg2+ levels detected at admission were significantly lower in AP patients with AKI than in non-AKI patients. Moreover, the low serum Mg2+ group had a longer ICU and hospital stay than the non-low serum Mg2+ group. Furthermore, serum Mg2+ was revealed as an independent risk factor for the development of AKI. Therefore, serum Mg2+ is an effective predictor of AKI after AP.
Mg2+ is a well-known divalent cation abundant in human cells and is concentrated in mitochondria. It mainly plays the role of a cofactor in enzyme reactions and a second messenger in cellular signaling pathways[19-21]. In the physiological state of acinar cells, Mg2+ plays an antagonistic role in the influx of Ca2+ channel ions and inhibits the secretion of intracellular enzymes[9,22]. In the acinar cell model of AP, the addition of Mg2+ mitigates the effects of AP by inhibiting Ca2+ influx into the mitochondria, thereby reducing the secretion of digestive enzymes and promoting ATP generation[14]. In conclusion, Mg2+ plays an important regulatory role in the pathophysiological state of acinar cells. Mitochondria are the key organelles for the energy supply in acinar cells. It is obvious that Mg2+ plays an important role in maintaining mitochondrial homeostasis and ATP generation from this perspective.
The persistent influx of Ca2+ into the mitochondria of acinar cells in AP leads to increased oxygen radicals further triggering cell necrosis, which in turn induces SIRS[23-25]. This imbalance leads to further inflammatory response and oxygen radical production, resulting in multiple organ dysfunction including AKI[26]. Therefore, it is important to prevent the continuous influx of Ca2+ into mitochondria to reduce acinar cell necrosis and inhibit trypsin activation in AP. This is consistent with research in animal experiments[8,9]. In a murine model, the risk of triggering AP was decreased by inhibiting Ca2+ release-activated Ca2+ channels[27]. To the best of our knowledge, hypomagnesemia is commonly seen in severely ill patients including those with SAP[28]. In our SAP patients, there was a significant negative correlation between the incidence of AKI and adjusted serum Mg2+ on admission.
AKI as a complication, which is associated with increased mortality, occurs in approximately 15%-70% of SAP patients[18,29]. Therefore, early prediction of AKI in hospitalized patients with AP is imperative, especially for screening graded treatment strategies[30]. Currently, there are various clinical methods to predict the occurrence of AKI in patients with AP. On the whole, current studies on biomarkers for AP-associated AKI are insufficient, and the number of patients included in the analysis was limited. In addition, from the latest clinical evidence on the markers of AKI in AP, PCT showed relatively better clinical predictive value than neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C[31-33]. At present, serum or urine NGAL and serum cystatin C are recognized as the best laboratory indicators for predicting AKI in AP with good diagnostic accuracy. However, these single-center clinical data are not convincing enough. Large multicenter clinical studies on biomarkers are of great clinical value in identifying AKI in AP.
However, the relationship between admission serum Mg2+ level and AKI incidence in patients with AP has not been fully elucidated. Our results are the first to show that reduced serum Mg2+ levels are significantly associated with an increased risk of AKI in patients with AP. We found that Mg2+ level of 0.755 mg/dL was an effective cut-off point for in-hospital AKI occurrence, with a sensitivity of 77.7%, and specificity of 63.5%.
However, there are some limitations to our analysis. Firstly, our study did not consider the value of peripheral blood Mg2+; thus, the reliability of the actual level of free Mg2+ in peripheral blood may be significantly reduced from this perspective. Secondly, the causal relationship between Mg2+ and AP-associated AKI still needs to be verified by a large number of prospective studies. Thirdly, our analysis included only one checkup at admission, and as serum Mg2+ is a dynamic state, it may not fully reflect the true status of Mg2+ in these patients. From this perspective, dynamic serum Mg2+ measurement after admission is more objective in predicting AP-associated AKI. Finally, there may be methodological bias in our analysis, it is necessary to explore new machine models (such as train-validation models) to verify the current analysis results.
Our analysis indicates that serum Mg2+ level at admission is independently associated the development of AKI in patients with AP and may be a potential prognostic factor.
There is a lack of effective predictors of acute kidney injury (AKI) after acute pancreatitis (AP) in clinical practice.
To investigate the association between serum Mg2+ on admission and AKI after AP.
To determine whether serum Mg2+ is a valid predictor of AP-associated AKI using clinical data from our severe acute pancreatitis center.
Our center is one of the largest severe acute pancreatitis treatment centers in China. A total of 233 patients with AP from August 2015 to February 2019 were included in a retrospective analysis. Almost all clinical and laboratory indicators were included in the study.
Lower serum Mg2+ was correlated with the occurrence of AKI (62.1% vs 21.2%, P < 0.001). Patients in the low serum Mg2+ level group had a longer intensive care unit (P < 0.001) and hospital stay (P < 0.001).
Serum Mg2+ on admission can effectively predict AKI in AP patients.
This study provides ideas and a basis for prospective observation of AKI after AP, and provides early warning for effective intervention of the disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: Member of the Standing Committee of Chinese Society of Critical Care Medicine; Vice Chairman of Pancreatic Disease Branch of Chinese Medical Doctor Association; and Chairman of the critical medicine branch of the whole army.
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar R S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Wu YXJ
| 1. | Windsor JA, Escott A, Brown L, Phillips AR. Novel strategies for the treatment of acute pancreatitis based on the determinants of severity. J Gastroenterol Hepatol. 2017;32:1796-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Talukdar R, Vege SS. Acute pancreatitis. Curr Opin Gastroenterol. 2015;31:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016;375:1972-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 558] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 4. | Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current Concepts in Severe Acute and Necrotizing Pancreatitis: An Evidence-Based Approach. Gastroenterology. 2019;156:1994-2007.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 252] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 5. | Krüger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol. 2000;157:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 218] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Feng S, Wei Q, Hu Q, Huang X, Zhou X, Luo G, Deng M, Lü M. Research Progress on the Relationship Between Acute Pancreatitis and Calcium Overload in Acinar Cells. Dig Dis Sci. 2019;64:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Ward JB, Petersen OH, Jenkins SA, Sutton R. Is an elevated concentration of acinar cytosolic free ionised calcium the trigger for acute pancreatitis? Lancet. 1995;346:1016-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Saluja AK, Bhagat L, Lee HS, Bhatia M, Frossard JL, Steer ML. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol. 1999;276:G835-G842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Mooren FCh, Hlouschek V, Finkes T, Turi S, Weber IA, Singh J, Domschke W, Schnekenburger J, Krüger B, Lerch MM. Early changes in pancreatic acinar cell calcium signaling after pancreatic duct obstruction. J Biol Chem. 2003;278:9361-9369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Song Y, Manson JE, Buring JE, Liu S. Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care. 2004;27:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Arch Intern Med. 1999;159:2151-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 264] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Kim DJ, Xun P, Liu K, Loria C, Yokota K, Jacobs DR Jr, He K. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care. 2010;33:2604-2610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Mooren FC, Turi S, Gunzel D, Schlue WR, Domschke W, Singh J, Lerch MM. Calcium-magnesium interactions in pancreatic acinar cells. FASEB J. 2001;15:659-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Schick V, Scheiber JA, Mooren FC, Turi S, Ceyhan GO, Schnekenburger J, Sendler M, Schwaiger T, Omercevic A, Brandt Cv, Fluhr G, Domschke W, Krüger B, Mayerle J, Lerch MM. Effect of magnesium supplementation and depletion on the onset and course of acute experimental pancreatitis. Gut. 2014;63:1469-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1029] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 16. | Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2063] [Cited by in RCA: 1839] [Article Influence: 183.9] [Reference Citation Analysis (0)] |
| 17. | Beker BM, Corleto MG, Fieiras C, Musso CG. Novel acute kidney injury biomarkers: their characteristics, utility and concerns. Int Urol Nephrol. 2018;50:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 18. | Zhou J, Li Y, Tang Y, Liu F, Yu S, Zhang L, Zeng X, Zhao Y, Fu P. Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis. Nephrology (Carlton). 2015;20:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Edmondson HA, BERNE CJ, HOMANN RE Jr, WERTMAN M. Calcium, potassium, magnesium and amylase disturbances in acute pancreatitis. Am J Med. 1952;12:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 78] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Ryzen E, Rude RK. Low intracellular magnesium in patients with acute pancreatitis and hypocalcemia. West J Med. 1990;152:145-148. [PubMed] |
| 21. | Krzewicki J. Clinical study on magnesium and calcium level in the blood during the acute pancreatitis. Magnes Res. 1998;11:19-23. [PubMed] |
| 22. | Wisdom DM, Salido GM, Baldwin LM, Singh J. The role of magnesium in regulating CCK-8-evoked secretory responses in the exocrine rat pancreas. Mol Cell Biochem. 1996;154:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Ammori BJ, Barclay GR, Larvin M, McMahon MJ. Hypocalcemia in patients with acute pancreatitis: a putative role for systemic endotoxin exposure. Pancreas. 2003;26:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Huang W, Cane MC, Mukherjee R, Szatmary P, Zhang X, Elliott V, Ouyang Y, Chvanov M, Latawiec D, Wen L, Booth DM, Haynes AC, Petersen OH, Tepikin AV, Criddle DN, Sutton R. Caffeine protects against experimental acute pancreatitis by inhibition of inositol 1,4,5-trisphosphate receptor-mediated Ca2+ release. Gut. 2017;66:301-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Frick TW. The role of calcium in acute pancreatitis. Surgery. 2012;152:S157-S163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Dumnicka P, Maduzia D, Ceranowicz P, Olszanecki R, Drożdż R, Kuśnierz-Cabala B. The Interplay between Inflammation, Coagulation and Endothelial Injury in the Early Phase of Acute Pancreatitis: Clinical Implications. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 27. | Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137:1509-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Shahbaz AU, Zhao T, Zhao W, Johnson PL, Ahokas RA, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Calcium and zinc dyshomeostasis during isoproterenol-induced acute stressor state. Am J Physiol Heart Circ Physiol. 2011;300:H636-H644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Lin HY, Lai JI, Lai YC, Lin PC, Chang SC, Tang GJ. Acute renal failure in severe pancreatitis: A population-based study. Ups J Med Sci. 2011;116:155-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Kuśnierz-Cabala B, Gala-Błądzińska A, Mazur-Laskowska M, Dumnicka P, Sporek M, Matuszyk A, Gil K, Ceranowicz P, Walocha J, Kucharz J, Pędziwiatr M, Bartuś K, Trąbka R, Kuźniewski M. Serum Uromodulin Levels in Prediction of Acute Kidney Injury in the Early Phase of Acute Pancreatitis. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Chai X, Huang HB, Feng G, Cao YH, Cheng QS, Li SH, He CY, Lu WH, Qin MM. Baseline Serum Cystatin C Is a Potential Predictor for Acute Kidney Injury in Patients with Acute Pancreatitis. Dis Markers. 2018;2018:8431219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Siddappa PK, Kochhar R, Sarotra P, Medhi B, Jha V, Gupta V. Neutrophil gelatinase-associated lipocalin: An early biomarker for predicting acute kidney injury and severity in patients with acute pancreatitis. JGH Open. 2019;3:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Huang HL, Nie X, Cai B, Tang JT, He Y, Miao Q, Song HL, Luo TX, Gao BX, Wang LL, Li GX. Procalcitonin levels predict acute kidney injury and prognosis in acute pancreatitis: a prospective study. PLoS One. 2013;8:e82250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |