Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10828
Peer-review started: July 23, 2021
First decision: August 19, 2021
Revised: August 31, 2021
Accepted: October 20, 2021
Article in press: October 20, 2021
Published online: December 16, 2021
Processing time: 140 Days and 3.2 Hours
Synthetic magnetic resonance imaging (MRI) MAGnetic resonance imaging compilation (MAGiC) is a new MRI technology. Conventional T1, T2, T2-fluid-attenuated inversion recovery (FLAIR) contrast images, quantitative images of T1 and T2 mapping, and MAGiC phase sensitive inversion recovery (PSIR) Vessel cerebrovascular images can be obtained simultaneously through post-processing at the same time after completing a scan. In recent years, studies have reported that MAGiC can be applied to patients with acute ischemic stroke. We hypothe
To explore the application value of vascular images obtained by synthetic MRI in diagnosing acute ischemic stroke.
A total of 64 patients with acute ischemic stroke were selected and examined by MRI in the current retrospective cohort study. The scanning sequences included traditional T1, T2, and T2-FLAIR, three-dimensional time-of-flight magnetic resonance angiography (3D TOF MRA), diffusion-weighted imaging (DWI), and synthetic MRI. Conventional contrast images (T1, T2, and T2-FLAIR) and intracranial vessel images (MAGiC PSIR Vessel] were automatically reconstructed using synthetic MRI raw data. The contrast-to-noise ratio (CNR) values of traditional T1, T2, and T2-FLAIR images and MAGiC reconstructed T1, T2, and T2-FLAIR images in DWI diffusion restriction areas were measured and compared. MAGiC PSIR Vessel and TOF MRA images were used to measure and calculate the stenosis degree of bilateral middle cerebral artery stenosis areas. The consistency of MAGiC PSIR Vessel and TOF MRA in displaying the degree of vascular stenosis with computed tomography angiography (CTA) was compared.
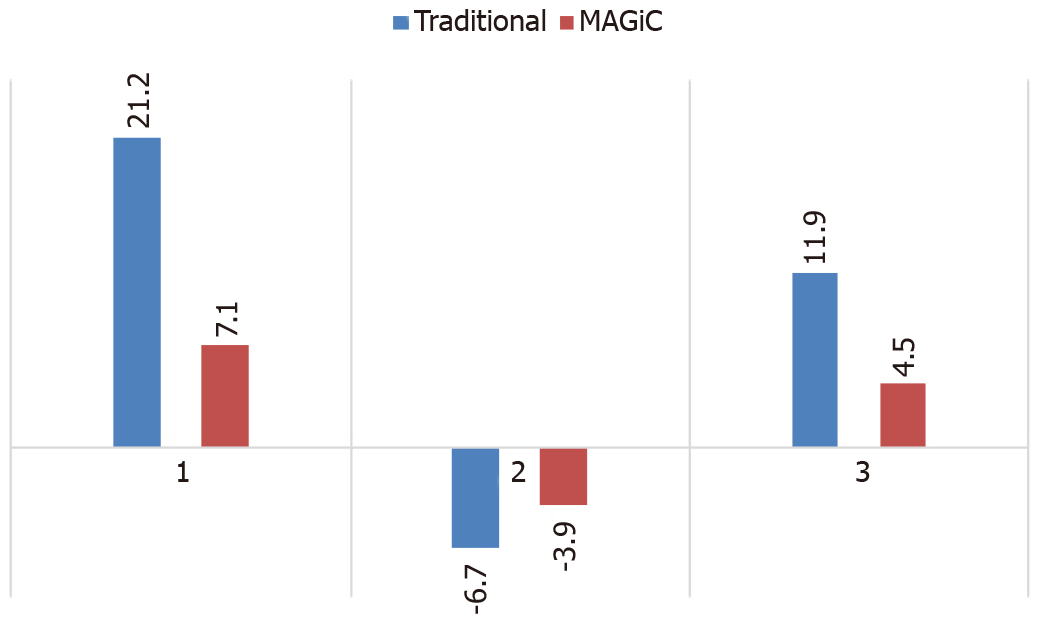
Among the 64 patients with acute ischemic stroke, 79 vascular stenosis areas showed that the correlation between MAGiC PSIR Vessel and CTA (r = 0.90, P < 0.01) was higher than that between TOF MRA and CTA (r = 0.84, P < 0.01). With a degree of vascular stenosis > 50% assessed by CTA as a reference, the area under the receiver operating characteristic (ROC) curve of MAGiC PSIR Vessel [area under the curve (AUC) = 0.906, P < 0.01] was higher than that of TOF MRA (AUC = 0.790, P < 0.01). Among the 64 patients with acute ischemic stroke, 39 were scanned for traditional T1, T2, and T2-FLAIR images and MAGiC images simultaneously, and CNR values in DWI diffusion restriction areas were measured, which were: Traditional T2 = 21.2, traditional T1 = -6.7, and traditional T2-FLAIR = 11.9; and MAGiC T2 = 7.1, MAGiC T1 = -3.9, and MAGiC T2-FLAIR = 4.5.
The synthetic MRI vascular screening scheme for patients with acute ischemic stroke can accurately evaluate the degree of bilateral middle cerebral artery stenosis, which is of great significance to early thrombolytic interventional therapy and improving patients’ quality of life.
Core Tip: This study evaluated the application value of vascular images obtained by synthetic magnetic resonance imaging in diagnosing acute ischemic stroke. Synthetic magnetic resonance imaging can obtain vascular images simultaneously as T2, T1, T2-fluid-attenuated inversion recovery and other contrast images. Compared with the results of computed tomography angiography examination, we found that the vascular images can be used to positively evaluate the degree of intracranial bilateral middle cerebral artery stenosis, which is of great significance to early thrombolytic interventional therapy and improving patients’ quality of life.
- Citation: Wang Q, Wang G, Sun Q, Sun DH. Application of MAGnetic resonance imaging compilation in acute ischemic stroke. World J Clin Cases 2021; 9(35): 10828-10837
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10828.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10828
Cerebrovascular disease is the second deadliest disease globally, and acute cerebro
MAGnetic resonance imaging compilation (MAGiC) is a newly emerging synthetic MRI technology. While completing one scan, the technician can acquire conventional T1, T2, T2-FLAIR, and other contrast images, quantitative T1 mapping and T2 mapping images, as well as MAGiC phase-sensitive inversion recovery (PSIR) Vessel cerebrovascular images simultaneously through post-processing, which significantly shortens the scanning time required for magnetic resonance examination[11-14]. In recent years, some researchers reported that MAGiC can be used to reconstruct various contrast images that can be applied in patients with acute ischemic stroke, and T2 mapping images acquired by MAGiC can more accurately evaluate stroke onset time[15,16]. However, studies evaluating the clinical application of intracranial vascular images acquired by MAGiC have not been reported.
This study aimed to compare the accuracy of MAGiC PSIR Vessel and TOF MRA in evaluating the stenosis degree of bilateral middle cerebral arteries, and to further explore the application value of MAGiC in acute ischemic stroke.
A total of 64 patients with acute ischemic stroke diagnosed at the neurology department of our hospital (The Stroke Hospital of Liaoning Province, Liaoning Province, China) from November 2020 to May 2021 were retrospectively analyzed (all conforming to the 2018 edition of Chinese Guidelines for Diagnosis and Treatment of Acute Ischemic Stroke), including 44 males and 20 females, aged from 41 to 78 years (average age: 58 years). Upon admission, all patients underwent multi-sequence brain MRI scanning (including DWI, TOF MRA, and MAGiC), and computed tomography angiography (CTA) scanning was performed within 3 d after MRI examination. The post-processing images of DWI, TOF MRA, synthetic MRI, and CTA were retrospectively analyzed. All patients signed an informed consent form before examinations.
In this study, a SIGNAPioneer 3.0T MR scanner (GE, USA) with a 21-channel head phased-array coil was used. The main scanning sequences are shown in Table 1.
| Slice thickness (mm) | Matrix (mm) | TR (ms) | TE (ms) | Acquisition time (min) | FOV (cm) | |
| DWI | 5 | 130 × 160 | 4221 | 80.2 | 0:34 | 24 |
| TOF MRA | 1.4 | 384 × 224 | 19 | 3.4 | 3:32 | 24 |
| Synthetic MRI (MAGiC) | 1.6 | 320 × 192 | 7365 | 12.9/90.1 | 4:25 | 24 |
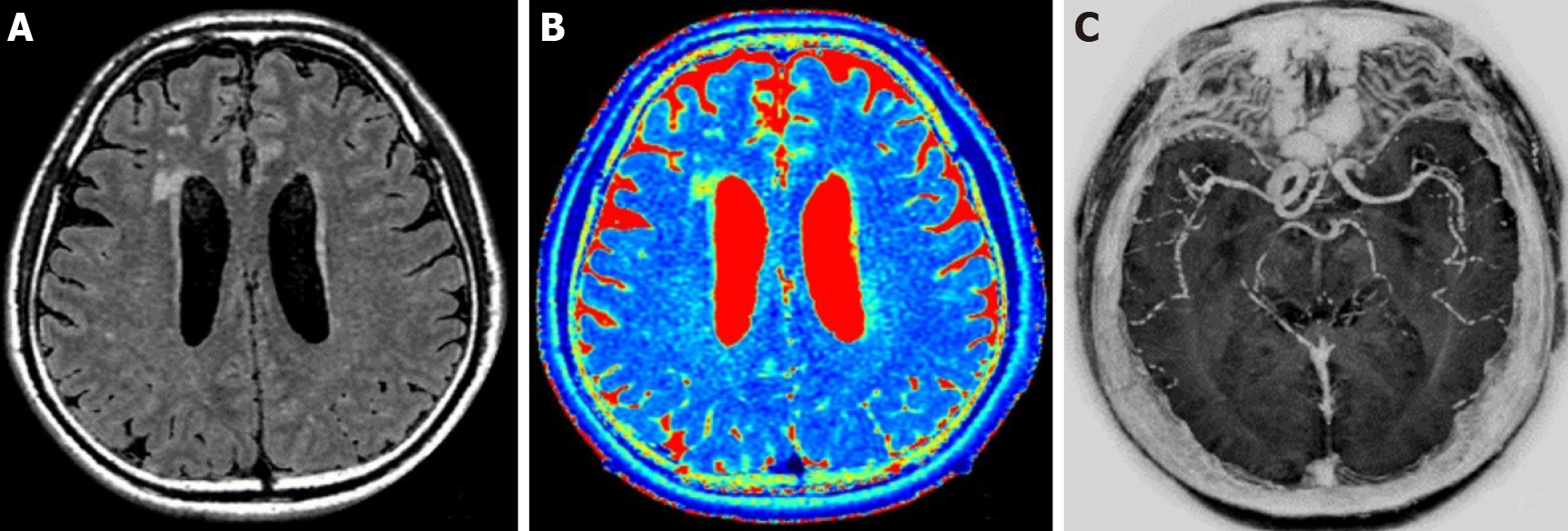
MAGiC original image and images (T1, T2, T2-FLAIR, T2 mapping, and MAGiC PSIR Vessel) automatically generated with the post-processing software supplied with the GE host after scanning were analyzed (Figure 1).
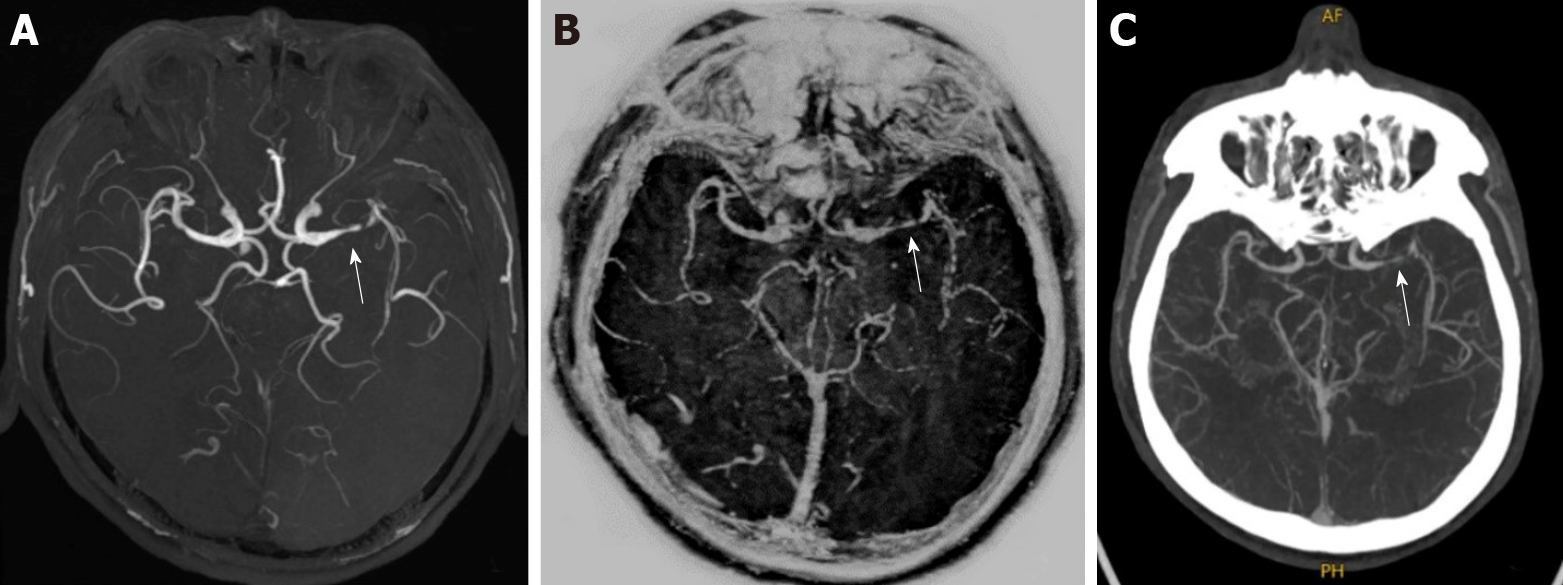
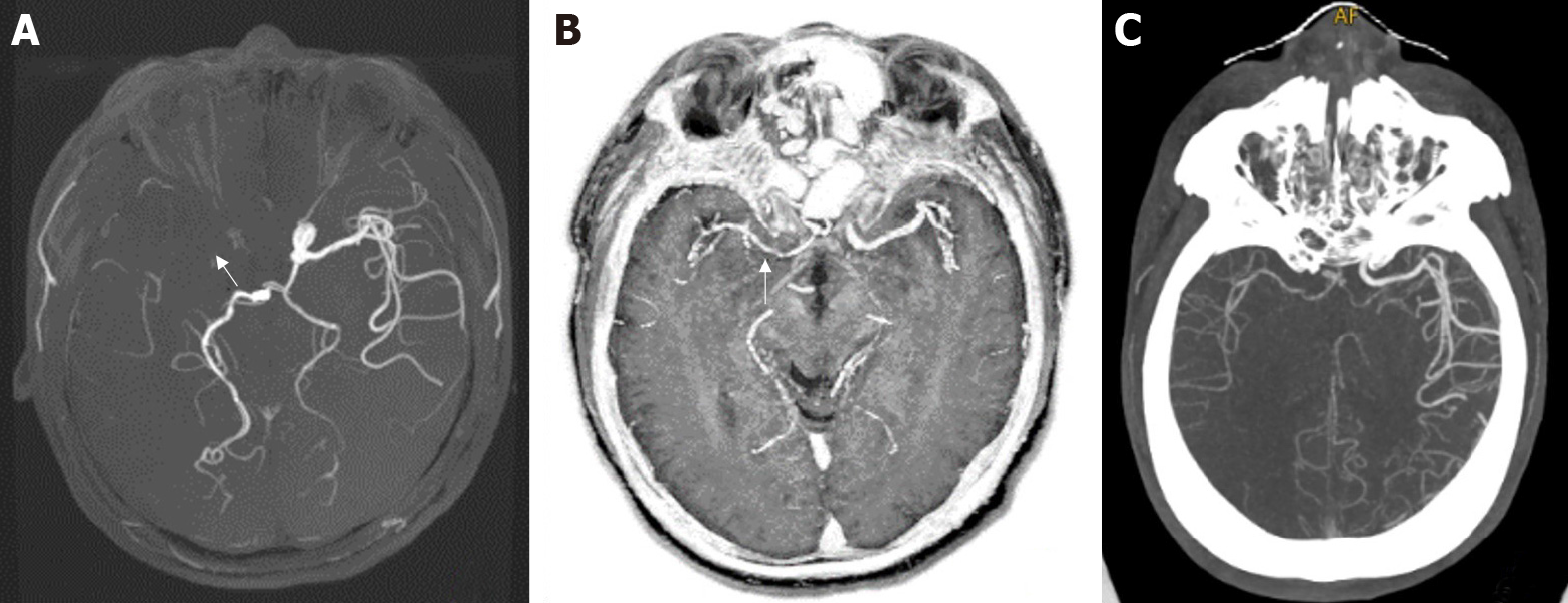
The 3D TOF MRA and MAGiC PSIR Vessel images were post-processed with Reformat software of GE ADW4.7 workstation. The consistency of the two examination methods with CTA in evaluating the degrees of intracranial vascular stenosis was investigated.
The degree of vascular stenosis was calculated as (1-diameter of the lumen at stenosis/diameter of the lumen of an adjacent normal blood vessel) × 100%.
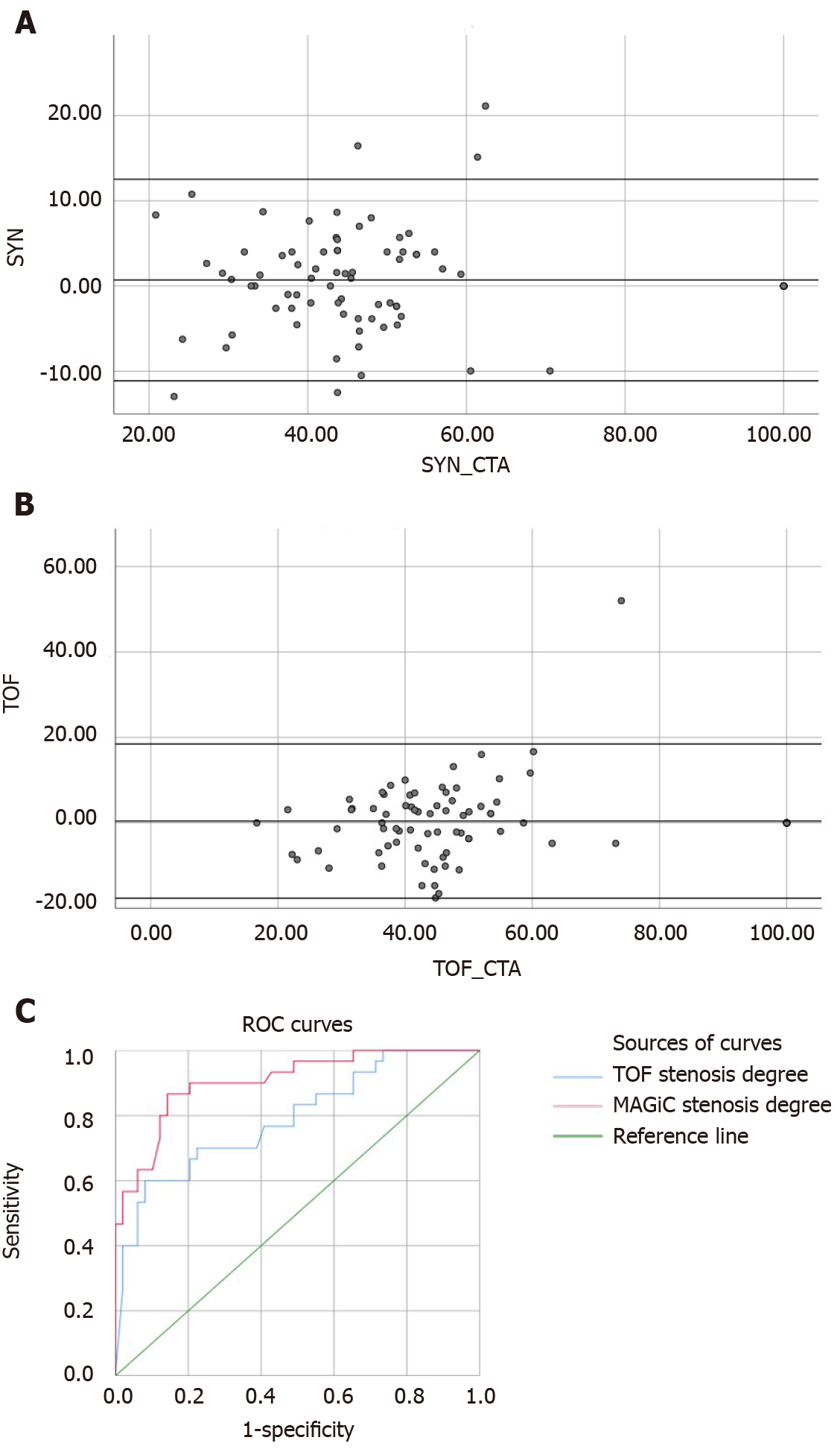
With CTA vascular stenosis degree greater than 50% as the classification point, receiver operating characteristic (ROC) curves were plotted for the two examination methods to calculate the area under the curve (AUC).
The method for measuring contrast-to-noise ratio (CNR) values in DWI diffusion restriction areas of traditional T1, T2, and T2-FLAIR images as well as MAGiC T1, T2, and T2-FLAIR images was as follows: CNR = (mean intensity of stroke lesion - mean intensity of thalamus)/standard deviation of the thalamus.
All quantitative data were analyzed and processed with SPSS25.0 statistical software. With CTA as the control, the consistency and correlation of the two examination methods TOF MRA and MAGiC PSIR Vessel with CTA in evaluating the degree of vascular stenosis were evaluated by Bland-Altman plots and Spearman correlation analysis, respectively. The significant difference was set as P < 0.05.
Figure 2 and Figure 3 show the evaluation of intracranial vascular stenosis degree by 3D TOF MRA, MAGiC PSIR Vessel, and CTA. The correlation between MAGiC PSIR Vessel and CTA (r = 0.90, P < 0.01) was higher than that between TOF MRA and CTA (r = 0.84, P < 0.01). The area under the ROC curve of MAGiC PSIR Vessel (AUC = 0.906, P < 0.01) was higher than that of TOF MRA (AUC = 0.790, P < 0.01), as shown in Figure 4.
MAGiC-reconstructed multi-contrast images had reduced CNR values of DWI diffusion restriction areas than the traditional multi-contrast images (traditional T2 = 21.2, traditional T1 = -6.7, and traditional T2-FLAIR = 11.9; MAGiC T2 = 7.1, MAGiC T1 = -3.9, and MAGiC T2-FLAIR = 4.5; Figure 5). In addition, two experienced diagnosticians respectively evaluated whether the images obtained by the two methods could meet the clinical diagnostic requirements of stroke. The results showed that both methods could meet the clinical diagnostic requirements.
Synthetic MRI MAGiC is a quantitative MRI technique, which can generate a variety of conventional contrast images (T1, T2, T2-FLAIR, etc.), quantitative images (T1 mapping and T2 mapping), and intracranial vessel images (PSIR Vessel) simultaneously in a single scan by acquiring the T1 relaxation rate, T2 relaxation rate, and PD density value of tissues, and has been applied in many sites such as nerves and joints[17-26]. Among them, research has been conducted on the application of T2 mapping in the nervous system, such as the evaluation of edema around tumors and showed epileptic lesions. In recent years, in the imaging diagnosis of stroke, many studies have been conducted to apply synthetic MRI imaging technology. For example, T2 mapping quantitative images acquired by MAGiC could more accurately evaluate stroke onset time[27-29].
MRI can provide important imaging evidence in the prevention, diagnosis, and treatment of cerebrovascular diseases. However, its long scanning time, especially in patients with hyperacute stroke, contradicts the clinical need to carry out treatment as soon as possible. For example, the evaluation of vascular stenosis degree in acute stroke is of great value in defining the etiology and responsible vessels and guiding the selection of subsequent clinical treatment regimens. However, traditional TOF MRA scanning usually takes 3 to 5 min; therefore, a vascular imaging technique with a shorter scanning time is needed in MRI examination. MAGiC PSIR Vessel intracranial blood vessel images are generated simultaneously with conventional T1, T2, and other contrast images, as well as T1 mapping, T2 mapping, and other quantitative images, which can be initially used for screening intracranial blood vessels without occupying additional scanning time. Its imaging principle is based on the use of difference in phase information between flowing blood flow and stationary tissues to image blood vessels, reducing the influence of hemodynamics on blood vessel imaging. In contrast, traditional TOF MRA imaging technology uses the enhancement effect of blood inflow to obtain blood vessel images, which will be affected by changes in hemodynamics. In the area of vascular stenosis, the degree of stenosis may be overestimated due to turbulent or slow blood flow[30-34]. This study revealed that with CTA results as a reference, the area under ROC curve of MAGiC PSIR Vessel examination was higher than that of traditional TOF MRA examinations in patients with vascular stenosis greater than 50%. Some patients with vascular occlusion not displayed by TOF MRA only showed moderate to severe stenosis on MAGiC PSIR Vessel and CTA images. In terms of scanning time, the scanning time of MAGiC was about 4.5 min, and the scanning time of MAGiC combined with DWI was about 5 min, which was significantly less than that of the traditional scanning schemes of TOF MRA and DWI combined with T1, T2, and T2-FLAIR (usually more than 10 min), which is conducive to improving the MRI examination efficiency for patients with acute stroke and treatment window for clinical thrombolytic intervention as soon as possible.
To obtain a clear enough blood vessel display, the slice thickness of the MAGiC image was set at 1.6 mm, which made the CNR values of T1, T2, and T2-FLAIR images generated by MAGiC decrease compared with traditional images due to the influence of the decrease in image signal-to-noise ratio. However, the overall display of lesions could meet the diagnostic needs of stroke. In addition, the slices in the MAGiC image were thinner.
There were some limitations to this study. First, this is a retrospective study, and some patients needed to complete CTA and MRI examinations simultaneously, which might result in selection bias. Meanwhile, due to this reason, not all the patients could be scored by the National Institute of Health stroke scale. Second, the number of cases was relatively small, which was mainly because MRI is not widely used in the diagnosis and treatment of acute stroke in clinical departments, as it is considered that the scanning time of this technique is relatively long, which may delay the diagnosis and treatment time of patients. Third, this study did not evaluate the long-term outcomes of patients, e.g., the proportion of recurrent stroke in patients treated with vascular recanalization. The evaluation of long-term outcomes of patients can further clarify the relationships of vascular stenosis degree judgment with cerebrovascular recanalization treatment and stroke recurrence.
In conclusion, MAGiC can simultaneously obtain a variety of conventional contrast images (T1, T2, T2-FLAIR, etc.), intracranial vessel images (MAGiC PSIR Vessel), and both T2 and T1 relaxation time quantitative images (T2 mapping and T1 mapping) in one scan, which can accurately determine the onset time of stroke, preliminarily screen intracranial vessels, and further shorten the MRI examination time in patients with acute stroke, thereby guiding clinical thrombolytic intervention as early as possible and improve patients’ quality of life.
Synthetic magnetic resonance imaging (MRI) MAGnetic resonance imaging compilation (MAGiC) is a new MRI technology. While completing one scan, the technician can acquire conventional T1, T2, T2-fluid-attenuated inversion recovery (FLAIR), and other contrast images, quantitative T1 mapping and T2 mapping images, as well as MAGiC phase-sensitive inversion recovery (PSIR) Vessel cerebrovascular images simultaneously through post-processing, which significantly shortens the scanning time required for MRI examination.
This study evaluated the application value of vascular images obtained by synthetic MRI in diagnosing acute ischemic stroke.
We hypothesized that the synthetic MRI vascular screening scheme can evaluate the degree of cerebral artery stenosis in patients with acute ischemic stroke.
The contrast-to-noise ratio (CNR) values of traditional T1, T2, and T2-FLAIR images and MAGiC reconstructed T1, T2, and T2-FLAIR images in DWI diffusion restriction areas were measured and compared. MAGiC PSIR Vessel and time-of-flight magnetic resonance angiography (TOF MRA) images were used to measure and calculate the stenosis degree of bilateral middle cerebral artery stenosis areas. The consistency of MAGiC PSIR Vessel and TOF MRA in displaying the degree of vascular stenosis with computed tomography angiography was compared.
Magnetic resonance imaging can provide important imaging evidence in the prevention, diagnosis, and treatment of cerebrovascular diseases. However, its long scanning time, especially in patients with hyperacute stroke, contradicts the clinical need to carry out treatment as soon as possible. MAGiC PSIR Vessel images are generated simultaneously with conventional T1, T2, and other contrast images, as well as T1 mapping, T2 mapping, and other quantitative images, which can be initially used for screening intracranial blood vessels without occupying additional scanning time.
MAGiC can simultaneously obtain a variety of conventional contrast images (T1, T2, T2-FLAIR, etc.), intracranial vessel images (MAGiC PSIR Vessel), and both T2 and T1 relaxation time quantitative images (T2 mapping, T1 mapping) in one scan, which can accurately determine the onset time of stroke, preliminarily screen intracranial vessels, and further shorten the magnetic resonance imaging examination time in patients with acute stroke.
The evaluation of long-term outcomes of patients can further clarify the relationships of vascular stenosis degree judgment with cerebrovascular recanalization treatment and stroke recurrence.
We would like to thank Mr. Fei Bie for MRI technical support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Radiology, Nuclear Medicine and Medical Imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Battaglini D S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Wang JL
| 1. | Mehanna R, Jankovic J. Movement disorders in cerebrovascular disease. Lancet Neurol. 2013;12:597-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Katan M, Luft A. Global Burden of Stroke. Semin Neurol. 2018;38:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 1255] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 3. | Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, Gorelick PB, Greenberg SM, Higashida RT, Kasner SE, Seshadri S; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Functional Genomics and Translational Biology; and Council on Hypertension. Prevention of Stroke in Patients With Silent Cerebrovascular Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2017;48:e44-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 4. | Smajlović D. Strokes in young adults: epidemiology and prevention. Vasc Health Risk Manag. 2015;11:157-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 5. | Mair G, Wardlaw JM. Imaging of acute stroke prior to treatment: current practice and evolving techniques. Br J Radiol. 2014;87:20140216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG; DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3099] [Cited by in RCA: 3812] [Article Influence: 544.6] [Reference Citation Analysis (0)] |
| 7. | Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG; DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2796] [Cited by in RCA: 3434] [Article Influence: 490.6] [Reference Citation Analysis (0)] |
| 8. | Rücker V, Heuschmann PU, O'Flaherty M, Weingärtner M, Hess M, Sedlak C, Schwab S, Kolominsky-Rabas PL. Twenty-Year Time Trends in Long-Term Case-Fatality and Recurrence Rates After Ischemic Stroke Stratified by Etiology. Stroke. 2020;51:2778-2785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Wheaton AJ, Miyazaki M. Non-contrast enhanced MR angiography: physical principles. J Magn Reson Imaging. 2012;36:286-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Anzalone N, Scomazzoni F, Castellano R, Strada L, Righi C, Politi LS, Kirchin MA, Chiesa R, Scotti G. Carotid artery stenosis: intraindividual correlations of 3D time-of-flight MR angiography, contrast-enhanced MR angiography, conventional DSA, and rotational angiography for detection and grading. Radiology. 2005;236:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Blystad I, Warntjes JBM, Smedby Ö, Lundberg P, Larsson EM, Tisell A. Quantitative MRI for analysis of peritumoral edema in malignant gliomas. PLoS One. 2017;12:e0177135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Hagiwara A, Hori M, Yokoyama K, Takemura MY, Andica C, Tabata T, Kamagata K, Suzuki M, Kumamaru KK, Nakazawa M, Takano N, Kawasaki H, Hamasaki N, Kunimatsu A, Aoki S. Synthetic MRI in the Detection of Multiple Sclerosis Plaques. AJNR Am J Neuroradiol. 2017;38:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Granberg T, Uppman M, Hashim F, Cananau C, Nordin LE, Shams S, Berglund J, Forslin Y, Aspelin P, Fredrikson S, Kristoffersen-Wiberg M. Clinical Feasibility of Synthetic MRI in Multiple Sclerosis: A Diagnostic and Volumetric Validation Study. AJNR Am J Neuroradiol. 2016;37:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Jung Y, Gho SM, Back SN, Ha T, Kang DK, Kim TH. The feasibility of synthetic MRI in breast cancer patients: comparison of T2 relaxation time with multiecho spin echo T2 mapping method. Br J Radiol. 2018;20180479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Duchaussoy T, Budzik JF, Norberciak L, Colas L, Pasquini M, Verclytte S. Synthetic T2 mapping is correlated with time from stroke onset: a future tool in wake-up stroke management? Eur Radiol. 2019;29:7019-7026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Li CW, Hsu AL, Huang CC, Yang SH, Lin CY, Shieh CC, Chan WP. Reliability of Synthetic Brain MRI for Assessment of Ischemic Stroke with Phantom Validation of a Relaxation Time Determination Method. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG; Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1204] [Cited by in RCA: 1179] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 18. | McGarry BL, Jokivarsi KT, Knight MJ, Grohn OHJ, Kauppinen RA. Magnetic Resonance Imaging Protocol for Stroke Onset Time Estimation in Permanent Cerebral Ischemia. J Vis Exp. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Seiler A, Lauer A, Deichmann R, Nöth U, You SJ, Pfeilschifter W, Singer OC, Pilatus U, Wagner M. Complete Restitution of the Ischemic Penumbra after Successful Thrombectomy : A Pilot Study Using Quantitative MRI. Clin Neuroradiol. 2019;29:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Drake-Pérez M, Delattre BMA, Boto J, Fitsiori A, Lovblad KO, Boudabbous S, Vargas MI. Normal Values of Magnetic Relaxation Parameters of Spine Components with the Synthetic MRI Sequence. AJNR Am J Neuroradiol. 2018;39:788-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Namer IJ, Waydelich R, Armspach JP, Hirsch E, Marescaux C, Grucker D. Contribution of T2 relaxation time mapping in the evaluation of cryptogenic temporal lobe epilepsy. Neuroimage. 1998;7:304-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Jackson GD, Connelly A, Duncan JS, Grünewald RA, Gadian DG. Detection of hippocampal pathology in intractable partial epilepsy: increased sensitivity with quantitative magnetic resonance T2 relaxometry. Neurology. 1993;43:1793-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 224] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Gonçalves FG, Serai SD, Zuccoli G. Synthetic Brain MRI: Review of Current Concepts and Future Directions. Top Magn Reson Imaging. 2018;27:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Kumar NM, Fritz B, Stern SE, Warntjes JBM, Lisa Chuah YM, Fritz J. Synthetic MRI of the Knee: Phantom Validation and Comparison with Conventional MRI. Radiology. 2018;289:465-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Hagiwara A, Warntjes M, Hori M, Andica C, Nakazawa M, Kumamaru KK, Abe O, Aoki S. SyMRI of the Brain: Rapid Quantification of Relaxation Rates and Proton Density, With Synthetic MRI, Automatic Brain Segmentation, and Myelin Measurement. Invest Radiol. 2017;52:647-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 26. | Sanoussi S, Comet C, Kaefer K, Attou R, De Bels D, Gazagnes MD, Honoré PM, Redant S. Can Magnetic Resonance Imaging make the Differential Diagnosis between Cerebral Ischemia and Epilepsy? J Transl Int Med. 2019;7:123-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Andica C, Hagiwara A, Hori M, Kamagata K, Koshino S, Maekawa T, Suzuki M, Fujiwara H, Ikeno M, Shimizu T, Suzuki H, Sugano H, Arai H, Aoki S. Review of synthetic MRI in pediatric brains: Basic principle of MR quantification, its features, clinical applications, and limitations. J Neuroradiol. 2019;46:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Ryu KH, Baek HJ, Moon JI, Choi BH, Park SE, Ha JY, Jeon KN, Bae K, Choi DS, Cho SB, Lee Y, Heo YJ. Initial clinical experience of synthetic MRI as a routine neuroimaging protocol in daily practice: A single-center study. J Neuroradiol. 2020;47:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Chougar L, Hagiwara A, Takano N, Andica C, Cohen-Adad J, Warntjes M, Maekawa T, Hori M, Koshino S, Nakazawa M, Abe O, Aoki S. Signal Intensity within Cerebral Venous Sinuses on Synthetic MRI. Magn Reson Med Sci. 2020;19:56-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Igase K, Igase M, Matsubara I, Sadamoto K. Mismatch between TOF MR Angiography and CT Angiography of the Middle Cerebral Artery may be a Critical Sign in Cerebrovascular Dynamics. Yonsei Med J. 2018;59:80-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Dündar TT, Aralaşmak A, Özdemir H, Seyithanoğlu MH, Uysal Ö, Toprak H, Kitiş S, Özek E, Alkan A. Comparison of TOF MRA, Contrast-Enhanced MRA and Subtracted CTA from CTP in Residue Evaluation of Treated Intracranial Aneurysms. Turk Neurosurg. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Tomura N, Kokubun M, Horiuchi K, Watanabe Z. Comparison of TOF-MRA and silent scan-MRA in depicting cerebral arteries in patients with Moyamoya disease. Acta Radiol. 2019;60:1321-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Zhang J, Ding S, Zhao H, Sun B, Li X, Zhou Y, Wan J, Degnan AJ, Xu J, Zhu C. Evaluation of chronic carotid artery occlusion by non-contrast 3D-MERGE MR vessel wall imaging: comparison with 3D-TOF-MRA, contrast-enhanced MRA, and DSA. Eur Radiol. 2020;30:5805-5814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Cirillo M, Scomazzoni F, Cirillo L, Cadioli M, Simionato F, Iadanza A, Kirchin M, Righi C, Anzalone N. Comparison of 3D TOF-MRA and 3D CE-MRA at 3T for imaging of intracranial aneurysms. Eur J Radiol. 2013;82:e853-e859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |