Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10494
Peer-review started: February 15, 2021
First decision: July 16, 2021
Revised: July 20, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: December 6, 2021
Processing time: 288 Days and 6.3 Hours
Anatomical segmentectomy has been proposed as a substitution for lobectomy for early-stage lung cancer. However, it requires technical meticulousness due to the complex anatomical variations of segmental vessels and bronchi.
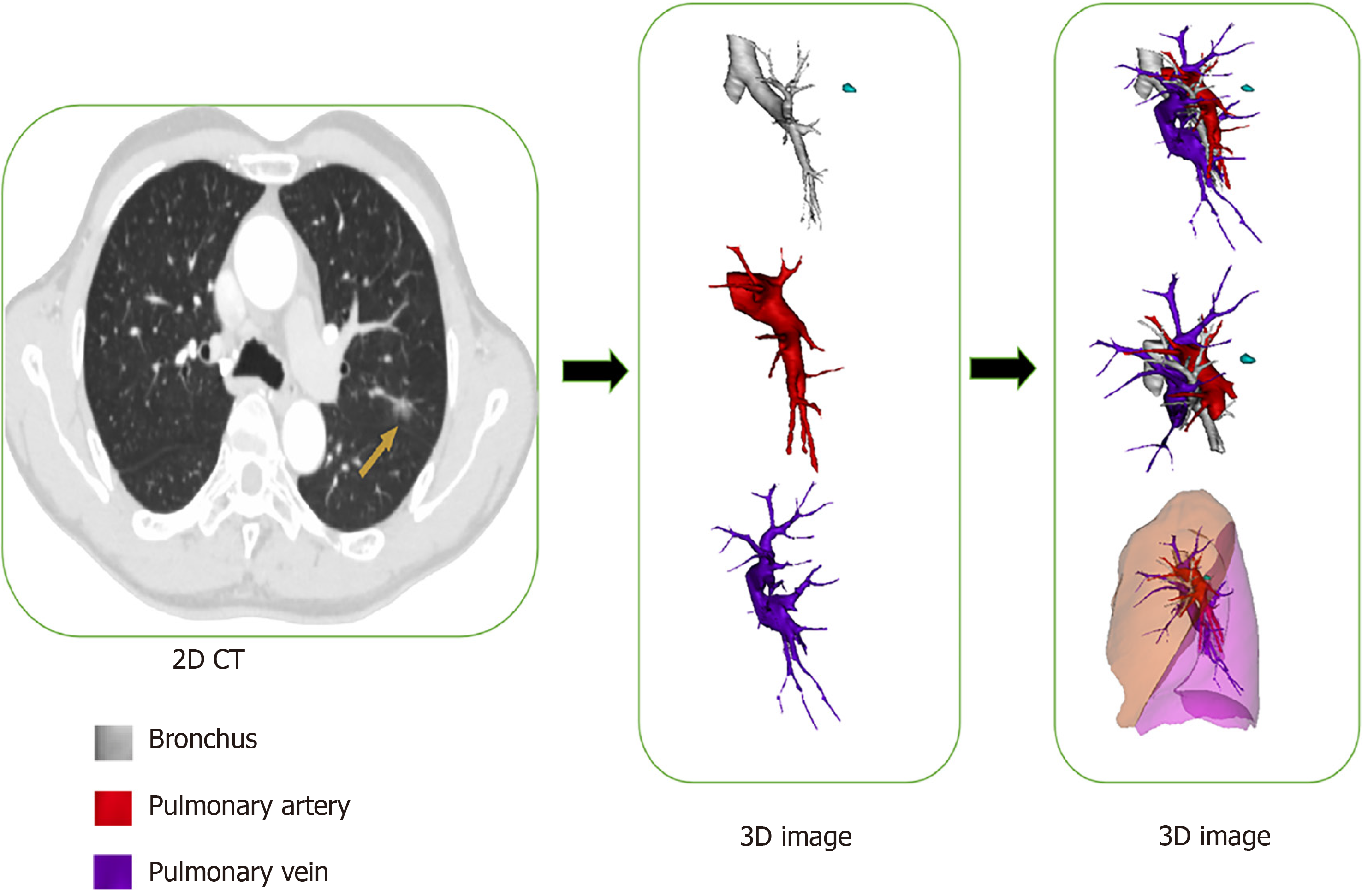
To assess the safety and feasibility of three-dimensional computed-tomography bronchography and angiography (3D-CTBA) in performing video-assisted thoracoscopic surgery (VATS) for lung cancers.
In this study, we enrolled 123 patients who consented to undergo thoracoscopic segmentectomy and lobectomy assisted by 3D-CTBA between May 2017 and June 2019. The image data of enhanced computed tomography (CT) scans was recons
A total of 59 women and 64 men were enrolled, of whom 57 (46.3%) underwent segmentectomy and 66 (53.7%) underwent lobectomy. The majority of tumor appearance on CT was part-solid ground-glass nodule (pGGN; 55.3%). The mean duration of chest tube placement was 3.5 ± 1.6 d, and the average length of postoperative hospital stay was 6.8 ± 1.8 d. Surgical complications included one case of pneumonia and four cases of prolonged air leak lasting > 5 d. Notably, there was no intraoperative massive hemorrhage, postoperative intensive-care unit stay, or 30-d mortality. Preoperative 3D-CTBA images can display clearly and vividly the targeted structure and the variations of vessels and bronchi. To reduce the risk of locoregional recurrence, the application of 3D-CTBA with a virtual 3D surgical margin help the VATS surgeon determine accurate distances and positional relations among the tumor, bronchial trees, and the intersegmental vessels. Three-dimensional navigation was performed to confirm the segmental structure, precisely cut off the targeted segment, and avoid intersegmental veins injury.
VATS and 3D-CTBA worked in harmony in our study. This combination also provided a new pattern of transition from lesion-directed location of tumors to computer-aided surgery for the management of early lung cancer.
Core Tip: To evaluate the therapeutic effect of video-assisted thoracoscopic surgery segmentectomy and lobectomy assisted by three-dimensional computed-tomography bronchography and angiography (3D-CTBA) on 123 patients. Using this method, surgeons can accurately identify and label targeted structures on the 3D-CTBA video and design meticulously to perform minimal unit resection with sufficient surgical margin.
- Citation: Wu YJ, Shi QT, Zhang Y, Wang YL. Thoracoscopic segmentectomy and lobectomy assisted by three-dimensional computed-tomography bronchography and angiography for the treatment of primary lung cancer. World J Clin Cases 2021; 9(34): 10494-10506
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10494.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10494
The increased popularity of health checkups and recent advances in high-resolution computed-tomography (CT) imaging have improved the early detection of lung cancer[1], ushering in a new era of less-invasive surgery for this disease. Currently, video-assisted thoracoscopic surgery (VATS) is being assessed as an alternative to thorac
Anatomical segmentectomy has been proposed as a substitution for lobectomy for early-stage lung cancer, which could produce oncological results equivalent to those of lobectomy[4]. However, there is an ongoing dispute over the safety and outcomes of VATS segmentectomy compared with those of lobectomy[5]. The segmentectomy can retain a maximal proportion of healthy lung tissue, which is beneficial to protecting postoperative lung function and improving quality of life. However, it requires technical meticulousness due to the complex anatomical variations of segmental vessels and bronchi. In addition, it remains highly controversial due to concerns about increased locoregional recurrence of lung cancer, higher rates of complications, and inadequate surgical margin[6]. Therefore, constructing a three-dimensional (3D) image that can provide stereoscopic vision and accurately map out targeted structures is desirable when planning for VATS segmentectomy[7].
Currently, the new 3D imaging software packages widely used across the world are not specifically designed for thoracic surgery. Vessel reconstruction by 3D computed tomography and angiography (3D-CTA) is now performed well, but reconstructions of the bronchus are influenced by multiple factors, and the distance from the lesion to the predetermined cutting margin is hard to measure accurately[8]. Our center is exploring reconstruction of preoperative 3D computed-tomography bronchography and angiography (3D-CTBA) using Mimics software (Materialise, Leuven, Belgium), which offers powerful 3D construction capability and allows for better 3D visualization of pulmonary anatomy. Therefore, we designed this study to evaluate the efficacy of 3D-CTBA using Mimics in performing accurate VATS segmentectomy and lobectomy, employing a series of typical examples.
The study protocol was approved and supervised by the Ethics Committee of the Affiliated Hospital of Yangzhou University, Yangzhou, China. All patients signed an informed-consent form. Between May 2017 and June 2019, we performed VATS segmentectomy and lobectomy via 3D-CTBA on 123 patients. Our inclusion criteria for VATS lobectomy in lung cancer were good lung reserve, clinical T1–T3 N0 stage. Patients with multi-lobe resection, lymph node metastasis, or small-cell lung cancer were excluded. Based on United States National Comprehensive Cancer Network (NCCN) guidelines for lung cancer[9], indications for VATS segmentectomy were as follows: (1) Poor lung reserve or other major comorbidity that contraindicated lobectomy; and (2) Peripheral nodule ≤ 2 cm with at least 1 of the following: (a) Pure adenocarcinoma in situ (AIS) histology; (b) Nodule had ≥ 50% ground-glass appearance (GGO) on CT; and (c) Radiological surveillance confirmed a long doubling time (≥ 400 d). Exclusion criteria included carcinoid tumor, small-cell lung cancer, lymph node metastasis, wedge resections, and multiple primary lung cancer. Data from patient medical records consisted of demographics, surgical technique, duration of surgery, blood loss, perioperative complications, length of postoperative hospital stay, tumor size, and histopathological subtype. The preoperative assessment included enhanced CT of chest and abdomen, flexible bronchoscopy, arterial blood gas analysis, spirometry, echocardiography (ECG), enhanced brain magnetic resonance imaging (EMRI), and positron emission tomography (PET). PET/CT for all patients showed N0 stage, and the final pathological N0-stage was confirmed by pathological examination. We obtained patients’ preoperative characteristics and surgical outcomes from the inpatient database for analysis. Mortality within 30 d was also recorded.
We performed preoperative enhanced-CT scans on all patients using a multidetector CT (MDCT) unit (Somatom Definition Flash; Siemens Healthcare, Erlangen, Germany). Scanning range was from the thoracic-inlet plane to the posterior costodiaphragmatic-angle plane. The thickness of the construction layer was 1.0 mm. Afterward, we transmitted the image data to the computer, saved it in Digital Imaging and Commu
Multidimensional 3D-CTBA views synergistically display the size, shape, and location of the tumor and its relationship to the surrounding structure. Because the lung should be resected with sufficient surgical margin, corresponding arteries, veins, and bronchi should be identified on the 3D images. During the simulation, surgeons can accurately identify and label targeted structures on the 3D-CTBA video and design meticulous plans to perform minimal unit resection with sufficient surgical margin and optimal intersegmental borders to protect postoperative lung function. Meanwhile, the reconstructed 3D-CTBA image can be rotated and displayed horizontally or vertically in the operating room for accurate intraoperative navigation.
We precisely located pulmonary nodules using CT-guided hookwire combined with 3D images. Afterwards, we performed VATS assisted by 3D-CTBA with patients under general anesthesia in the lateral-decubitus position with 1-lung ventilation. We made a 1.5-cm incision for thoracoscopic observation in the 7th or 8th intercostal space (ICS) at the midaxillary line, through which we inserted a 30° thoracoscope. A second incision, about 4 cm long, was made through the 4th or 5th ICS between the anterior axillary and midclavicular lines as the main operating hole. Next, a 2-cm incision was made through the 7th or 8th ICS between the posterior scapular and posterior axillary lines as the auxiliary operating hole. The 3D-CTBA image enabled the surgeon to secure a sufficient surgical margin within which the involved structure could be meticulously resected. Oriented by 3D-CTBA, we maintained the intersegmental veins and defined intersectional boundaries using an improved inflation–deflation method[8]. Anatomical resection of the pulmonary parenchyma was performed along inflation–deflation lines with 2–3 endoscopic staplers. During the procedure of VATS Lobectomy, systematic lymph node dissection was mandatory. The #10, #11, and #5-7 nodes of the left lung were all dissected, while the #10, #11, #2, #4, and #7 nodes of the right lung accepted routine dissection. In segmentectomy, the #10-12 lymph nodes should be dissected. Mediastinal lymph node sampling was performed for #5-7 nodes on the left, and #2, #4, and #7 nodes on the right. We incised the targeted structure using optimal surgical manipulation in order to reduce lung compression and air leakage and to preserve maximal postoperative lung function. At the end of surgery, we inserted a 28 French (28F) chest tube.
The demographic and preoperative characteristics of patients enrolled in this study are listed in Table 1. Patients included a total of 59 women and 64 men, of whom 57 (46.3%) underwent VATS segmentectomy and 66 (53.7%) underwent lobectomy. One patient whose tumor size was 25 mm consented to a compromised segmentectomy due to poor pulmonary reserve. The median age was 61.4 ± 9.8 years (range: 39.0–80.0 years). Tumor appearance on CT was mostly part-solid ground-glass nodule (pGGN; 55.3%); solid nodules were second most common (34.1%), followed by pure ground glass nodule (GGN; 10.6%). Mean duration of chest tube placement was 3.5 ± 1.6 d and the average postoperative hospital stay was 6.8 ± 1.8 d without intensive-care unit (ICU) stay. We pathologically diagnosed 112 cases as adenocarcinoma, including minimally invasive adenocarcinoma (MIA; n = 21), invasive adenocarcinoma (IA; n = 80), and AIS (n = 11). All lymph nodes were pathological negative.
| Variable | Segmentectomy (n = 57) | Lobectomy(n = 66) | Total (n = 123) |
| Age, mean (range), yr | 59.3 ± 10.8 (39.0-75.0) | 63.2 ± 8.5 (44.0-80.0) | 61.4 ± 9.8 (39.0-80.0) |
| Gender | |||
| Female | 31 (54.4%) | 28 (42.4%) | 59 (48.0%) |
| Male | 26 (45.6%) | 38 (57.6%) | 64 (52.0%) |
| Smoking history | 19 (33.3%) | 31 (47.0%) | 50 (40.7%) |
| Comorbidity | |||
| Hypertension | 14 (24.6%) | 11 (16.7%) | 25 (20.3%) |
| Diabetes mellitus | 6 (10.5%) | 4 (6.1%) | 10 (8.1%) |
| COPD | 5 (8.8%) | 7 (10.6%) | 12 (9.8%) |
| Arrhythmia | 1 (1.8%) | 2 (3.0%) | 3 (2.4%) |
| Operative time, mean (range), min | 129.8 ± 16.1 (105-180) | 125.2 ± 11.7 (105-160) | 113.4 ± 19.7 (20-150) |
| Blood loss, mean (range), mL | 48.8 ± 26.2 (20-150) | 79.5 ± 34.0 (20-220) | 65.3 ± 34.2 (20-220) |
| Duration of chest tube placement, mean (range), d | 3.1 ± 1.1 (2.0-9.0) | 3.8 ± 1.9 (2.0-10.0) | 3.5 ± 1.6 (2.0-10.0) |
| Postoperative hospital stay, mean (range), d | 6.4 ± 1.3 (5.0-12.0) | 7.1 ± 2.2 (5.0-15.0) | 6.8 ± 1.8 (5.0-15.0) |
| Tumor diameter, mean (range), mm | 10.5 ± 4.9 (5.0-25.0) | 23.4 ± 12.1 (8.0-60.0) | 17.4 ± 11.4 (5.0-60.0) |
| Appearance on CT | |||
| Pure GGO | 12 (21.1%) | 1 (1.5%) | 13 (10.6) |
| p GGO | 45 (78.9%) | 23 (34.8%) | 68 (55.3%) |
| Solid | 0 (0%) | 42 (63.6%) | 42 (34.1%) |
| Histological types | |||
| Adenocarcinoma | 56 (98.2%) | 56 (84.8%) | 112 (91.1%) |
| AIS | 8 (14.0%) | 3 (4.5%) | 11 (8.9%) |
| MIA | 20 (35.1%) | 1 (1.5%) | 21 (17.1%) |
| IA | 28 (49.1%) | 52 (78.8%) | 80 (65.0%) |
| Squamous cell | 1 (1.8%) | 7 (10.6%) | 8 (6.5%) |
| Others | 0 (0%) | 3 (4.5%) | 3 (2.4%) |
| Postoperative complications | 2 (3.5%) | 8 (12.1%) | 10 (8.1%) |
| Pneumonia | 0 (0%) | 1 (1.5%) | 1 (0.8%) |
| Prolonged air leakage (> 5 d) | 2 (3.5%) | 1 (1.5%) | 3 (2.4%) |
| Chylothorax | 0 (0%) | 6 (9.1%) | 6 (4.9%%) |
| Absent | 55 (96.5%) | 58 (87.9%) | 113 (91.9%) |
| Conversion rate | 0 (0%) | 0 (0%) | 0 (0%) |
| 30-d mortality | 0 (0%) | 0 (0%) | 0 (0%) |
| Postoperative ICU stay | 0 (0%) | 0 (0%) | 0 (0%) |
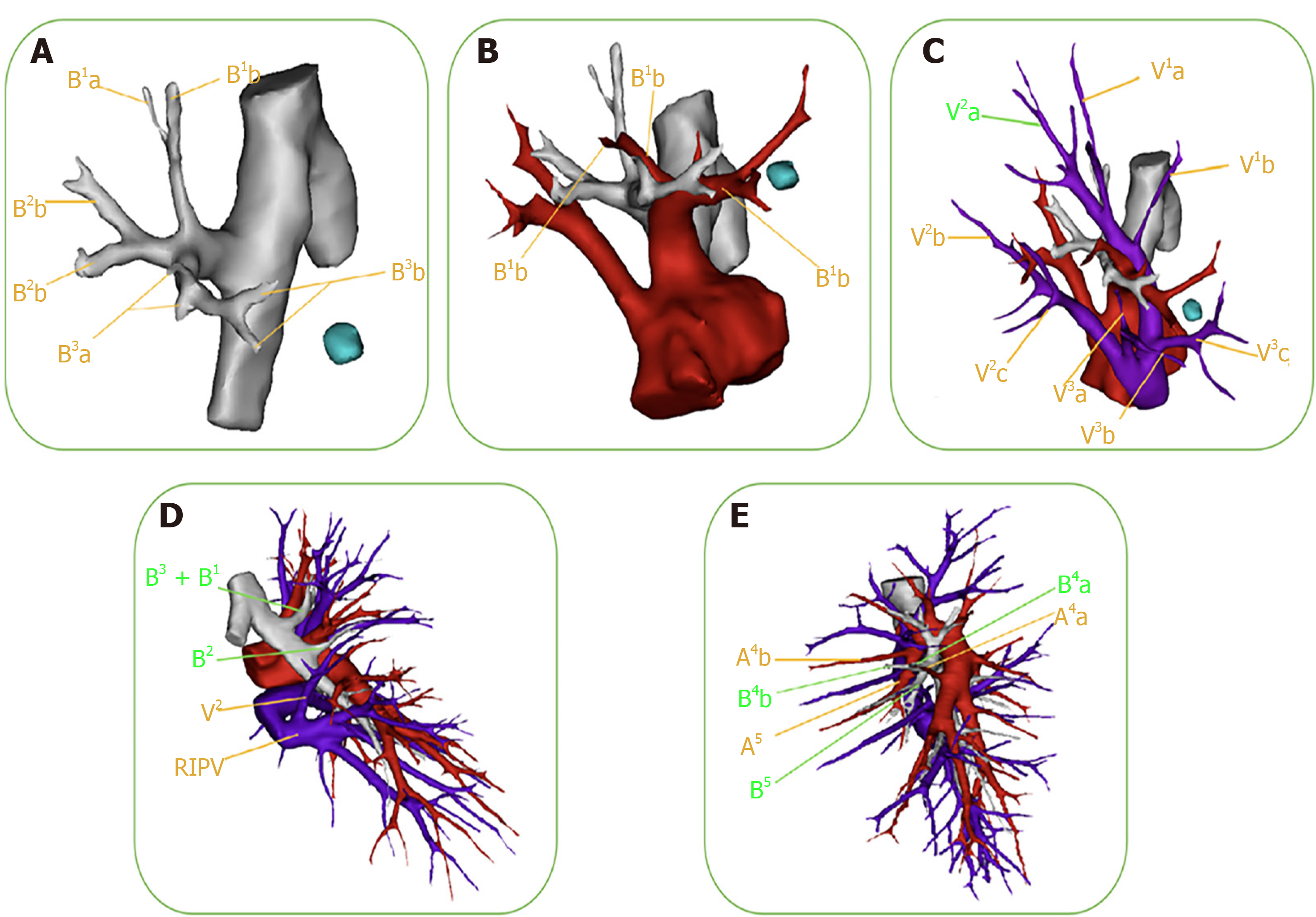
Owing to the complex anatomical variations of segmental vessels and bronchi, thoracoscopic segmentectomy requires more technical meticulousness than lobectomy. Preoperative 3D-CTBA images can clearly and vividly display the targeted structure and variations of vessels and bronchi (Figure 2). One example is variation of the right upper-lobe artery. In the case shown in Figure 2B, the A3a originated from the distal end of the A1, while the enlarged A3b coexisted with the proximal end of the A1. Care should be taken to protect the A1 when dealing with the A3b during the surgery. The 3D image in Figure 2D shows variations of the B2 and V2 in the right upper lobe. The posterior bronchus (B2) branched out separately, but the apical bronchus (B1) and the anterior bronchus (B3) originated from a common stem. When the B1 is resected, the surgeon often accidentally resects the B3 as well. In the same case, the right superior posterior pulmonary vein (V2) merged into the right inferior pulmonary vein (RIPV). When the surgeon deals with the RIPV, the V2 can be mistakenly cut off altogether, leading to postoperative hemoptysis. Confirming anatomical variations before surgery can enable careful surgeon performance and better accuracy and security during the operation.
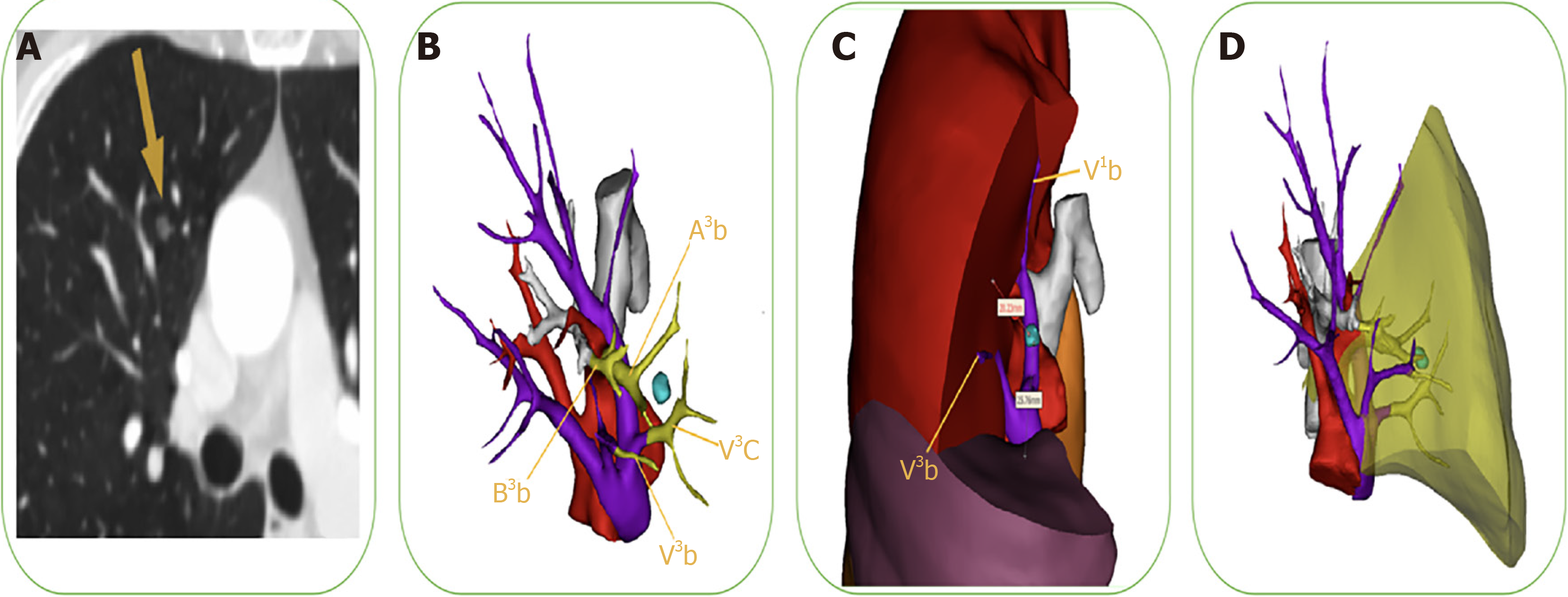
To reduce the risk of locoregional recurrence, preoperative planning is critical to securing a sufficient margin and minimizing anatomical resection of the targeted structure. The safety margin is defined as a sphere extending at least 2 cm outside the lesion or 2 cm greater than tumor size. When the cutting line is beyond the intersegmental plane, extended or combined segmentectomies are required, especially for intersegmental pulmonary nodules. The application of 3D-CTBA with a virtual 3D surgical margin helps the VATS surgeon determine accurate distances and positional relations among the tumor, bronchial trees, and intersegmental vessels. As shown in Figure 3, we precisely identified sufficient surgical margin after calculating the distance from the lesion to the predetermined cutting margin on the 3D-CTBA image. The extent of targeted lung parenchyma was labeled in yellow to facilitate adequate and precise resection.
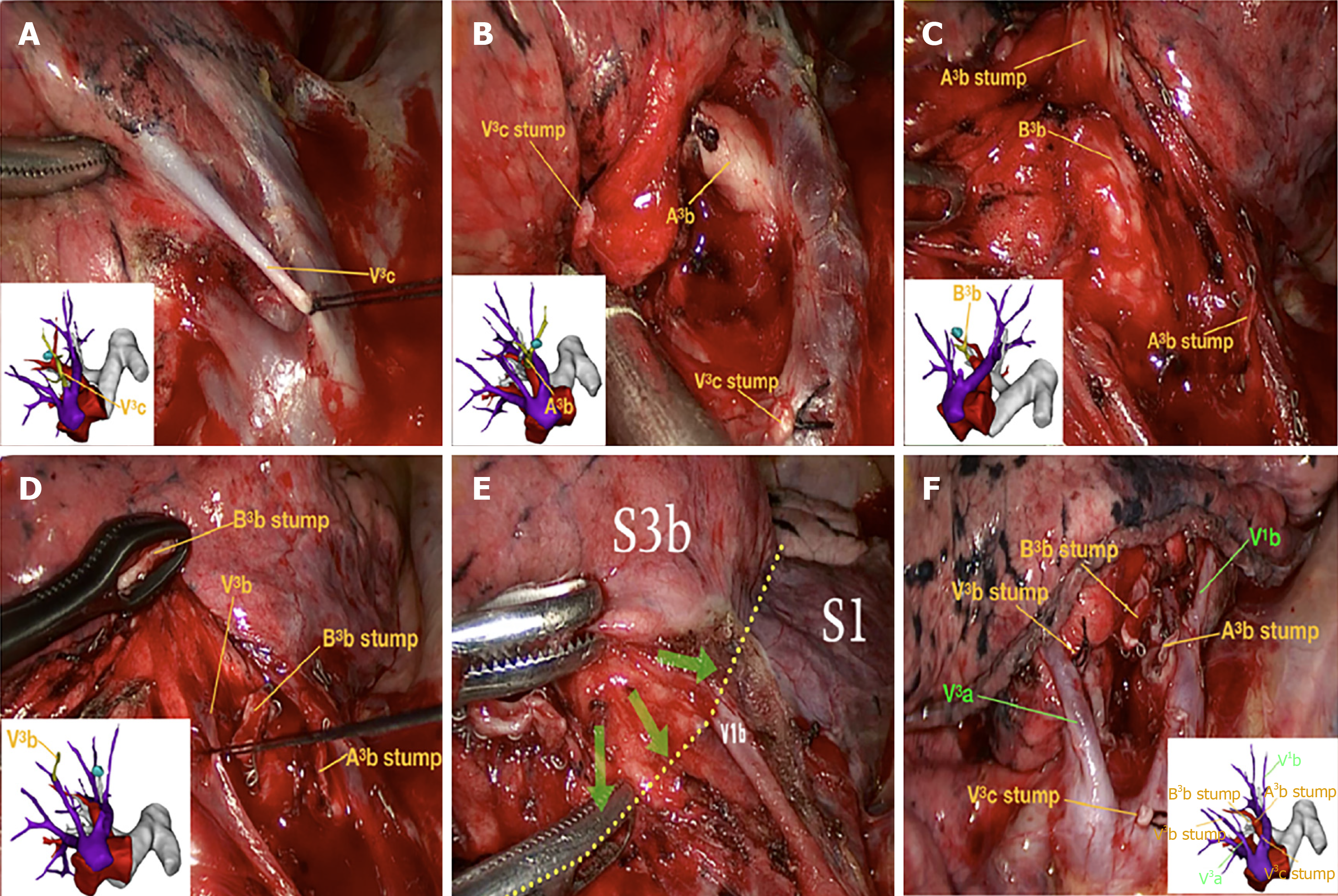
Surgery was conducted according to the designed surgical procedure and with accurate intraoperative guidance by 3D-CTBA. As illustrated in Figure 4, we carefully performed segmentectomy of the RS3b, navigating by 3D-CTBA. A series of techniques were involved in the thoracoscopic segmentectomy, including location of pulmonary nodules, resection of the targeted vessels and bronchi, preservation of intersegmental veins, and identification of the intersegmental demarcation. Images 4A–D (respectively the V3c, A3b, B3b and V3b) indicate the resection sequence for the targeted vessels and bronchi. We defined the intersegmental demarcation (yellow dotted line) using the improved inflation–deflation method, with assistance from 3D-CTBA. Finally, surgeons precisely identified, separated, and dissected the targeted segment based on the cone-shaped principle.
As shown in Table 1, we observed no conversion from segmentectomy or lobectomy to open thoracotomy. The predominant pathology was adenocarcinoma, representing 98.2% of segmentectomy cases and 84.8% of lobectomy cases. Surgical results for all 123 cases were as follows: duration of surgery, mean 113.4 ± 19.7 min (range: 20–150 min); blood loss, mean 65.3 ± 34.2 mL (range: 20–220 mL); duration of chest tube placement, mean 3.5 ± 1.6 d (range: 2–10 d); length of postoperative hospital stay, mean 6.8 ± 1.8 d (range: 5–15 d); and tumor diameter, mean 17.4 ± 11.4 mm (range: 5–60 mm). Surgical complications included one patient with pneumonia and4 patients with prolonged air leak lasting > 5 d, which prolonged their hospital stays. No other complications were observed. Notably, there was no intraoperative massive hemorrhages, postoperative ICU stays, or 30-d mortalities.
Table 2 shows the sites and VATS surgical procedures for the 123 patients who underwent segmentectomy and lobectomy. Anatomical resection of the right upper lobe (n = 20) was the most frequently performed lobectomy, followed by resection of the right lower (n = 12), left lower (n = 12), left upper (n = 12), and right middle (n = 10) lobes. The following segmentectomies were performed: single segmentectomy (n = 36), combined segmentectomy (n = 3), single subsegmentectomy (n = 9), segmentectomy combined with subsegmentectomy (n = 4), combined subsegmentectomy (n = 4), and sub-subsegmentectomy (n = 1). The top three single-segmentectomy sites were the RS2 in the right upper lobe (n = 6), the RS6 in the right lower lobe (n = 5), and the LS4+5 in the left upper lobe (n = 5).
| Site | Segmentectomy (n = 57) | Lobectomy (n = 66) | |||||
| Single seg. | Combined seg. | Single subseg. | Seg. combined with subseg. | Combined subseg. | Sub-subseg. | ||
| Left lung | |||||||
| Upper lobe | 12 | ||||||
| LS1+2b | 1 | ||||||
| LS1+2c | 2 | ||||||
| LS1+2 | 1 | ||||||
| LS1+2 + LS3a | 1 | ||||||
| LS1+2+3 | 3 | ||||||
| LS2 | 1 | ||||||
| LS3a | 1 | ||||||
| LS3a + LS4b | 1 | ||||||
| LS3b | 1 | ||||||
| LS3a+b | 1 | ||||||
| LS3b+c | 2 | ||||||
| LS3 | 2 | ||||||
| LS4+5 | 5 | ||||||
| Lower lobe | 12 | ||||||
| LS6 | 4 | ||||||
| LS8 | 2 | ||||||
| LS8+9+10 | 1 | ||||||
| Right lobe | |||||||
| Upper lobe | 20 | ||||||
| RS1b | 1 | ||||||
| RS1 + RS2a | 3 | ||||||
| RS1 + RS2 | 2 | ||||||
| RS2 | 6 | ||||||
| RS3 | 2 | ||||||
| RS3b | 2 | ||||||
| RS3bii | 1 | ||||||
| Middle lobe | 10 | ||||||
| RS5 | 1 | ||||||
| Lower lobe | 12 | ||||||
| RS6 | 5 | ||||||
| RS6 + RS* | 1 | ||||||
| RS8a | 1 | ||||||
| RS8 | 2 | ||||||
| RS7+8+9+10 | 1 | ||||||
This study analyzed the safety and feasibility of 3D-CTBA in performing VATS segmentectomy and lobectomy for primary lung cancers, including preoperative confirmation of anatomical variations, identification of sufficient surgical margin and intraoperative navigation. The purpose of our study is to assess the use of Mimics software in 3D-CTBA to help surgeons determine accurate distances and positional relations among the tumor, bronchial trees, and intersegmental vessels.
The higher frequency of diagnosis for small lung abnormalities elicits multiple questions about the optimal surgical approach in these patients, which has led to segmentectomy’s reaffirmation as an alternative to traditional lobectomy[10]. Many retrospective studies have shown that the efficacy of minimally invasive segmen
The total mean tumor diameter in our study was 17.4 ± 11.4 mm (range: 5–60 mm). The mean tumor size was 10.5 ± 4.8 mm (range: 5–25 mm) in the segmentectomy group and 23.4 ± 12.1 mm (range: 8–60 mm) in the lobectomy groups. The majority of tumor appearance on CT was pGGN (55.3%) among all patients. The point of controversy is the best management policy for small tumors presenting as pure GGN and pGGN. First, many researchers worry that these small lesions are often overtreated. A recent article by Zhu et al[16] revealed that patients with micro-sized lung adenocarcinomas (≤ 1 cm in diameter) had better 5-year overall and disease-specific survival rates than those with small lung adenocarcinomas (1.1–2.0 cm in diameter), and that a sublobar surgical procedure was feasible. Second, it is difficult to palpate and pinpoint small nodules, some as small as a few millimeters in diameter, due to their nonsolid composition and deep location within the parenchyma. A variety of methods have been used to confirm the location of pulmonary nodules, such as methylene blue and CT-guided hookwire; with these methods, surgeons can solve the problem of wedge resection and lobectomy, rather than segmentectomy and subsegmentectomy[17]. Furthermore, surgeons are often confused by labyrinthine segment structures that cannot even be sufficiently mutually distinguished for segmentectomy or subsegmentectomy, resulting in high frequency of intraoperative and postoperative complications[18]. With 3D-CTBA assistance, the surgeons at our center can ascertain pulmonary-segment anatomy and confirm the location of small nodules; therefore, a segmentectomy can be preoperatively planned and simulated, and then precisely navigated step by step during the operation. In our study, VATS segmentectomy was a meticulously performed procedure with fewer complications and better therapeutic benefit.
Despite the development of high-resolution 2D-CT, it is hard to achieve a vivid composition of the length, angle, dimensions, and direction of the targeted segment[19]. The advent of 3D-CTBA provides more-accurate and detailed 3D imaging of the bronchi and pulmonary vessels of the regional anatomy, which consequently helps thoracic surgeons perfect anatomical orientation for VATS segmentectomy[8,14,15]. The 3D-CTBA method is remarkable not only for its accuracy but also for its feasibility and visualization. Several studies have evaluated the feasibility of using different 3D-imaging software packages for thoracic surgery[8,14,15,20-22]. She et al[15] and Wu et al[14] recommend DeepInsight software (Northeastern University, Shenyang, China). OsiriX is a powerful 3D reconstruction software with which surgeons can easily manipulate and process 2D-CT data into 3D images[15]. Thanks to its stronger 3D reconstruction, Mimics provides a higher quality and completely realistic vision of the bronchi and the vessels, dramatically enhancing the dynamic range of 3D display and thus extending its ability to present images with a high degree of realism and a vivid stereoscopic feeling. This user-friendly software with its advanced image processing tools allows comprehensive information to be generated from images, meeting VATS requirements for surgeons worldwide.
With the application of 3D-CTBA, a series of thoracoscopic-surgery techniques have been gradually developed, such as location of pulmonary nodules, dissection of targeted vessels and bronchi, preservation of intersegmental veins, and identification of the intersegmental demarcation[23]. First, we used CT-guided hookwire combined with 3D-CTBA images to precisely locate nodules before surgery. Combining different methods to accomplish this task is of vital importance, especially for small, deep nodules or pure GGO[24,25]. Notably, we encountered no intraoperative massive hemorrhage, thanks to our accurate determination of pulmonary vascular anatomy and variations. Additionally, we defined the intersegmental demarcation using the improved inflation–deflation method assisted by 3D-CTBA[14]. The inflation–deflation interface was anatomically separated from the hilum to the distal region along the intersegmental veins and dissected using an electrotome and/or endoscopic staplers. The intersegmental vein and the inflation–deflation demarcation were identified as the markers of the intersegmental plane. Finally, with the help of Dr. Liang Chen, our institution explored the technique called “cone-shaped segmentectomy,” with which surgeons could precisely identify, separate, and dissect the targeted segment based on the cone-shaped principle[8].
There are several limitations to the utility of 3D-CTBA for surgical guidance. First, nearly all 3D-reconstruction software packages are designed for general business and industrial use, not specifically for medical applications, let alone thoracic surgery. Therefore, the 3D reconstruction procedure for pulmonary vessels and bronchi is not fully automated and is time consuming[26]. Second, designing surgical procedures using 3D-CTBA technology depends on computer processing ability and the operational technique of the image software. The thoracic surgeon must not only cooperate with the radiologist, but also master radiological knowledge and ability[27]. In addition, lesions are often detected by preoperative CT with the lung fully inflated, but during surgery the lung is often deflated. Understanding how preoperative conditions correlate with interoperative conditions requires significant experience and the ability to accurately identify anatomical structures. Furthermore, the size of the cohort in our study is a bit small, and more cases need to be collected in future. A final limitation is that we need more time to observe the postoperative recurrence and mortality rates in future studies. In the future, new high-quality software packages will hopefully facilitate the utility and diffusion of 3D technology among thoracic surgeons.
The advent of 3D-CTBA could dramatically change the VATS procedure for lung cancers, leading to a simpler, shorter, and more-accurate surgical process. The 3D-CTBA method enables the surgeon to visualize the anatomical relationship between the pulmonary nodule and the surrounding structure, which is valuable for a thoracoscopic-surgery strategy. The combination of VATS and 3D-CTBA worked in harmony in our study, and it also provided a new pattern of transition from the lesion-directed location of tumors to computer-aided surgery for the management of early-stage lung cancer.
Performance of video-assisted thoracoscopic surgery (VATS) segmentectomy and lobectomy for primary lung cancer has currently increased. For small lung lesions, identification of the anatomical variation and intersegmental line is often difficult, and ensuring a sufficient surgical margin is more likely to be uncertain.
A lack of stereoscopic vision and the existence of anatomical variations create problems for surgeons during VATS, which can lead to unexpected complications.
The purpose of this study was to evaluate the therapeutic effect of VATS segmen
The 3D-CTBA during VATS segmentectomy and lobectomy was used for identifying the location of lesions, confirming anatomical variations, and securing the resection margins.
There was no intraoperative massive hemorrhages, postoperative intensive-care unit stays or 30-d mortalities. Three-dimensional navigation was performed to confirm the segmental structure, precisely cut off the targeted segment, and avoid intersegmental veins injury.
The combination of VATS and 3D-CTBA worked in harmony in our study. This combination also demonstrated a new pattern of transition from lesion-directed location of tumors to computer-aided surgery for the management of small lung lesions.
Intraoperative 3D-CTBA navigation could enable a more definitive VATS segmentectomy and lobectomy for early lung cancer.
We thank Dr. Liang Chen from Jiangsu Province Hospital for his helpful support and surgical skills.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yajima T S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Yu HG
| 1. | Balata H, Fong KM, Hendriks LE, Lam S, Ostroff JS, Peled N, Wu N, Aggarwal C. Prevention and Early Detection for NSCLC: Advances in Thoracic Oncology 2018. J Thorac Oncol. 2019;14:1513-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 2. | Handa Y, Tsutani Y, Mimae T, Miyata Y, Okada M. Complex segmentectomy in the treatment of stage IA non-small-cell lung cancer. Eur J Cardiothorac Surg. 2020;57:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Akiba T. Utility of three-dimensional computed tomography in general thoracic surgery. Gen Thorac Cardiovasc Surg. 2013;61:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Qu X, Wang K, Zhang T, Shen H, Dong W, Liu Q, Du J. Long-term outcomes of stage I NSCLC (≤3 cm) patients following segmentectomy are equivalent to lobectomy under analogous extent of lymph node removal: a PSM based analysis. J Thorac Dis. 2017;9:4561-4573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Dai C, Shen J, Ren Y, Zhong S, Zheng H, He J, Xie D, Fei K, Liang W, Jiang G, Yang P, Petersen RH, Ng CS, Liu CC, Rocco G, Brunelli A, Shen Y, Chen C. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol. 2016;34:3175-3182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Roman M, Labbouz S, Valtzoglou V, Ciesla A, Hawari M, Addae-Boateng E, Thorpe JA, Duffy JP, Majewski A. Lobectomy vs. segmentectomy. A propensity score matched comparison of outcomes. Eur J Surg Oncol. 2019;45:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Le Moal J, Peillon C, Dacher JN, Baste JM. Three-dimensional computed tomography reconstruction for operative planning in robotic segmentectomy: a pilot study. J Thorac Dis. 2018;10:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Wu WB, Xu XF, Wen W, Xu J, Zhu Q, Pan XL, Xia Y, Chen L. Three-dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis. 2016;8:S710-S715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer (Version 6.2019). Available from: http://www.nccn.org/patients. |
| 10. | Nakazawa S, Shimizu K, Mogi A, Kuwano H. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg. 2018;66:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Shapiro M, Weiser TS, Wisnivesky JP, Chin C, Arustamyan M, Swanson SJ. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg. 2009;137:1388-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Nomori H, Mori T, Ikeda K, Yoshimoto K, Iyama K, Suzuki M. Segmentectomy for selected cT1N0M0 non-small cell lung cancer: a prospective study at a single institute. J Thorac Cardiovasc Surg. 2012;144:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Ojanguren A, Recuero JL, Pardina M, Milla L, Santamaría M. Three dimensional computed tomography for preoperative assessment of the pulmonary artery in patients undergoing endoscopic lobectomy or segmentectomy. Cir Esp. 2017;95:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Wu WB, Xia Y, Pan XL, Wang J, He ZC, Xu J, Wen W, Xu XF, Zhu Q, Chen L. Three-dimensional navigation-guided thoracoscopic combined subsegmentectomy for intersegmental pulmonary nodules. Thorac Cancer. 2019;10:41-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | She XW, Gu YB, Xu C, Li C, Ding C, Chen J, Zhao J. Three-dimensional (3D)- computed tomography bronchography and angiography combined with 3D-video-assisted thoracic surgery (VATS) versus conventional 2D-VATS anatomic pulmonary segmentectomy for the treatment of non-small cell lung cancer. Thorac Cancer. 2018;9:305-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Zhu WY, Tan LL, Wang ZY, Wang SJ, Xu LY, Yu W, Chen ZJ, Zhang YK. Clinical characteristics and advantages of primary peripheral micro-sized lung adenocarcinoma over small-sized lung adenocarcinoma. Eur J Cardiothorac Surg. 2016;49:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Klinkenberg TJ, Dinjens L, Wolf RFE, van der Wekken AJ, van de Wauwer C, de Bock GH, Timens W, Mariani MA, Groen HJM. CT-guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol. 2017;115:898-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Ghaly G, Kamel M, Nasar A, Paul S, Lee PC, Port JL, Stiles BM, Altorki NK. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg. 2016;101:465-72; discussion 472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Nomura Y, Higaki T, Fujita M, Miki S, Awaya Y, Nakanishi T, Yoshikawa T, Hayashi N, Awai K. Effects of Iterative Reconstruction Algorithms on Computer-assisted Detection (CAD) Software for Lung Nodules in Ultra-low-dose CT for Lung Cancer Screening. Acad Radiol. 2017;24:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Li X, Wang X, Dai Y, Zhang P. Supervised recursive segmentation of volumetric CT images for 3D reconstruction of lung and vessel tree. Comput Methods Programs Biomed. 2015;122:316-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Coenen A, Honda O, van der Jagt EJ, Tomiyama N. Computer-assisted solid lung nodule 3D volumetry on CT: influence of scan mode and iterative reconstruction: a CT phantom study. Jpn J Radiol. 2013;31:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Yao F, Wang J, Yao J, Hang F, Lei X, Cao Y. Three-dimensional image reconstruction with free open-source OsiriX software in video-assisted thoracoscopic lobectomy and segmentectomy. Int J Surg. 2017;39:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Mun M, Nakao M, Matsuura Y, Ichinose J, Nakagawa K, Okumura S. Novel techniques for video-assisted thoracoscopic surgery segmentectomy. J Thorac Dis. 2018;10:S1671-S1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Kleedehn M, Kim DH, Lee FT, Lubner MG, Robbins JB, Ziemlewicz TJ, Hinshaw JL. Preoperative Pulmonary Nodule Localization: A Comparison of Methylene Blue and Hookwire Techniques. AJR Am J Roentgenol. 2016;207:1334-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Nakajima J. Advances in techniques for identifying small pulmonary nodules. Surg Today. 2019;49:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Reeves AP, Xie Y, Jirapatnakul A. Automated pulmonary nodule CT image characterization in lung cancer screening. Int J Comput Assist Radiol Surg. 2016;11:73-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Shimizu K, Nakazawa S, Nagashima T, Kuwano H, Mogi A. 3D-CT anatomy for VATS segmentectomy. J Vis Surg. 2017;3:88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |