Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10484
Peer-review started: April 8, 2021
First decision: April 28, 2021
Revised: May 9, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: December 6, 2021
Processing time: 235 Days and 19.1 Hours
Multiple primary malignancies (MPM) are characterized by two or more primary malignancies in the same patient, excluding relapse or metastasis of prior cancer. We aimed to elucidate the clinical features and survival of MPM patients.
To elucidate the clinical features and survival of MPM patients.
A retrospective study of MPM patients was conducted in our hospital between June 2016 and June 2019. Overall survival (OS) was calculated using the Kaplan-Meier method. The log-rank test was used to compare the survival of different groups.
A total of 243 MPM patients were enrolled, including 222 patients with two malignancies and 21 patients with three malignancies. Of patients with two malignancies, 51 (23.0%) had synchronous MPM, and 171 (77.7%) had metachronous MPM. The most common first cancers were breast cancer (33, 14.9%) and colorectal cancer (31, 14.0%). The most common second cancers were non-small cell lung cancer (NSCLC) (66, 29.7%) and gastric cancer (24, 10.8%). There was no survival difference between synchronous and metachronous MPM patients (36.4 vs 35.3 mo, P = 0.809). Patients aged > 65 years at diagnosis of the second cancer had a shorter survival than patients ≤ 65 years (28.4 vs 36.4 mo, P = 0.038). Patients with distant metastasis had worse survival than patients without metastasis (20.4 vs 86.9 mo, P = 0.000). Following multivariate analyses, age > 65 years and distant metastasis were independent adverse prognostic factors for OS.
During follow-up of a first cancer, the occurrence of a second or more cancers should receive greater attention, especially for common concomitant MPM, to ensure early detection and treatment of the subsequent cancer.
Core Tip: In the paper we investigated the clinical features and survival of 243 patients with multiple primary malignancies (MPM), including 222 patients with two malignancies and 21 patients with three malignancies. There was no survival difference between synchronous and metachronous MPM patients. After multivariate analyses, age > 65 years and distant metastasis were independent adverse prognostic factors for overall survival. In clinical procedure and follow-up of initial cancer, the occurrence of second or more cancer should be paid great attention to.
- Citation: Wang XK, Zhou MH. Clinical features and survival of patients with multiple primary malignancies. World J Clin Cases 2021; 9(34): 10484-10493
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10484.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10484
Multiple primary malignancies (MPM) are characterized by two or more different primary malignancies in the same patient, excluding relapse or metastases of prior cancer[1]. An increased incidence of MPM has been observed with the rapid development of medical techniques and prolonged life expectancy[2,3]. The causes of MPM may be associated with genetic alterations, the environment and iatrogenic factors[4].
Colorectal cancer, breast cancer and head and neck cancer have been reported as the most common first primary malignancies, and lung cancer, breast cancer and colorectal cancer as the most common subsequent primary malignancies[5,6].The most common MPM pairs were head and neck-lung cancer, and breast-gynecologic cancer[7]. According to the interval between initial cancer and subsequent cancer, MPM can be divided into synchronous MPM (within 6 mo) and metachronous MPM (more than 6 mo)[8]. Metachronous MPM account for most of the patients with MPM, with a 5-year OS, ranging from 61% to 68%[7,9]. In some reports, patients with metachronous MPM had better survival than patients with synchronous MPM[7,9], while this survival advantage was not observed in the study by Xu and Gu[10].
In the present study, we retrospectively analyzed the clinical features and survival of patients with MPM in our hospital over the past 3 years, in order to provide helpful information for the diagnosis, treatment, prognosis and follow-up of these patients.
A retrospective study of MPM patients was conducted in our hospital between June 2016 and June 2019. The diagnosis of each malignancy in MPM patients was identified by histopathology. Hematological malignancies were excluded in our study, and only solid malignant tumors were included. A total of 27055 patients with solid malignant tumors were consecutively identified. Of these patients, 260 had MPM. After further review, 17 patients were excluded from our study, including 11 patients with no pathological diagnosis of one tumor, and 6 patients who were lost to follow-up. Therefore, a total of 243 MPM patients with complete clinical and follow-up data were enrolled in the study, including 222 patients with two malignancies and 21 patients with three malignancies. In patients with two primary malignancies, synchronous MPM was defined as two malignancies diagnosed within 6 mo, and metachronous MPM as two malignancies diagnosed within more than 6 mo between the first and second cancer. The study was approved by the institutional review board of our hospital (2020KY018-KS001).
Continuous and categorical variables were summarized as the median with range, and the count with percentage, respectively. For patients with two malignancies, overall survival (OS) was defined as the time from diagnosis of the second malignancy to death due to any cause, or to the last follow-up. For patients with three malignancies, OS was defined as the time from diagnosis of the third malignancy to death due to any cause, or to the last follow up. The end of follow-up was December 2019. OS was calculated using the Kaplan-Meier method. The log-rank test was used to compare the survival of different groups. Multivariate Cox regression models were used to find the prognostic factors for OS. All the data were analyzed using IBM SPSS statistics software (version 22). P value < 0.05 was considered statistically significant.
A total of 26795 patients with one solid malignant tumor were found. The five most common cancers were non-small cell lung cancer (NSCLC) (18.3%), colorectal cancer (12.5%), breast cancer (10.6%), gastric cancer (9.4%) and liver cancer (7.3%). Patients with MPM accounted for 0.96% (260/27055) of all patients with solid malignant tumor.
A total of 243 patients with MPM were included in our study. Of these patients, 222 with two malignancies were identified, and the demographics and clinical characteristics of these patients are shown in Table 1. Fifty-one patients (23.0%) had synchronous MPM, and 171 patients (77.7%) had metachronous MPM. In the synchronous, metachronous and total MPM groups, 32 patients (62.7%), 87 patients (50.9%) and 119 patients (53.6%) were male, respectively; the median age at diagnosis of the first cancer was 62 years, 55 years and 56 years respectively; the median interval between the first and second cancer diagnoses was 0.2 mo, 73.2 mo and 43.6 mo, respectively; distant metastasis was found in 16 (31.4%) patients, 83 (48.5%) patients and 99 (44.6%) patients, respectively.
| Characteristics | Synchronous MPM | Metachronous MPM | Total |
| Patients | 51 (23.0) | 171 (77.0) | 222 (100) |
| Gender | |||
| Male | 32 (62.7) | 87 (50.9) | 119 (53.6) |
| Female | 19 (37.3) | 84 (49.1) | 103 (46.4) |
| Age in yr, median (range) | |||
| First cancer | 62 (32-84) | 55 (19-87) | 56 (19-87) |
| Second cancer | 62 (32-84) | 64 (28-90) | 64 (28-90) |
| The most common sites in first primary cancers | |||
| 1st | Esophagus, 9 (17.6) | Breast, 31 (18.1) | Breast, 33 (14.9) |
| 2nd | Colorectum, 8 (15.7) | Colorectum, 23 (13.7) | Colorectum, 31 (14.0) |
| 3rd | Bladder/thyroid/hypopharynx, 4 (7.8), respectively | Stomach, 14 (8.2) | Stomach, 17 (7.7) |
| The most common sites in second primary cancers | |||
| 1st | NSCLC, 12 (23.5) | NSCLC, 54 (31.6) | NSCLC,66 (29.7) |
| 2nd | Stomach, 9 (17.6) | Stomach, 15 (8.8) | Stomach, 24 (10.8) |
| 3rd | Esophagus/kidney, 6 (11.8), respectively | Liver, 13 (7.6) | Esophagus/liver, 16 (7.2), respectively |
| Median interval (range) between the first and second cancers (mo) | 0.2 (0-5.9) | 73.2 (6.3-536.8) | 43.6 (0-536.8) |
| Metastasis | 16 (31.4) | 83 (48.5) | 99 (44.6) |
| Median overall survival in mo | 36.4 | 35.3 | 35.4 |
In 222 patients with two malignancies, the most common first cancers were breast cancer (33, 14.9%), colorectal cancer (31, 14.0%), and gastric cancer (17, 7.7%). The most common second cancers were NSCLC (66, 29.7%), gastric cancer (24, 10.8%) and esophageal cancer, liver cancer (16, 7.2%), respectively. In 51 synchronous MPM patients, the most common first cancer was esophageal cancer (9, 17.6%), colorectal cancer (8, 15.7%) and bladder cancer, thyroid cancer, hypopharyngeal cancer (4, 7.8%, respectively). The most common second cancers were NSCLC (12, 23.5%), gastric cancer (9, 17.6%) and esophageal cancer, renal cancer (6, 11.8%, respectively). In 171 metachronous MPM patients, the most common first cancers were breast cancer (31, 18.1%), colorectal cancer (23, 13.7%) and gastric cancer (14, 8.2%). The most common second cancers were NSCLC (54, 31.6%), gastric cancer (15, 8.8%) and liver cancer (13, 7.6%).
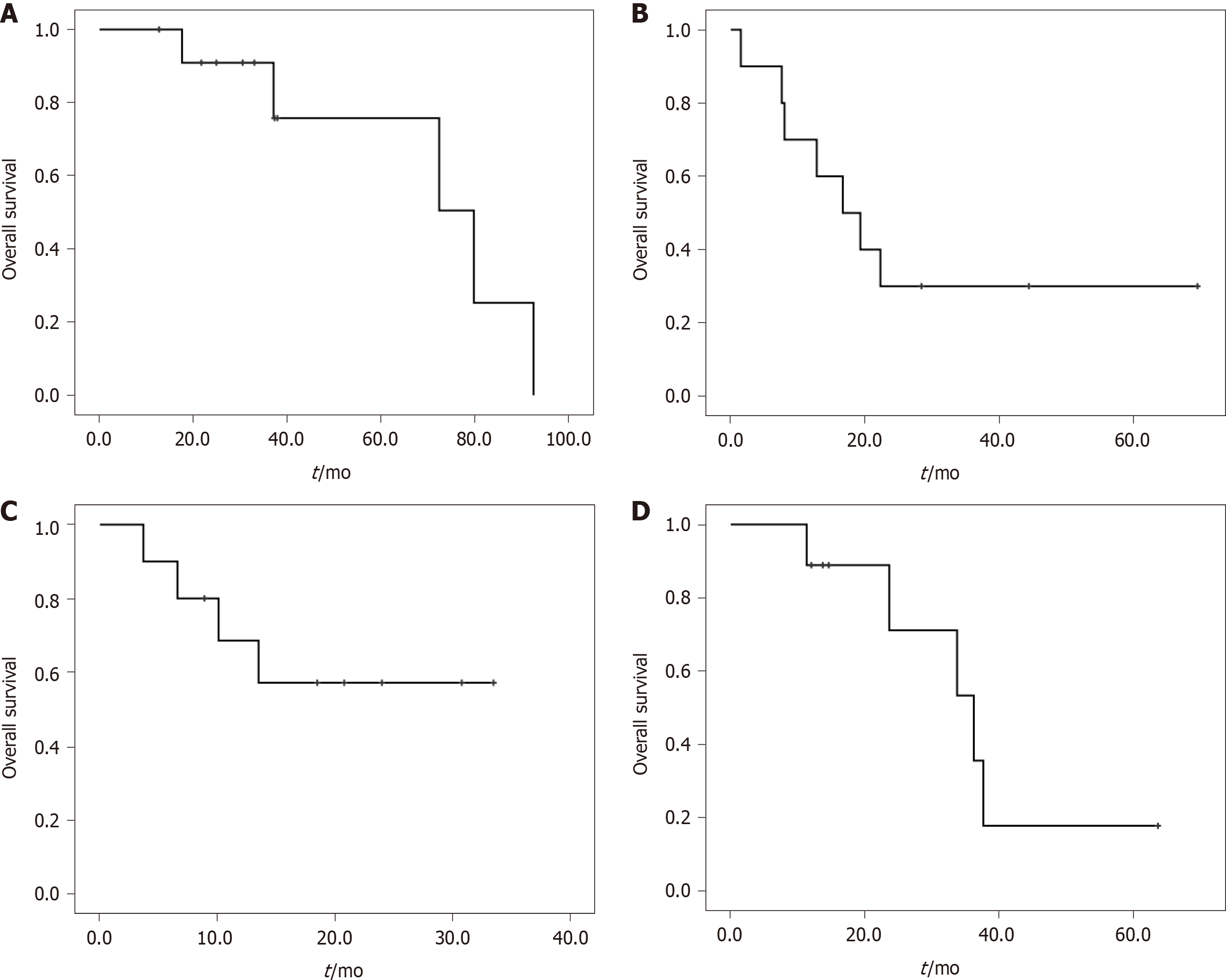
In the 222 patients with two malignancies, the most common MPM and their median OS are shown in Table 2. Twelve patients were found to have NSCLC and breast cancer, and the median OS of these patients was 79.8 mo (Figure 1A). Ten patients were found to have NSCLC and gastric cancer, and the median OS was 16.7 mo (Figure 1B). Colorectal cancer and gastric cancer were identified in ten patients, with the median OS not reached (Figure 1C). Esophageal cancer and gastric cancer were identified in nine patients, with a median OS of 36.2 mo (Figure 1D).
| Malignancies | Total | Synchronous MPM | Metachronous MPM | Median OS in mo |
| NSCLC and breast cancer | 12 | 2 | 10 | 79.8 |
| NSCLC and gastric cancer | 10 | 0 | 10 | 16.7 |
| Colorectal cancer and gastric cancer | 10 | 3 | 7 | Not reached |
| Esophageal cancer and gastric cancer | 9 | 6 | 3 | 36.2 |
| NSCLC and bladder cancer | 7 | 2 | 5 | 31.2 |
| NSCLC and cervical cancer | 6 | 1 | 5 | 35.5 |
| NSCLC and thyroid cancer | 6 | 2 | 4 | Not reached |
| NSCLC and colorectal cancer | 6 | 1 | 5 | Not reached |
| NSCLC and esophageal cancer | 6 | 3 | 3 | Not reached |
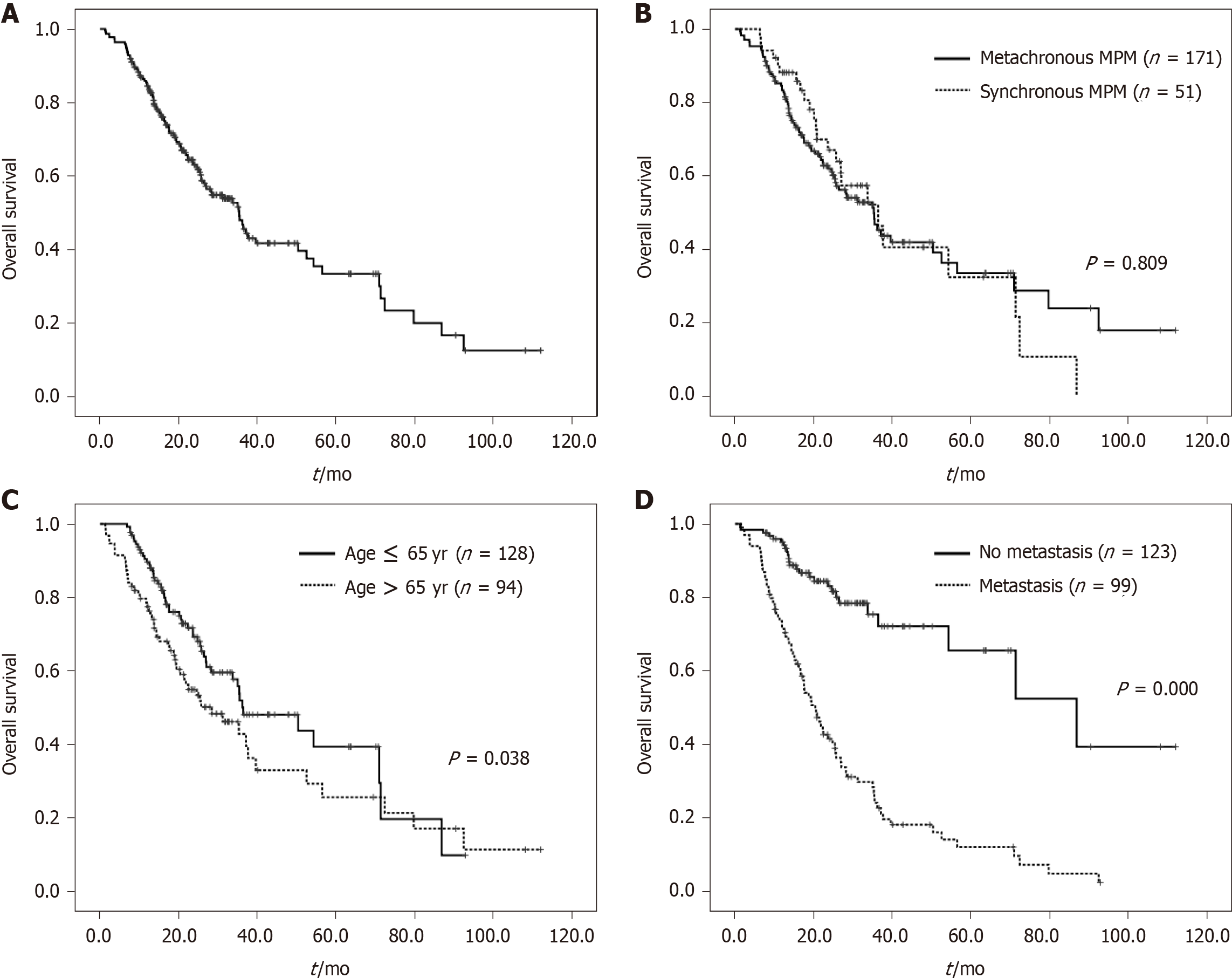
The univariate and multivariate analyses of prognostic factors for OS inpatients with two malignancies are presented in Table 3. The median OS in all 222 patients was 35.4 mo (Figure 2A). The median OS in synchronous MPM and metachronous MPM patients were 36.4 mo and 35.3 mo, respectively, which was not significantly different (Figure 2B). The median OS between male and female patients was also not significantly different. However, patients aged > 65 years at the second cancer diagnosis had a shorter survival than patients ≤ 65 years (28.4 mo vs 36.4 mo, P = 0.038; Figure 2C). Patients with distant metastasis had worse survival than patients without metastasis (20.4 mo vs 86.9 mo, P = 0.000; Figure 2D). Furthermore, multivariate analyses showed that age and metastases remained statistically different, indicating that age > 65 years and distant metastasis were independent adverse prognostic factors for OS.
| Factors | Cases | Univariate analysis | Multivariate analysis | |||
| Median OS in mo | P | HR | 95%CI | P | ||
| Gender | 0.114 | |||||
| Male | 119 | 31.2 | ||||
| Female | 103 | 37.1 | ||||
| Age at second cancer (yr) | 0.038 | 1.016 | 1.000-1.032 | 0.046 | ||
| ≤ 65 | 128 | 36.4 | ||||
| > 65 | 94 | 28.4 | ||||
| Metastasis | 0.000 | 4.291 | 2.743-6.710 | 0.000 | ||
| No | 123 | 86.9 | ||||
| Yes | 99 | 20.4 | ||||
| MPM | 0.809 | |||||
| Synchronous | 51 | 36.4 | ||||
| Metachronous | 171 | 35.3 | ||||
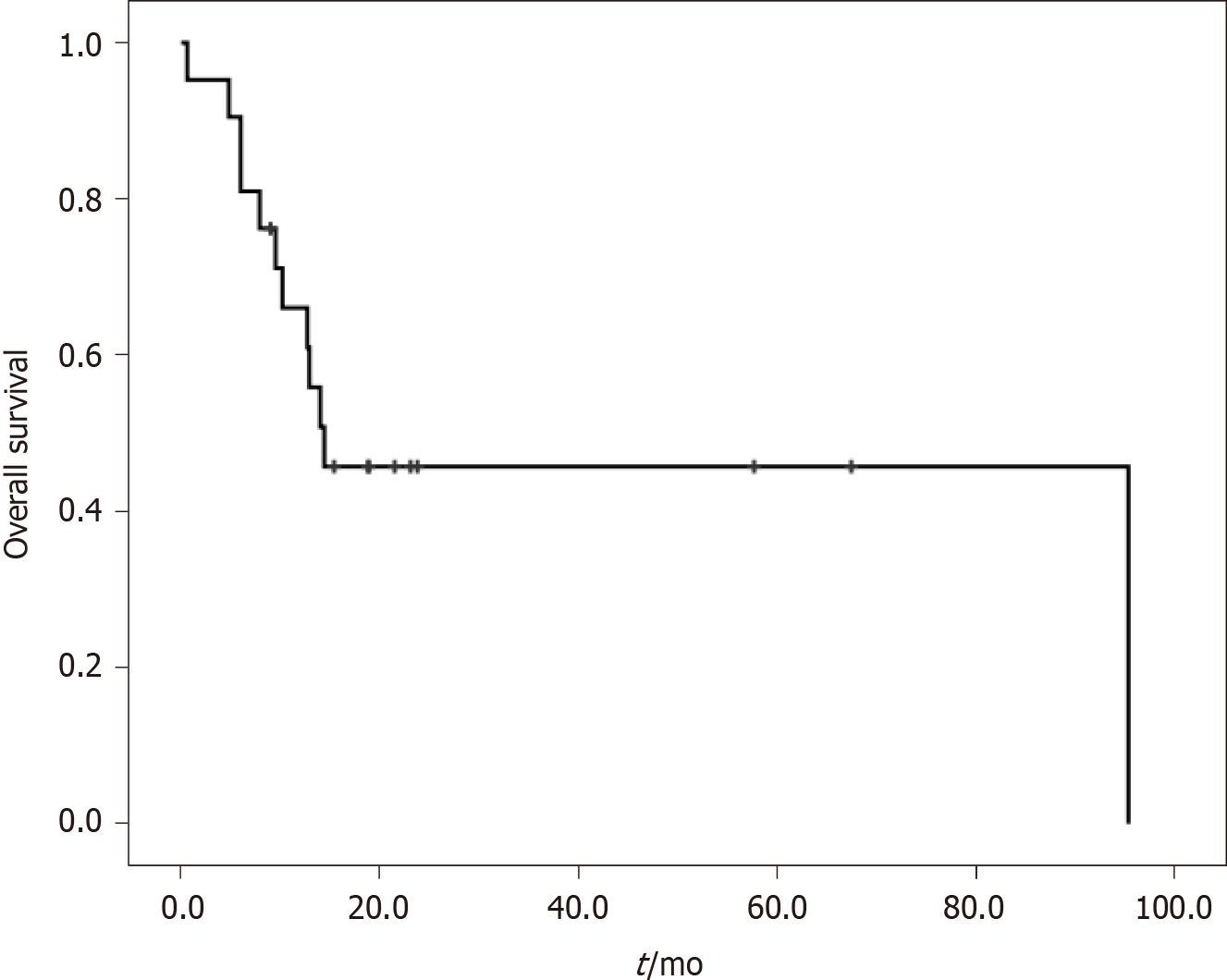
A total of 21 patients with three malignancies were identified in our study. Their clinical features are shown in Table 4 and Table 5. Eleven patients (52.4%) were male, and 12 patients (57.1%) had distant metastases. The median age at the first, second and third cancer was 47 years, 54 years and 57 years, respectively. The most common cancers in all patients were colon cancer (9, 14.3%), rectal cancer (7, 11.1%) and breast cancer (5, 7.9%). The median OS from diagnosis of the third cancer was 14.4 mo (Figure 3).
| Case | Sex | First cancer | Second cancer | Third cancer | Metastases | Outcome | OS since third cancer in mo |
| 1 | M | Parotid cancer | Colon cancer | Penile cancer | Yes | Dead | 0.6 |
| 2 | F | Small cell lung cancer | Colon cancer | Soft tissue sarcoma | No | Dead | 14.4 |
| 3 | M | Gastric cancer | Liver cancer | Rectal cancer | Yes | Dead | 14.0 |
| 4 | M | Rectal cancer | Renal cancer | Colon cancer | No | Alive | 23.1 |
| 5 | M | Colon cancer | Bladder cancer | Renal pelvis cancer | No | Alive | 67.5 |
| 6 | F | Soft tissue sarcoma | Colon cancer | Bladder cancer | Yes | Dead | 4.8 |
| 7 | F | Colon cancer | Endometrial cancer | Breast cancer | Yes | Alive | 23.8 |
| 8 | F | Breast cancer | Parotid cancer | Neuroendocrine carcinoma | Yes | Dead | 9.5 |
| 9 | M | Bladder cancer | Thyroid cancer | Ureteral cancer | No | Alive | 18.9 |
| 10 | M | Rectal cancer | Colon cancer | Cholangiocarcinoma | Yes | Dead | 6.0 |
| 11 | M | Laryngeal cancer | Esophageal cancer | Non-small cell lung cancer | No | Alive | 21.5 |
| 12 | M | Thyroid cancer | Laryngeal cancer | Rectal cancer | Yes | Dead | 10.2 |
| 13 | M | Bladder tumor | Colon cancer | Non-small cell lung cancer | No | Dead | 12.7 |
| 14 | M | Laryngeal cancer | Renal pelvis cancer | Bladder cancer | No | Dead | 12.9 |
| 15 | F | Rectal cancer | Thyroid cancer | Breast cancer | Yes | Dead | 95.4 |
| 16 | F | Skin squamous cell carcinoma | Skin basal cell carcinoma | Endometrial cancer | Yes | Alive | 57.7 |
| 17 | F | Gallbladder cancer | Endometrial cancer | Rectal cancer | No | Alive | 15.4 |
| 18 | F | Endometrial cancer | Rectal cancer | Pancreatic cancer | Yes | Dead | 6.0 |
| 19 | F | Breast cancer | Thymic cancer | Choriocarcinoma | No | Alive | 18.8 |
| 20 | F | Breast cancer | Thyroid cancer | Liver cancer | Yes | Alive | 9.0 |
| 21 | M | Prostate cancer | Gastric cancer | Colon cancer | Yes | Dead | 7.9 |
| Characteristics | n (%) |
| Total | 21 |
| Gender | |
| Male | 11 (52.4) |
| Female | 10 (47.6) |
| Age in yr, median (range) | |
| First cancer | 47 (18-74) |
| Second cancer | 54 (33-87) |
| Third cancer | 57 (34-89) |
| The most common cancers | |
| Colon cancer | 9 (14.3) |
| Rectal cancer | 7 (11.1) |
| Breast cancer | 5 (7.9) |
| Bladder/endometrial/thyroid cancer | 4 (6.3), respectively |
| Laryngeal cancer | 3 (4.8) |
| Metastasis | 12 (57.1) |
| Median OS since third cancer in mo | 14.4 |
In this study, we retrospectively analyzed the clinical features and survival of 243 MPM patients, including 222 patients with two malignancies and 21 patients with three malignancies. The most common MPM were NSCLC and breast cancer (12 cases), NSCLC and gastric cancer (10 cases), and colorectal cancer and gastric cancer (10 cases), with the median OS of 79.8 mo, 16.7 mo and not reached, respectively. The median OS of patients with two malignancies and three malignancies were 35.4 mo and 14.4 mo, respectively. Age > 65 years and distant metastases were independent adverse prognostic factors for OS in patients with two malignancies.
In a retrospective study of 278 MPM patients, 120 (43%) patients presented with synchronous MPM, and 158 patients (57%) with metachronous MPM[9]. In our study, we had fewer patients with synchronous MPM (51, 23%) and more patients with three malignancies. The median interval between two cancers in patients with metachronous MPM in the above study was 30.98 mo, less than the median interval in our study of 73.2 mo. In the above study, the most common first cancers were breast, head and neck, and colorectal cancer; the most common second cancers were breast, colorectal and uterine body cancer. In our study, breast and colorectal cancer were also the most common. In addition, other common cancers in our study were NSCLC and digestive system malignancies, such as stomach, esophagus and liver, which were different from those in the above report.
The survival differences of synchronous and metachronous MPM have been reported inconsistently in different studies. Some reported that survival in patients with metachronous MPM was better than in synchronous MPM[7,9]. However, no survival differences between synchronous and metachronous MPM were noted in our study, and in another study[10]. Of note, the starting point of calculated survival time in metachronous MPM was not consistent in previous studies, some were calculated from the date of the first cancer diagnosis and some from the date of the last cancer diagnosis. Besides, different cancer constituents may account for the inconsistent results.
In a report of 350 MPM patients with lung cancer, the most common associated malignancies were esophageal cancer, breast cancer, gastric cancer and colorectal cancer[11]. In another report of 268 metachronous MPM patients with lung cancer, colorectal cancer, breast cancer and gastric cancer were the most common associated primary cancers[12]. Unfortunately, the survival of patients with lung MPM was not reported. Similarly, in our study, the most common concomitant malignancies in MPM patients with NSCLC were breast cancer, gastric cancer and bladder cancer, with a median OS of 79.8 mo, 16.7 mo and 31.2 mo, respectively.
In a report of 55 MPM patients with colorectal cancer, stomach cancer was the most commonly associated lesion[13]. In another study of 117 MPM patients with colorectal cancer, the most commonly associated cancer was gastric cancer, followed by lung and breast cancer[14]. In our study, gastric cancer was also the most frequently observed associated cancer in patients with colorectal cancer. A total of ten patients with colorectal and gastric cancer were found in our 222 MPM patients with two malignancies. The median OS of patients with colorectal and gastric cancer was not reached.
In a respective study of 170 MPM patients[10], 17 cases with esophageal and gastric cancer were found. The median survival of 42 gastrointestinal MPM, including the above 17 cases, was 40 mo, which was close to our 36.2 mo in nine patients with esophageal and gastric cancer. Chen et al[15] analyzed 192 patients with esophageal and gastric cancer from a database in the United States, and found that the median OS of these patients was approximately 59 mo, but with longer follow-up time, none of these patients survived.
Following univariate and multivariate analyses, older age and distant metastasis were independent poor prognostic factors for OS. In the study by Etiz et al[7], elderly and young patients showed no differences in survival. However, Wang et al[11] reported that the OS of patients < 60 years was significantly better than that of patients ≥ 60 years, which was also showed in the multivariate analysis. It is generally believed that cancer patients with distant metastasis have a poor prognosis[16,17]. Distant metastasis was also an adverse prognostic factor in our MPM patients.
In our 243 MPM patients, 21 patients with three primary malignancies were identified, whereas only two patients with three primary malignancies in 170 MPM patients were found in the study by Xu and Gu[10]. Compared with our study, the study by Xu and Gu[10] was conducted at least 6 years ago, and insufficient diagnosis and therapy may have resulted in the fewer patients being diagnosed with three malignancies. In another report of 30 patients with three primary malignancies[18], the median OS from the initial cancer diagnoses was 11.2 years. Our 21 patients with three malignancies had a median OS of 14.4 mo from the third cancer diagnoses. Due to the 10-years interval between the first and third cancer in our study, it is justified to consider that these two patient groups had a similar survival.
There are two limitations in our respective study. Firstly, the prognostic characteristics and treatment response of different cancers vary widely. Therefore, cancer types and treatment methods are important prognostic factors. However, these two factors were not included in our survival analysis. Secondly, MPM patients, especially those with three primary malignancies, may have potential genetic and environmental pathogenic factors, which are important in cancer prevention and treatment. However, these underlying pathogenic factors were not investigated in our study and deserve further detailed study.
During the diagnosis, treatment and follow-up of the initial cancer, more attention should be paid to the occurrence of a second, or even a third cancer in patients with MPM, to ensure early detection and treatment of the subsequent cancer. In particular, for common MPM pairs, such as NSCLC and breast/gastric cancer, colorectal and gastric cancer, the risk of concomitant MPM should be closely monitored.
Multiple primary malignancies (MPM) are characterized by two or more primary malignancies in the same patient, excluding relapse or metastasis of prior cancer.
The clinical features and survival of MPM patients are not clear.
We aimed to elucidate the clinical features and survival of MPM patients.
A retrospective study of MPM patients was conducted in our hospital between June 2016 and June 2019. Overall survival (OS) was calculated using the Kaplan-Meier method.
A total of 243 patients with MPM, including 222 patients with two malignancies and 21 patients with three malignancies. Following multivariate analyses, age > 65 years and distant metastasis were independent adverse prognostic factors for OS.
During the diagnosis, treatment and follow-up of the initial cancer, more attention should be paid to the occurrence of a second, or even a third cancer in patients with MPM.
For common MPM pairs, such as NSCLC and breast/gastric cancer, colorectal and gastric cancer, the risk of concomitant MPM should be closely monitored, to ensure early detection and treatment of the subsequent cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Szakács Z S-Editor: Liu M L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Nemes A, Nagy V. The impact of multiple primary neoplasms in daily practicea systematic review of the literature. J BUON. 2018;23:14-18. [PubMed] |
| 2. | Mariotto AB, Rowland JH, Ries LA, Scoppa S, Feuer EJ. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev. 2007;16:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Carlomagno N, Santangelo ML, Mastromarino R, Calogero A, Dodaro C, Renda A. Rare multiple primary malignancies among surgical patients-a single surgical unit experience. Ecancermedicalscience. 2014;8:438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Papaconstantinou I, Mantzos DS, Asimakoula K, Michalaki V, Kondi-Pafiti A. A 12-year experience at a tertiary hospital on patients with multiple primary malignant neoplasms. J BUON. 2015;20:332-337. [PubMed] |
| 5. | Jiao F, Yao LJ, Zhou J, Hu H, Wang LW. Clinical features of multiple primary malignancies: a retrospective analysis of 72 Chinese patients. Asian Pac J Cancer Prev. 2014;15:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Bagri PK, Singh D, Singhal MK, Singh G, Mathur G, Jakhar SL, Beniwal S, Sharma N, Kumar HS, Sharma A, Bardia MR. Double primary malignancies: a clinical & pathological analysis report from a regional cancer institute in India. Iran J Cancer Prev. 2014;7:66-72. [PubMed] |
| 7. | Etiz D, Metcalfe E, Akcay M. Multiple primary malignant neoplasms: A 10-year experience at a single institution from Turkey. J Cancer Res Ther. 2017;13:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Jena A, Patnayak R, Lakshmi AY, Manilal B, Reddy MK. Multiple primary cancers: An enigma. South Asian J Cancer. 2016;5:29-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Nemes A, Todor N, Nagy V. Clinicopathological characteristics of patients with multiple primary neoplasms-a retrospective analysis. J BUON. 2018;23:1846-1854. [PubMed] |
| 10. | Xu LL, Gu KS. Clinical retrospective analysis of cases with multiple primary malignant neoplasms. Genet Mol Res. 2014;13:9271-9284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Wang H, Hou J, Zhang G, Zhang M, Li P, Yan X, Ma Z. Clinical characteristics and prognostic analysis of multiple primary malignant neoplasms in patients with lung cancer. Cancer Gene Ther. 2019;26:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Shan S, She J, Xue ZQ, Su CX, Ren SX, Wu FY. Clinical characteristics and survival of lung cancer patients associated with multiple primary malignancies. PLoS One. 2017;12:e0185485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Li Q, Zhang B, Niu FN, Ye Q, Chen J, Fan XS. [Clinicopathological characteristics, MSI and K-ras gene mutations of double primary malignancies associated with colorectal cancer]. Zhonghua Yi Xue Za Zhi. 2020;100:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Kato T, Suzuki K, Muto Y, Sasaki J, Tsujinaka S, Kawamura YJ, Noda H, Horie H, Konishi F, Rikiyama T. Multiple primary malignancies involving primary sporadic colorectal cancer in Japan: incidence of gastric cancer with colorectal cancer patients may be higher than previously recognized. World J Surg Oncol. 2015;13:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Chen D, Fan N, Mo J, Wang W, Wang R, Chen Y, Hu J, Wen Z. Multiple primary malignancies for squamous cell carcinoma and adenocarcinoma of the esophagus. J Thorac Dis. 2019;11:3292-3301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Zhang N, Deng J, Sun W, Du Y, Guo S, Bai H, Liu H, Liang H. Extranodal soft tissue metastasis as an independent prognostic factor in gastric cancer patients aged under 70 years after curative gastrectomy. Ann Transl Med. 2020;8:376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Suzuki H, Nishikawa D, Beppu S, Terada H, Sawabe M, Hanai N. Prognostic Value of Age and Distant Metastasis in Differentiated Thyroid Carcinoma Undergoing Salvage Surgery. Anticancer Res. 2020;40:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Amer MH. Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res. 2014;6:119-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |