Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10337
Peer-review started: July 8, 2021
First decision: August 18, 2021
Revised: August 27, 2021
Accepted: September 22, 2021
Article in press: September 22, 2021
Published online: November 26, 2021
Processing time: 137 Days and 7.8 Hours
Although the incidence and cure rate of spinal hydatidosis are low, the recurrence rate of spinal hydatidosis is high, and the prognosis of spinal hydatidosis is poor. Therefore, we report a typical case of refractory spinal hydatidosis to increase spine surgeons’ awareness of the disease and reduce misdiagnosis and recurrence.
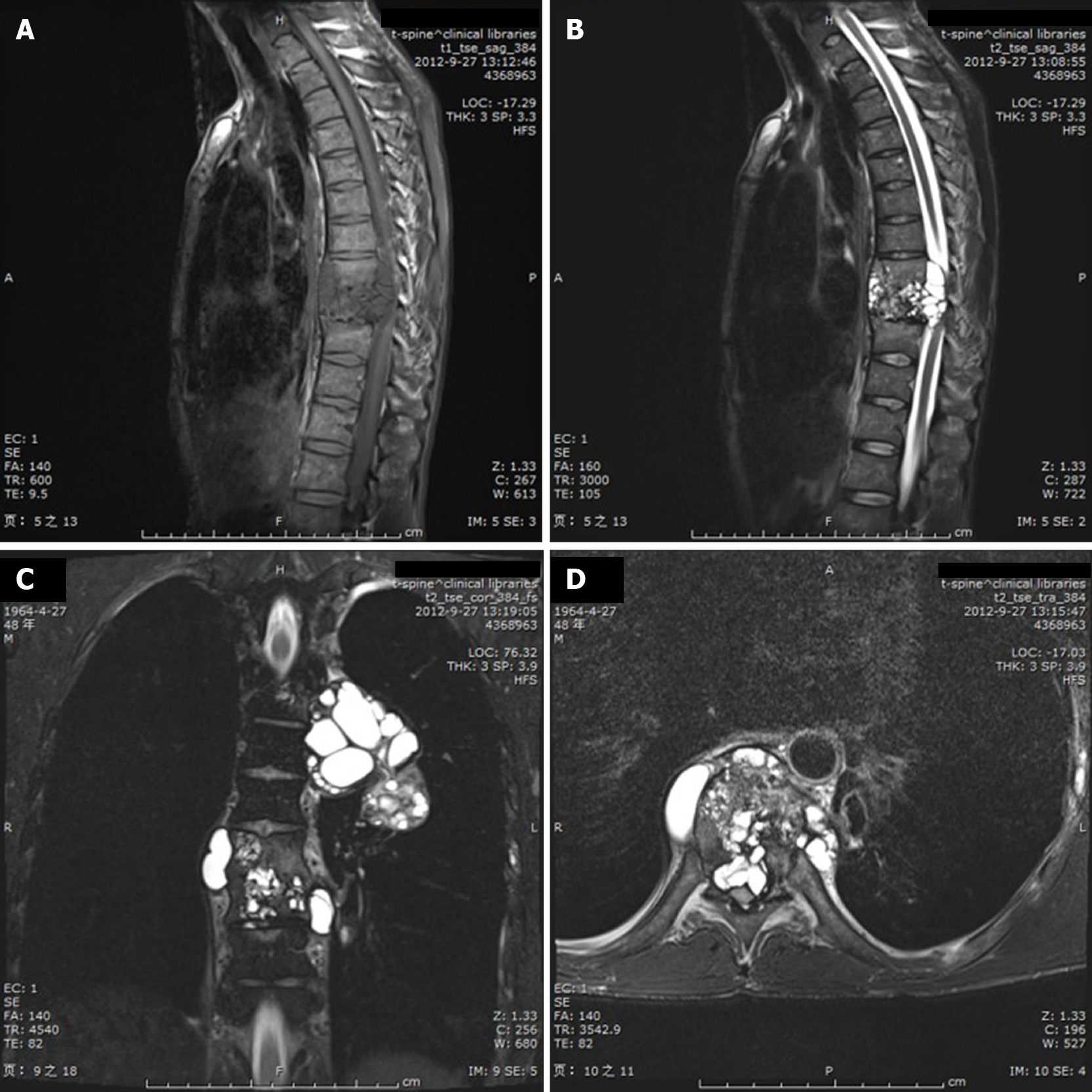
A 48-year-old man presented with back pain, significant weight loss, and paralysis of both lower limbs. The patient was misdiagnosed with spinal tuberculosis in an outside hospital. However, spinal magnetic resonance imaging (MRI) showed hyperintense cystic components on T2-weighted images and hypointensity on T1-weighted images. A lobulated, multiocular, honeycomb-appearance, septated cystic mass protruding intraspinally and compressing the spinal cord at segments T8–T9 was present. Paravertebral polycystic lobular lesions presented as a “bunch of grapes”. The ELISA test result for Echinococcus granulosus was positive. Then, a diagnosis of spinal hydatidosis and lung hydatid disease was made, and the patient underwent left transthoracic approach lobectomy, paravertebral lesion debridement, and subtotal vertebrectomy with vertebral body replacement of segments T8 and T9 by a mesh cage. The patient also underwent albendazole chemotherapy before and after surgery. One year after stopping the drug therapy, the patient developed recurrent T5 vertebral lesions and underwent a second subtotal vertebrectomy surgery. The patient is currently in good condition and is receiving long-term medication and follow-up.
The MRI feature of a “bunch of grapes” is a typical imaging indication of spinal hydatidosis. Subtotal vertebrectomy is a risk factor for postoperative recurrence. Total spondylectomy makes it possible to cure spinal hydatidosis, but antiparasitic drug therapy is also an important supplementary therapy to multimodal therapy. It is preferable for patients with spinal hydatidosis to receive life-long antiparasitic medication therapy and follow-up.
Core Tip: We report a rare case of typical refractory spinal hydatidosis. The magnetic resonance imaging finding of a “bunch of grapes” is a typical imaging feature of spinal hydatidosis. Subtotal vertebrectomy is a risk factor for postoperative recurrence. Total spondylectomy makes it possible to cure spinal hydatidosis, but antiparasitic drug therapy is also an important supplementary therapy to multimodal therapy. Preferably, patients with spinal hydatidosis should receive life-long antiparasitic medication therapy and follow-up.
- Citation: Zhang T, Ma LH, Liu H, Li SK. Incurable and refractory spinal cystic echinococcosis: A case report. World J Clin Cases 2021; 9(33): 10337-10344
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10337.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10337
Cystic echinococcosis (hydatidosis) is caused by Echinococcus granulosus. Most cases of cystic echinococcosis are asymptomatic and are found in the liver (55%), lung (28%), spleen, kidneys, and brain[1]. The incidence of osseous cystic echinococcosis is much lower, accounting for approximately 0.5%–4% of the total reported cases[2]. The most commonly affected bone is the spine (45%), followed by the ribs[3]. In spinal cystic echinococcosis, the lesions are predominantly located in the thoracic spine (47%), followed by the thoracolumbar spine (17.7%) and the lumbosacral spine (17.7%)[4,5].
Although the prevalence of spinal cystic echinococcosis is very low, it often causes back pain or motor dysfunction in clinical practice. The imaging findings of spinal cystic echinococcosis are easily misdiagnosed as spinal tuberculosis. Primary spinal cystic echinococcosis must be considered in the differential diagnosis of atypical manifestations of vertebral lesions, especially in patients with risk factors. Early diagnosis, preferably followed by anterior radical surgery combined with antiparasitic therapy of sufficient duration, is the solution to at least prevent the progression of symptoms. Surgery and chemotherapy can improve the symptoms in most cases but may not completely cure the patient’s condition or prevent recurrences[6].
Multimodal therapy for spinal cystic echinococcosis includes drug therapy and surgical treatment. Surgery is still recognized as the “gold standard” treatment[7]. The only cure for spinal cystic echinococcosis is radical resection. However, this is rarely possible. Here, we present a case in which a patient underwent two thorough surgical treatments but was still not completely cured.
In September 2012, a 48-year-old man presented with progressive back pain, weakness in the lower limbs, significant weight loss for 1 year, and paralysis of both lower limbs for 1 mo.
Patient’s symptoms started 1 year ago with progressive back pain, paralysis of both lower limbs for 1 mo. The patient was misdiagnosed with spinal tuberculosis in an outside hospital and underwent anti-tuberculosis treatment for 9 mo.
His past medical history was normal.
The patient was a herder living in a pastoral area for a long time.
Local examination of the spine revealed tenderness in the spinous process of the T8 and T9 vertebrae. There was hypoesthesia below the umbilical plane. Neurological examination revealed spastic paralysis with lower extremity motor powers of 0/5. Deep tendon flexes revealed hyperreflexia, and the Babinski sign was positive. Anal reflex, anal tonus, and voluntary anal contraction were present.
The initial laboratory examination showed normal leukocytes (6.24 × 109/L), neutrophils (4.14 × 109/L, 66.3%), and eosinophils (0.21 × 109/L, 3.4%). C-reactive protein was 0.6 mg/dL, and the erythrocyte sedimentation rate was 1 mm/h. The T-SPOT test result for tuberculosis was negative, but the ELISA test result for Echinococcus granulosus was positive.
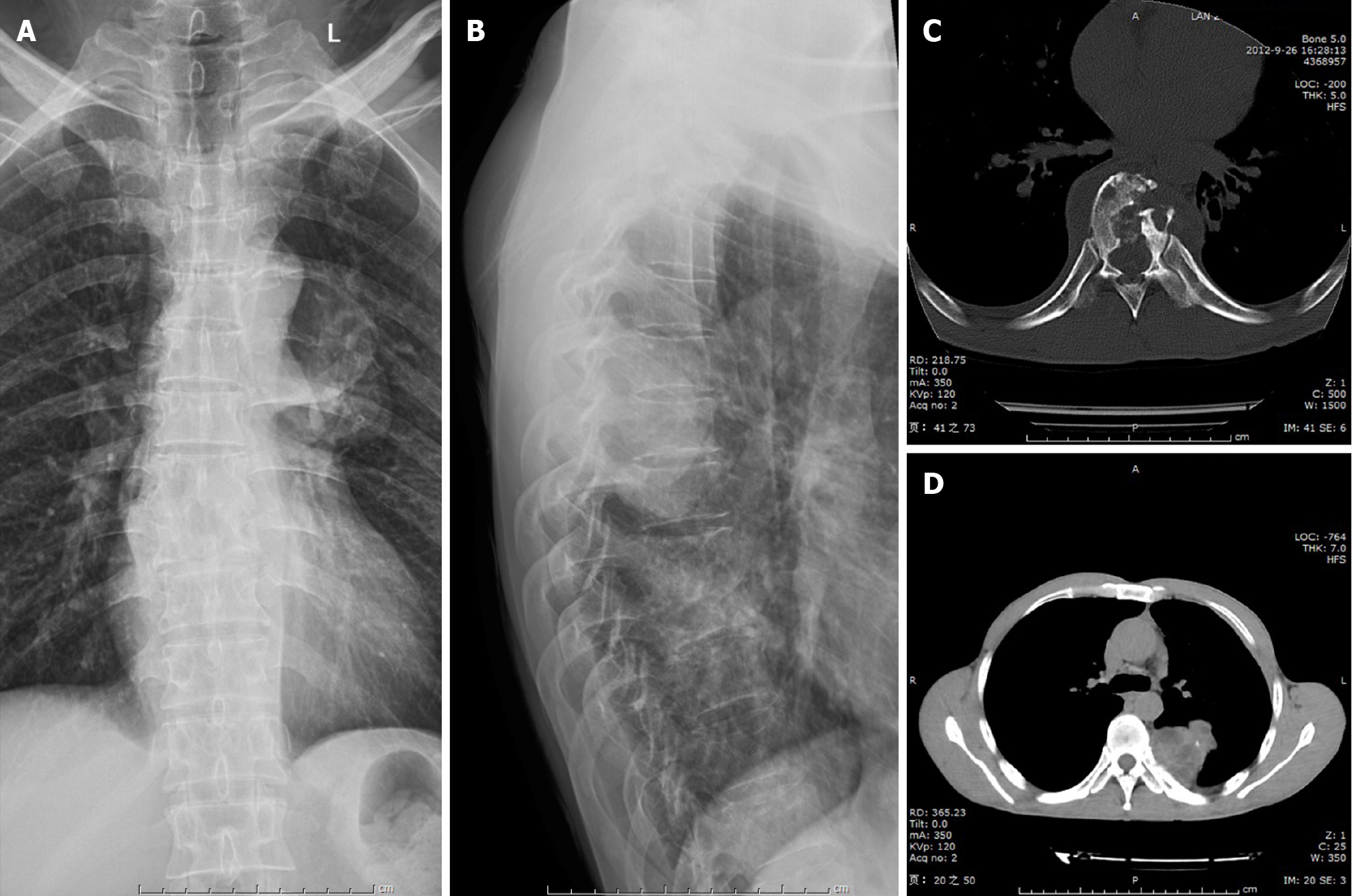
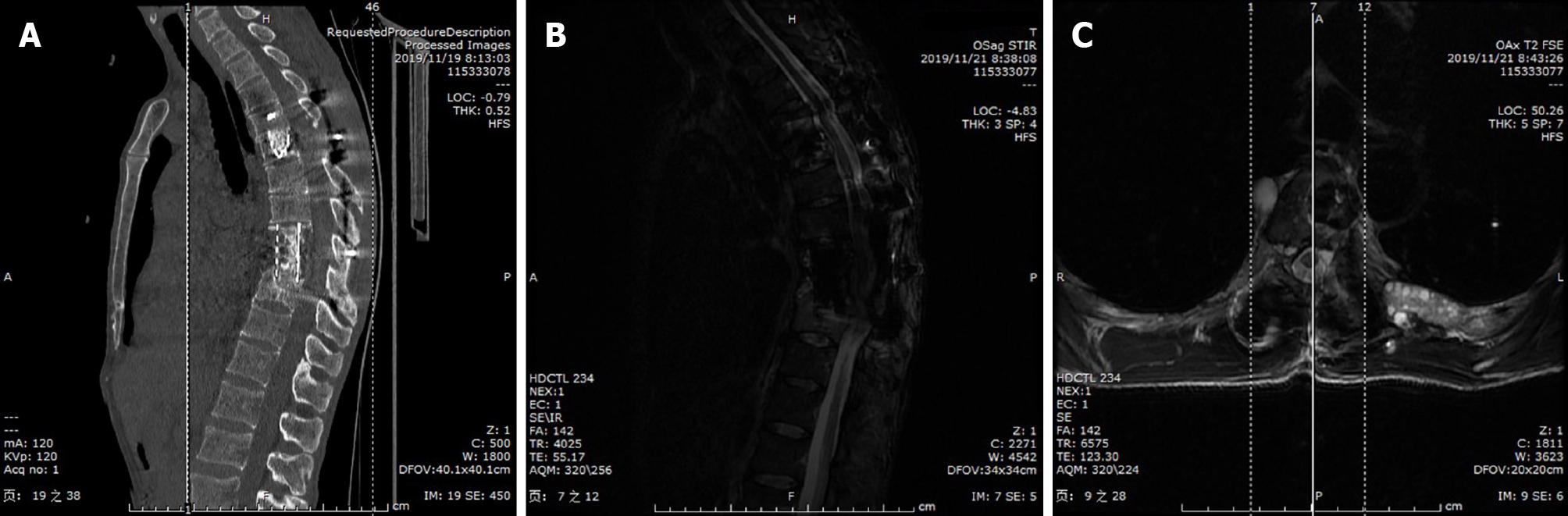
Radiography images revealed that the T8–9 disc space narrowed and the vertebral body height of T9 decreased (Figure 1). Axial computed tomography (CT) of the thoracic spine confirmed osteolytic destruction of the T8 and T9 vertebrae and the posterior part of the 4th, 5th, and 6th ribs on the left. Heterogeneous lesions containing hypodense cystic areas and partial calcification were also detected on the left paravertebra (Figure 1). Thoracic magnetic resonance imaging (MRI) showed hyper
The diagnosis of spinal hydatidosis and lung hydatid disease was based on laboratory findings and typical imaging findings.
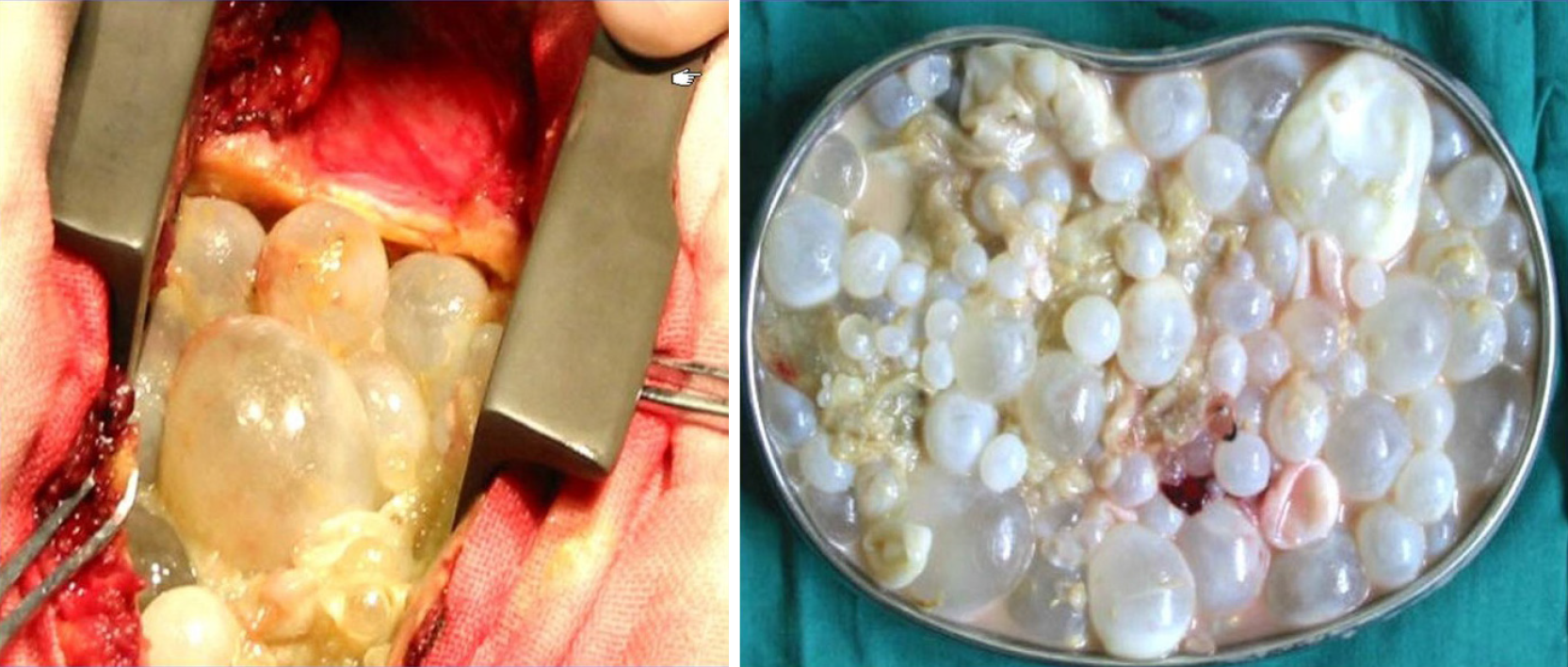
An operation to achieve dorsal spine arthrodesis of segments T7–T10 was performed. Then, the patient underwent left transthoracic approach lobectomy, paravertebral lesion debridement, and subtotal vertebrectomy with vertebral body replacement of T8 and T9 by a mesh cage. Intraoperatively, the cystic lesion had a white crystal-like appearance (Figure 3). The entire vertebral body and spinal canal were infiltrated by white granular tissue, destroying the integrity of the vertebral body. The surgical wound was soaked in 10% hypertonic sodium chloride solution for 30 min. Postoperative histopathology confirmed cystic echinococcosis.
The patient underwent albendazole chemotherapy before and after surgery; the patient received two doses a day for a total of 15 mg/kg/d. Hemopoiesis and liver enzymes were monitored as recommended during treatment. Two weeks after the rehabilitation program, the muscle strength of both of the patient’s lower extremities recovered to 3/5. The senses were normal. After 6 wk, the patient was walking independently.
Two years later (in 2014, one year after voluntarily stopping the medication), the patient experienced increased spinal cord dysfunction, insufficient motor function, numbness, and decreased peripheral sensitivity. However, the patient did not exhibit significant sphincter dysfunction. MRI showed new lesions in the T5 vertebra and spinal canal. After continuing to take albendazole, the patient's sensorimotor function gradually recovered. Therefore, it was recommended that the patient take the albendazole drug treatment for life.
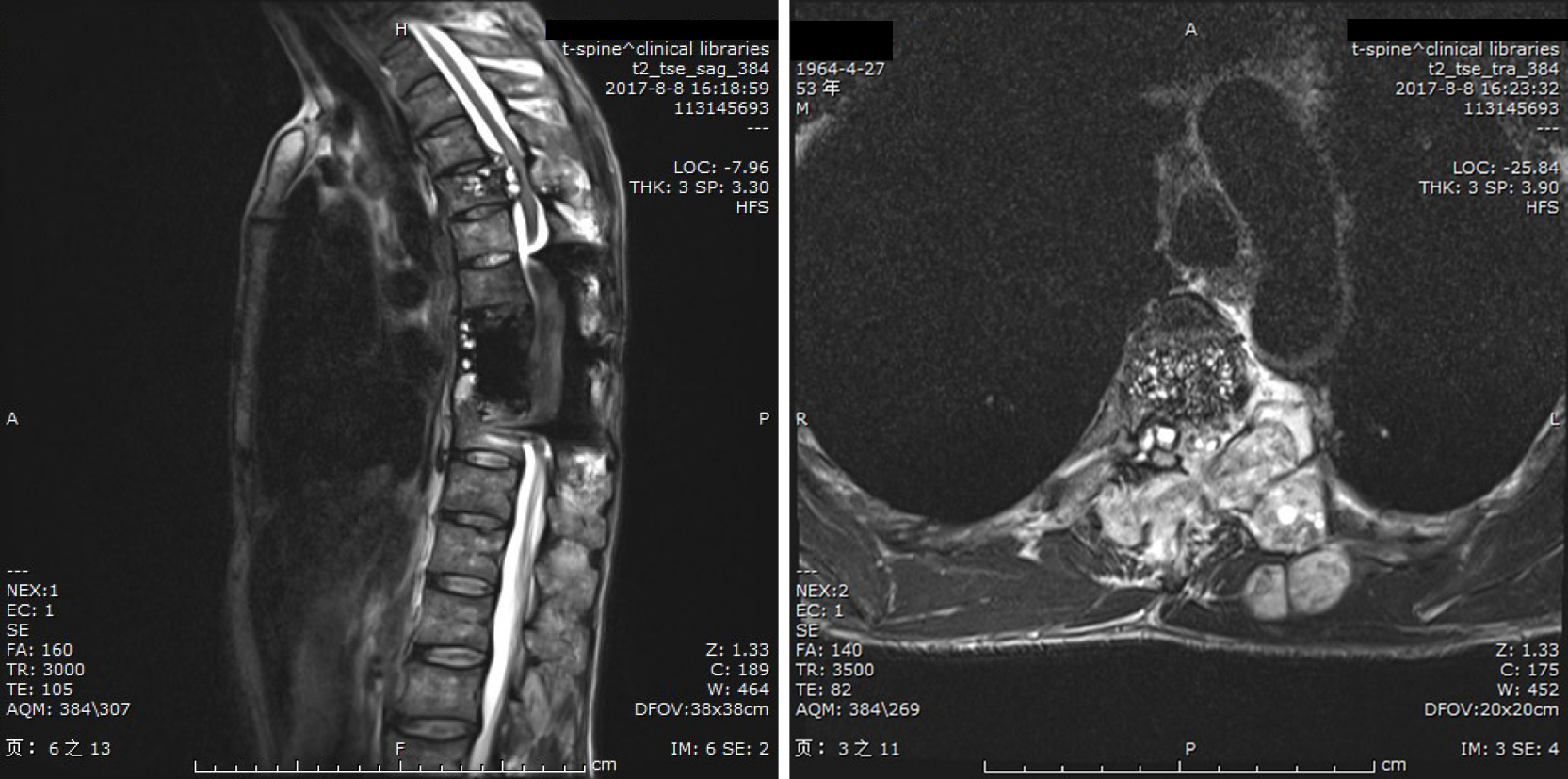
During continued chemotherapy, the patient relapsed with partial paralysis of both lower extremities in 2017, and the muscle strength of both lower extremities was 2/5. Patella and Achilles reflexes were hyperactive. Thoracic MRI revealed anomalous cystic lesions with extradural expansion and invasion of the T5 vertebral body and left pedicle and transverse processes of the T5-T6 vertebrae that extended to the ribs and spinal canal and compressed the spinal cord (Figure 4). A second posterior surgical procedure was performed on the patient to remove spinal cysts. Laminectomy, costotransversectomy, and vertebrectomy were all performed at the T5 level, and the epidural cyst was removed at the same time. The patient was discharged in an independent ambulatory state 2 wk later. During the 3-year follow-up period after the second operation, the patient's sensory and motor functions were normal. Postoperative CT showed that the intervertebral bone graft had been fused, but MRI showed that there were still hyperintense lesions in the paravertebral region (Figure 5). This patient is still undergoing long-term albendazole chemotherapy.
Spinal cystic echinococcosis has no characteristic signs or symptoms. Therefore, the diagnosis of such cases is difficult and is frequently delayed until signs and symptoms of spinal cord or nerve compression appear. The most common clinical manifestation is pain (59.2%–75%), followed by loss of leg strength (37%–50%)[4,5]. This patient was misdiagnosed with spinal tuberculosis in another hospital. Therefore, in the initial diagnosis of spinal tuberculosis or spinal pyogenic infection, according to the patient’s clinical symptoms and signs, a differential diagnosis should consider spinal hydatidosis, malignancies, giant cell tumors of bone, etc.[8,9]. The overall incidence of spinal hydatidosis is very low, and it is more likely to be misdiagnosed and missed. Therefore, surgeons should have sufficient knowledge about spinal hydatidosis.
Another way to reduce the misdiagnosis of spinal hydatidosis is to fully understand the imaging manifestations of hydatid disease. The most common radiologic feature of osseous lesions is a combination of multilocular cysts and reactive sclerosis[10]. When spinal hydatidosis is suspected, MRI is the first choice for evaluation. T2 images often show hyperintense cysts with clear boundaries that are rounded or oval in shape and flow together, while cyst septations produce “wheel-like” structures, and the presence of daughter cysts is indicated by “rosette-like” or honeycomb-like” structures[2,10-12]. Spinal cystic echinococcosis has a characteristic appearance of images resembling a bunch of grapes (Figure 2)[4,13]. In the typical MRI findings of this patient, would not difficult for a physician with experience in the diagnosis and treatment of hydatid disease to make a preliminary diagnosis of spinal hydatidosis.
The theoretical cure for spinal hydatidosis is radical resection, but the cure rate is zero when surgical intervention is performed alone[4]. The main goal of spinal hydatidosis surgery should be to remove the infected vertebrae in a craniocaudal fashion from healthy bones to healthy bones[14] and to eradicate cysts and scolexes. The best operation is radical resection with a safety margin of 2 cm[15], but this is almost impossible for patients with vertebral disease. Similar to what was done for this patient, the paravertebral lesions around the T5 and T6 vertebrae can be completely resected, but we cannot initially expand the excision to the T5 and T6 vertebrae because this will cause spinal instability. During this patient’s operation, we resected the spinal and paravertebral lesions as integrally and completely as possible. The cyst was carefully removed to avoid overflow, and hypertonic saline was used to extinguish the scolex. However, we still could not prevent recurrence. After the first resection of paravertebral lesions, the patient had recurrent lesions in the T5 vertebra. Relapse of spinal hydatidosis is very common[16], and the recurrence rate reported in the literature is as high as 89%–92.6%[4,17]. If surgery cannot prevent recurrence, then pre- and postoperative antiparasitic drug therapy is a very important supplementary treatment.
Subtotal vertebral resection of hydatid lesions may be the main reason for the high recurrence rate of spinal hydatidosis. Therefore, even if only a part of the vertebral body is involved, some authors still recommend total vertebrectomy to prevent recurrence. Liang et al[18] reported that there was no recurrence after total en bloc spondylectomy of spinal hydatidosis, and the patients were free of disease[19]. This suggests that expanded total en bloc spondylectomy may be a better choice for this patient. However, for patients who cannot undergo total vertebrectomy, the intraoperative use of scolicidals and the use of albendazole before and after surgery are currently considered to be the standard for the treatment of spinal hydatidosis[20].
It is generally believed that the combination of medical treatment and surgery to prevent intraoperative cyst spillover can reduce the risk of spinal hydatidosis recurrence. Albendazole medication may not cure or prevent the recurrence of spinal hydatidosis, but it is still the only treatment option currently available for inoperable patients. Albendazole is administered to patients twice a day for a total dose of 10–15 mg/kg per day and is regarded by the World Health Organization as the first-choice and mainstay drug for the treatment of hydatid disease[2,15,21]. Praziquantel appears to have a synergistic effect by increasing plasma levels of albendazole, and there is some evidence to support the use of praziquantel in combination with albendazole during surgery[4,22]. This patient has been taking albendazole for 8 years and has not been completely cured, suggesting that the combination of praziquantel and albendazole may be a better choice.
Recurrence of spinal hydatidosis may occur 20 years after initial occurrence[23,24], and albendazole withdrawal is a decisive factor in the dramatic evolution of patients. Since radical therapy for spinal hydatidosis is rarely feasible, follow-up should be performed throughout the patient’s lifetime[25]. It may also require life-long antiparasitic drug treatment. Patients with spinal hydatidosis require long-term follow-up to understand the potential for recurrence and possible complications or sequelae associated with surgery or antiparasitic treatment. The prognosis of spinal hydatidosis is still poor, and early recognition is essential for optimal recovery.
We report a rare case of typical refractory spinal hydatidosis, and the MRI finding of a “bunch of grapes” imaging feature is typical of spinal hydatidosis. Subtotal vertebrectomy is a risk factor for postoperative recurrence. Total spondylectomy makes it possible to cure spinal hydatidosis, but antiparasitic drug therapy is also an important supplementary therapy to multimodal therapy. Preferably, patients with spinal hydatidosis should receive life-long antiparasitic medication therapy and follow-up.
We thank the patient and the members of the team.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beiromvand M S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Mahmoudi S, Mamishi S, Banar M, Pourakbari B, Keshavarz H. Epidemiology of echinococcosis in Iran: a systematic review and meta-analysis. BMC Infect Dis. 2019;19:929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Song XH, Ding LW, Wen H. Bone hydatid disease. Postgrad Med J. 2007;83:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Steinmetz S, Racloz G, Stern R, Dominguez D, Al-Mayahi M, Schibler M, Lew D, Hoffmeyer P, Uçkay I. Treatment challenges associated with bone echinococcosis. J Antimicrob Chemother. 2014;69:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Monge-Maillo B, Olmedo Samperio M, Pérez-Molina JA, Norman F, Mejía CR, Tojeiro SC, López-Vélez R. Osseous cystic echinococcosis: A case series study at a referral unit in Spain. PLoS Negl Trop Dis. 2019;13:e0007006.. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Gezercan Y, Ökten AI, Çavuş G, Açık V, Bilgin E. Spinal Hydatid Cyst Disease. World Neurosurg. 2017;108:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Hall WA. Spinal parasites. World Neurosurg. 2015;83:39-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Celik C, Sasmaz MF, Oktay F, Ucan H, Kaptanoglu E. Paraplegia associated with spinal hydatid cyst: a case report. Spine (Phila Pa 1976). 2010;35:E356-E358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Butt A, Khan J. The Maverick Disease: Cystic Echinococcosis in Unusual Locations: A Ten Year Experience from an Endemic Region. Cureus. 2019;11:e5939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Saul D, Seitz MT, Weiser L, Oberthür S, Roch J, Bremmer F, Perske C, Viezens L, Sehmisch S, Lehmann W. Of Cestodes and Men: Surgical Treatment of a Spinal Hydatid Cyst. J Neurol Surg A Cent Eur Neurosurg. 2020;81:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Teke M, Göçmez C, Hamidi C, Gündüz E, Göya C, Çetinçakmak MG, Hattapoğlu S, Durmaz MS. Imaging features of cerebral and spinal cystic echinococcosis. Radiol Med. 2015;120:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Majmundar N, Patel PD, Dodson V, Tran A, Goldstein I, Assina R. Parasitic infections of the spine: case series and review of the literature. Neurosurg Focus. 2019;46:E12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Zalaquett E, Menias C, Garrido F, Vargas M, Olivares JF, Campos D, Pinochet N, Luna A, Dahiya N, Huete Á. Imaging of Hydatid Disease with a Focus on Extrahepatic Involvement. Radiographics. 2017;37:901-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Zhang Z, Li F, Zhao G, Sun T. "Bunch of grapes" on the spine-spinal hydatidosis. Braz J Infect Dis. 2012;16:313-314. [PubMed] |
| 14. | Caglar YS, Ozgural O, Zaimoglu M, Kilinc C, Eroglu U, Dogan I, Kahilogullari G. Spinal Hydatid Cyst Disease : Challenging Surgery - an Institutional Experience. J Korean Neurosurg Soc. 2019;62:209-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Meinel TR, Gottstein B, Geib V, Keel MJ, Biral R, Mohaupt M, Brügger J. Vertebral alveolar echinococcosis-a case report, systematic analysis, and review of the literature. Lancet Infect Dis. 2018;18:e87-e98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Xia Y, Ju Y, Liu JP, Chen LY. Common Spinal Parasites. Turk Neurosurg. 2019;29:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Kafaji A, Al-Zain T, Lemcke J, Al-Zain F. Spinal manifestation of hydatid disease: a case series of 36 patients. World Neurosurg. 2013;80:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Liang Q, Xiang H, Xu L, Wen H, Tian Z, Yunus A, Wang C, Jiang D, Abuduwaili M, Chen J, Song X. Treatment experiences of thoracic spinal hydatidosis: a single-center case-series study. Int J Infect Dis. 2019;89:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Kandwal P, Vijayaraghavan G, Upendra BN, Jayaswal A. Single-stage vertebrectomy for hydatid disease involving L3 vertebra: Five year follow-up. Neurol India. 2018;66:1499-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Neumayr A, Tamarozzi F, Goblirsch S, Blum J, Brunetti E. Spinal cystic echinococcosis--a systematic analysis and review of the literature: part 2. Treatment, follow-up and outcome. PLoS Negl Trop Dis. 2013;7:e2458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Kern P, Menezes da Silva A, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C, Vuitton DA. The Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. Adv Parasitol. 2017;96:259-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 310] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 22. | Mohammadi A, Sabet B, Amini M, Mohammadi M. Short-Course Combination therapy with Albendazole and Praziquantel Chemotherapy in recurrent complicated case of vertebral hydatidosis. Iran Red Crescent Med J. 2008;10:241-243. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | González-Redondo R, DiCaudo C, García-García D, Zubieta JL, Viteri-Torres C. Spinal hydatidosis relapse related to albendazole withdrawal after 20-year treatment. Spine J. 2013;13:715-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Neumayr A, Tamarozzi F, Goblirsch S, Blum J, Brunetti E. Spinal cystic echinococcosis--a systematic analysis and review of the literature: part 1. Epidemiology and anatomy. PLoS Negl Trop Dis. 2013;7:e2450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Baldo S, Tacconi L. Spinal hydatid cysts: a long-life disease? J Neurosurg Sci. 2020;64:117-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |