Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10300
Peer-review started: July 8, 2021
First decision: August 18, 2021
Revised: August 31, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: November 26, 2021
Processing time: 137 Days and 0.4 Hours
Glioblastoma has a high degree of malignancy and poor prognosis. It is common to have in situ recurrence and intracranial metastasis, while extracranial metastasis is rare, and extracranial multiorgan metastasis is extremely rare. We report a case of glioblastoma with extracranial multiorgan metastasis, which will strengthen clinicians’ attention to the extracranial metastasis of glioblastoma and its treatment.
A male patient visited our hospital for treatment of dizziness and headache. Magnetic resonance imaging of the brain revealed a space-occupying lesion in the right temporoparietal occipital region. Chest computed tomography and abdominal ultrasound were normal, and no space-occupying lesions were observed in other organs of the body. The patient underwent surgery and diagnosed with glioblastoma. Postoperative concurrent radiotherapy and chemotherapy were completed. During the follow-up, the tumor was found to have metastasized to the scalp and neck, and a second tumor resection was performed. Postoperative follow-up revealed extracranial metastases to multiple extracranial organs including skull, scalp, ribs, spine, liver and lung. His family members refused further treatment, and requested only symptomatic treatment such as pain relief, and the patient died of systemic multiple organ failure. Survival time from diagnosis to death was 13 mo and from extracranial metastasis to death was 6 mo.
Glioblastoma extracranial metastasis is extremely rare, clinicians should always pay attention to its existence. The mechanism of glioblastoma extracranial metastasis is still unclear, and genetic and molecular studies are required.
Core Tip: Glioblastoma is common to have in situ recurrence and intracranial metastasis, while extracranial metastasis is rare, and extracranial multiorgan metastasis is extremely rare. We reported a case of extracranial multiple organ metastasis of glioblastoma, reviewed relevant literature, and discussed the related mechanism and treatment plan. This report may provide more possibilities for the related mechanism and treatment of extracranial metastasis of glioblastoma.
- Citation: Luan XZ, Wang HR, Xiang W, Li SJ, He H, Chen LG, Wang JM, Zhou J. Extracranial multiorgan metastasis from primary glioblastoma: A case report. World J Clin Cases 2021; 9(33): 10300-10307
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10300.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10300
Glioblastoma is the most common primary malignant tumor of the central nervous system (CNS). It often recurs in situ and rarely metastases, especially extracranially. Extracranial multiorgan metastasis is even rarer. We report a case of postoperative glioblastoma with not only extracranial metastasis but also multiorgan metastasis; the relevant literature is subsequently reviewed.
A 47-year-old man presented with dizziness for 1 mo and headache for 1 wk without other discomfort.
The patient developed dizziness without obvious cause 1 mo ago, and was obviously dizzy and uncomfortable when squatting and suddenly standing up. The head distension and pain occurred 1 wk ago, and the symptoms persisted, but there was no relief after rest.
The patient had a history of right elbow injury caused by a car accident 20 years ago.
Denial of personal and family history.
Physical examination showed no obvious abnormality and blood pressure was 130/80 mmHg.
Blood analysis revealed mild leukocytosis 12.5 × 109/L, with predominant neutrophils (80%) with normal hematocrit and platelet count. Other tests were within the normal range.
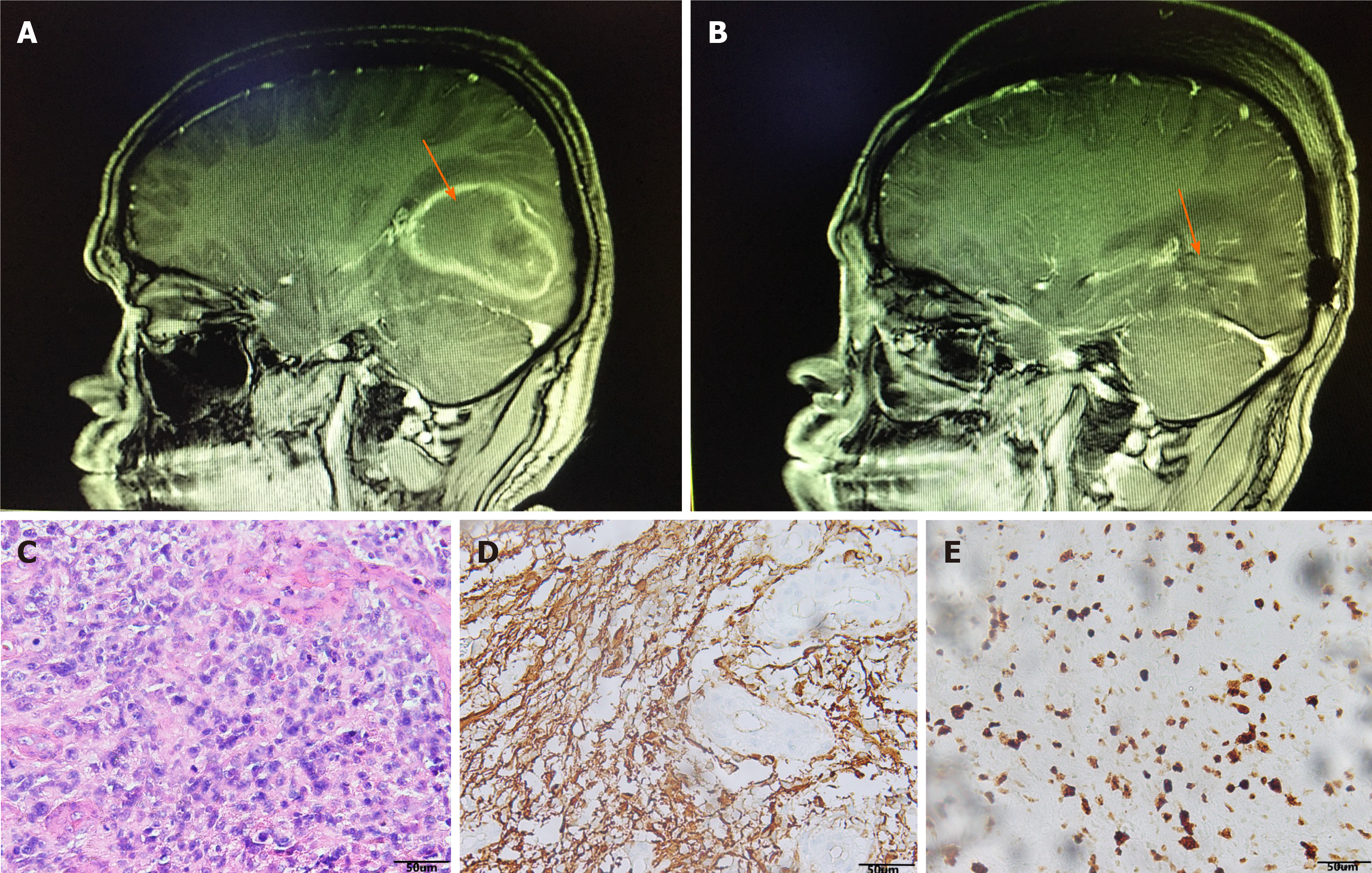
Magnetic resonance imaging (MRI) of the brain revealed a space-occupying lesion of 62 mm × 40 mm × 43 mm in the right temporoparietal occipital region. T1-weighted and T2-weighted images showed high signal. The contrast-enhanced scan showed irregular annular enhancement, and the cerebral line shifted about 0.8 cm to the left (Figure 1A). Chest computed tomography (CT) and abdominal ultrasound were normal, and no space-occupying lesions were observed in other organs of the body.
After admission, the lesion was extensively excised and the ventricle was opened intraoperatively (Figure 1B). The pathological results of the patient suggested that hematoxylin-eosin staining showed a large amount of necrosis and scattered heteromorphic cells, considering high-grade glioblastoma (Figure 1C), glial fibrillary acidic protein (+) (Figure 1D), Ki67 (+20%) (Figure 1E). Furthermore, pathological examination revealed that immune phenotype IDH1 wild type, IDH2 wild type, MGMT unmethylated, and the diagnosis was glioblastoma (World Health Organ
The patient was diagnosed with glioblastoma (IDH-wild type).
Postoperative concurrent chemoradiotherapy with the following regimen was administered: Radiotherapy (daily fractions of 2 Gy given 5 d/wk for 6 wk, for a total of 60 Gy) plus continuous daily temozolomide (75 mg/m2 body-surface area per day, 7 d/wk from the first to the last day of radiotherapy), followed by six cycles of adjuvant temozolomide (150-200 mg/m2 for 5 d during each 28-d cycle, and regular follow-up.
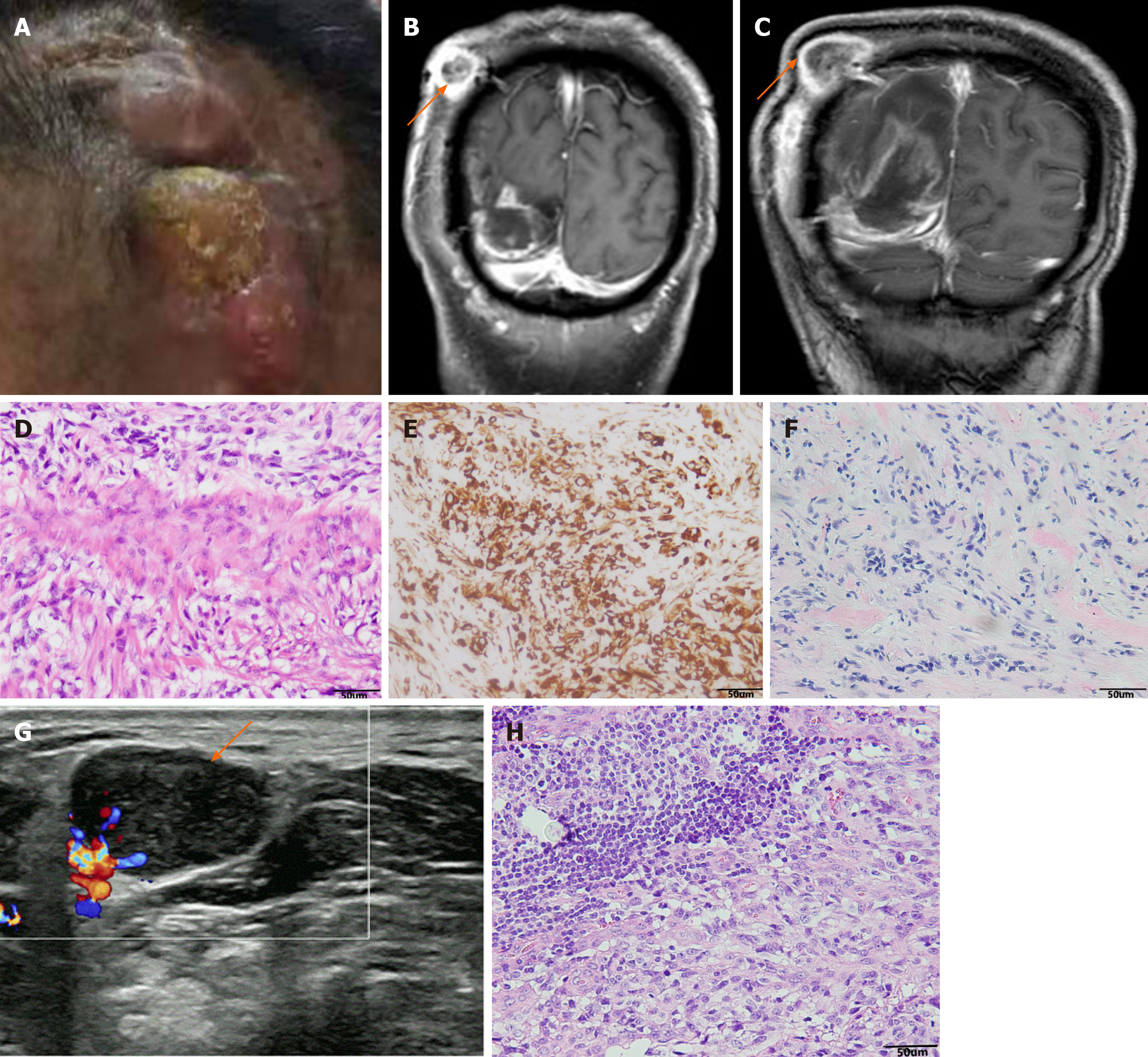
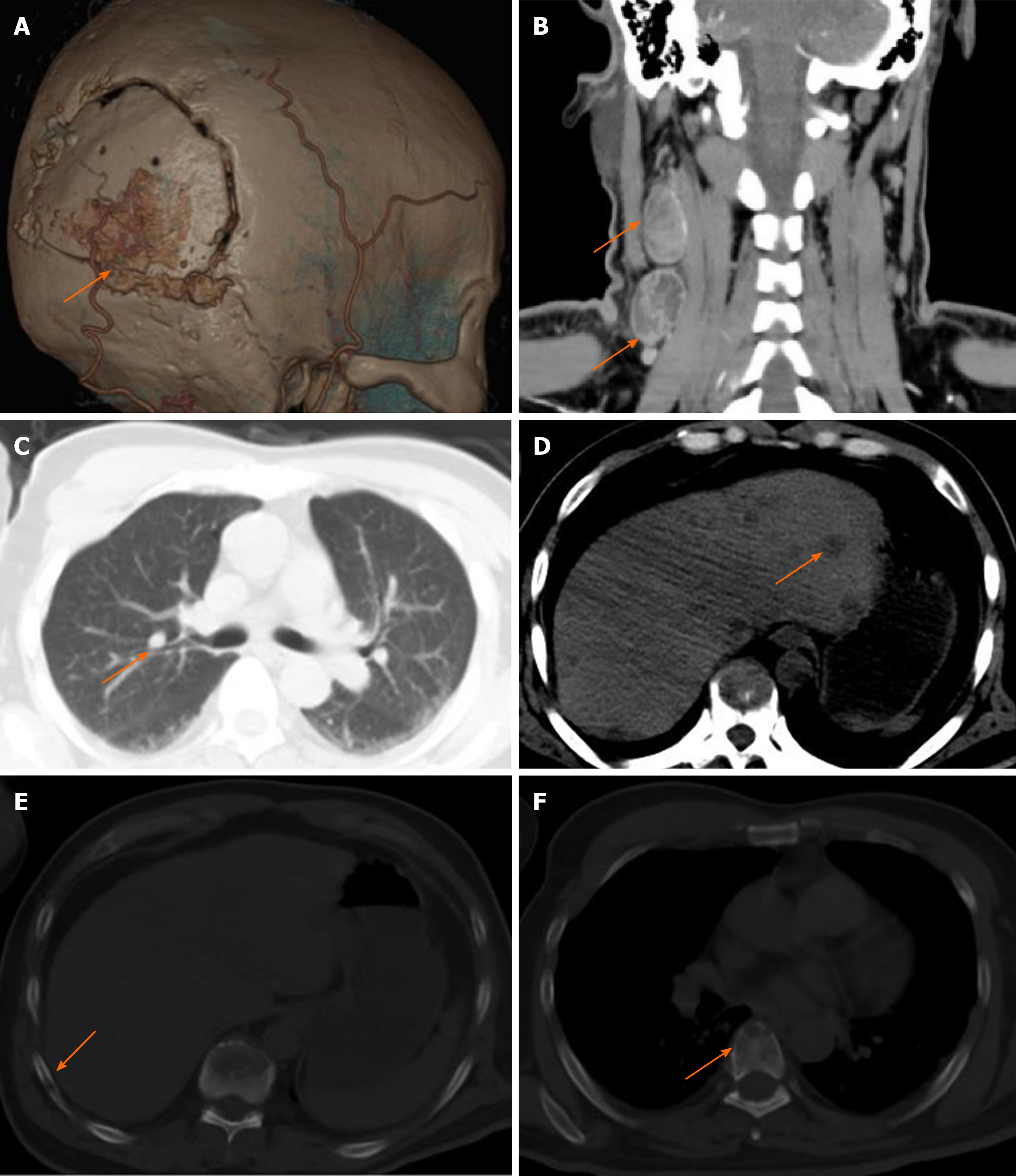
Six months after surgery, the patient had a mass at the site of surgical incision (Figure 2A), and intracranial recurrence and subcutaneous metastasis were considered in the re-examination of enhanced MRI (Figure 2B). Family members required temporary observation and simultaneous radiotherapy and chemotherapy. Nine months postoperatively, the patient was readmitted due to enlargement of the scalp incision and a new neck mass (Figure 2C). After the second admission, the patient underwent maximum lesion resection and scalp mass resection. Pathological examination of the scalp mass revealed glioblastoma, which was confirmed as extracranial metastasis (Figure 2D-F). Ultrasound indicated that the cervical mass was cervical lymph node enlargement, and we performed excision of the mass (Figure 2G). The pathological examination was consistent with glioblastoma metastasis to the cervical lymph nodes (Figure 2H). Follow-up head and chest CT showed metastases in the skull, scalp, ribs, spine, liver and lungs, and the patient died of systemic organ failure (Figure 3A-F). We suggested that the patient complete positron emission tomography/CT examination and further surgical treatment, but the patient and his family members refuse further examination and surgery, and only palliative treatment. Survival time from diagnosis to death was 13 mo and from extracranial metastasis to death was 6 mo.
Postoperative recurrence of glioblastoma is common, but metastasis is rare and extracranial multiorgan metastasis is extremely rare[1].
Weiss[2] proposed the diagnostic criteria for extraencephalic metastasis of malignant tumors in 1955: (1) The histological features of the CNS neoplasm must be demonstrated to be unitary; (2) The initial symptoms must be caused by the tumor in the clinical history; (3) A full autopsy is necessary and there is sufficient evidence to rule out the possibility of other tumors; and (4) The pathomorphology of distant metastases and CNS neoplasm must be consistent, and appropriate degrees of difference can be considered. In view of the histological features of brain glioblastoma and the histological manifestations of scalp and neck lymph nodes, the present case met the diagnostic criteria proposed by Weiss (except autopsy).
To further explore the characteristics of glioblastoma extracranial metastases, we reviewed cases of intracranial tumors with extracranial metastases reported in the past 5 years (Table 1). We found the following features: (1) Glioblastoma is the most common intracranial tumor with extracranial metastasis, although astrocytomas and diffuse glioblastoma have also been reported; (2) The most common site of the disease is the temporal lobe, and the frontal and occipital lobes have also been reported; (3) The most common site of extracranial metastasis is the spinal canal, followed by liver, lungs, lymph nodes and other sites, while extracranial multiorgan metastasis is relatively rare; and (4) The average time from diagnosis to death for intracranial tumors was 15.2 mo, and the discovery of extracranial metastases was approximately 4 mo. Our patient had glioblastoma located in the right temporal lobe with extracranial metastases to the skull, scalp, ribs, spine, liver and lungs. The survival time from diagnosis to death was 13 mo, and the survival time from extracranial metastases to death was 6 mo.
| No. | Ag/sex | Location primary | Location metastasis | Diagnose | Interval | Survival | Adjuvant therapy |
| 1 | 65/M | R occipital lobe | Bones (femur, ilium, sacrum) | GBM | 13 | 11 | Concurrent radiochemotherapy |
| 2 | 20/W | L temporal lobe | Lung, lymph gland | GBM | NS | NS | Chemotherapy (temozolomide) |
| 3 | 75/W | L temporal lobe | Lung, pleura | Diffuse astrocytoma | NS | NS | NS |
| 4 | 48/W | L temporal lobe | Bone, lung, pleura, liver, mesentery | GBM | 13 | 11 | Concurrent radiochemotherapy |
| 5 | 43/W | L frontal lobe | Lung, pleura | GBM | 38 | 2 | Concurrent radiochemotherapy |
| 6 | 49/M | R temporal lobe | Lung, cerebrospinal fluid | GBM | 12 | 10 | Concurrent radiochemotherapy |
| 7 | 56/W | R temporal lobe | Parotid, lymph node, lung | GBM | 14.5 | 11 | Concurrent radiochemotherapy |
| 8 | 32/M | L basal ganglia region | Lymph nodes, bones (ribs, scapula, spine) | GBM | 22 | 3 | Concurrent radiochemotherapy |
| 9 | 41/W | R temporal lobe | Lymphatic, spinal column | GBM | 10 | 1 | Concurrent radiochemotherapy |
| 10 | 43/M | R temporal lobe | Multiple bone metastases | GBM | 3 | 2 | Radiotherapy |
| 11 | 38/W | R temporal lobe | Lymph gland, bones | GBM | 10 | 6 | Concurrent radiochemotherapy |
| 12 | 23/W | L temporal lobe | Lung, multiple bone metastases | Oligoastrocytoma | 36 | 5 | Concurrent radiochemotherapy |
| 13 | 47/M | R temporal lobe | Scalp, lymph gland ribs, spine, liver, lungs | GBM | 13 | 6 | Concurrent radiochemotherapy |
The mechanisms of glioblastoma extracranial metastasis are still not well known. Many reports of extracranial glioblastoma metastases show a strong correlation between these lesions and preceding intracranial operation such as aggressive surgical resection, biopsy, and ventriculopleural shunting, in particular, the intraoperative opening of the ventricle. Extracranial metastases have been attributed commonly to tumor cells depositing into the bloodstream or to surgical defects in the dura and skull[3]. However, Anzil[4] found that > 10% of all cases occurred in the absence of prior surgical intervention. He believed that aggressive operations are not prerequisites for extracranial metastasis of glioblastoma, also suggesting that early hematogenous spread may be a mechanism. While intracranial operations are not necessary in the development of extracranial glioblastoma metastases, such operations provide opportunities for vascular invasion and cerebrospinal fluid dissemination of tumor cells, thereby increasing the risk of systemic spread and extracranial tumor metastasis[5,6]. The most common mechanism of glioblastoma metastasis outside the CNS/neuraxis is likely vascular invasion, which indicates the existence of circulating tumor cells in the bloodstream. Therefore, the tumor-free principle becomes especially important during surgery and ensures dura mater integrity. In addition, the fraction of tumor cells in the circulation may provide an opportunity for early detection and genetic analysis of the intracranial glioblastoma[7]. In our patient, the ventricle was opened and the dura was destroyed during the operation. The opening of the ventricular system led to tumor invasion into the ventricular system, coupled with the destruction of the dura and invasion of the epidural blood vessels, leading to the systemic metastasis of multiple organs. However, beyond these factors, is there a deeper genetic connection to extracranial metastasis?
Our case suggests that MGMT is not methylated, and whether extracranial diffusion is affected by MGMT methylation status remains unknown. A significant study by Hegi et al[8] suggested that MGMT promoter methylation is an independent prognostic factor, and adjuvant TMZ can be beneficial in glioblastoma patients treated with methylated MGMT promoter. It has been reported that MGMT status is potentially in contact with extracranial metastasis, and distant metastasis of oligoendrocytes and melanoma in tumors has been reported[9,10]. MGMT promoter methylation is closely associated with distant metastasis, and there is considerable heterogeneity. However, whether MGMT promoter methylation status is associated with poor prognosis in GBM has not been found. MGMT promoter status with glioblastoma extracranial metastasis merits further research.
Currently, the standard initial approach for most primary glioblastomas is maximum safe surgical resection followed by postoperative concurrent radiotherapy and chemotherapy[11]. The same is true of our patient treatment regimens. In the case of extracranial metastases, there is no standard treatment; surgery, radiation and systemic chemotherapy or bevacizumab are all potential options, depending on the patient’s condition[11]. Studies have suggested a possible beneficial pairing between immunotherapy and TMZ therapy, and radiation-driven evolution may have therapeutic implications for recurrent GBM[12,13]. Moreover, Draaisma et al[14] found that MGMT promoter methylation had a prognostic effect in tumor recurrence, and the high-mutated phenotype only occurred in 6%-8% of TMZ-treated IDH WT GBMs. Therefore, for patients with extracranial multiple organ metastasis of glioblastoma, simultaneous radiotherapy and chemotherapy combined with immunotherapy is worth a try, and the study of targeted genes is a direction worthy of profound exploration.
Malignant glioblastoma with extracranial multiorgan metastasis is rare, and survival is short. Surgeons should always be alert to its occurrence. At present, surgical treatment and concurrent chemoradiotherapy are still the primary options for glioblastoma, so attention should be paid to achieving tumor-free status during surgery to reduce the possibility of extracranial metastasis. The mechanism of extracranial metastasis of glioblastoma is still not clear, thus genetic and molecular pathology is still worthy of further exploration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kołat D, Li C S-Editor: Gao CC L-Editor: A P-Editor: Li JH
| 1. | Kalokhe G, Grimm SA, Chandler JP, Helenowski I, Rademaker A, Raizer JJ. Metastatic glioblastoma: case presentations and a review of the literature. J Neurooncol. 2012;107:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Mavroudis C, Townsend JJ, Wilson CB. A metastasizing ependymoma of the cauda equina. Case report. J Neurosurg. 1977;47:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Figueroa P, Lupton JR, Remington T, Olding M, Jones RV, Sekhar LN, Sulica VI. Cutaneous metastasis from an intracranial glioblastoma multiforme. J Am Acad Dermatol. 2002;46:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Anzil AP. Glioblastoma multiforme with extracranial metastases in the absence of previous craniotomy. Case report. J Neurosurg. 1970;33:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Frank S, Kuhn SA, Brodhun M, Mueller U, Romeike B, Kosmehl H, Regenbrecht CR, Ewald C, Reichart R, Kalff R. Metastatic glioblastoma cells use common pathways via blood and lymphatic vessels. Neurol Neurochir Pol. 2009;43:183-190. [PubMed] |
| 6. | Thorsen F, Tysnes BB. Brain tumor cell invasion, anatomical and biological considerations. Anticancer Res. 1997;17:4121-4126. [PubMed] |
| 7. | Adamczyk LA, Williams H, Frankow A, Ellis HP, Haynes HR, Perks C, Holly JM, Kurian KM. Current Understanding of Circulating Tumor Cells - Potential Value in Malignancies of the Central Nervous System. Front Neurol. 2015;6:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5037] [Cited by in RCA: 5274] [Article Influence: 263.7] [Reference Citation Analysis (0)] |
| 9. | Li G, Zhang Z, Zhang J, Jin T, Liang H, Gong L, Cui G, Yang H, He S, Zhang Y, Gao G. Occipital anaplastic oligodendroglioma with multiple organ metastases after a short clinical course: a case report and literature review. Diagn Pathol. 2014;9:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Kohonen-Corish MR, Cooper WA, Saab J, Thompson JF, Trent RJ, Millward MJ. Promoter hypermethylation of the O(6)-methylguanine DNA methyltransferase gene and microsatellite instability in metastatic melanoma. J Invest Dermatol. 2006;126:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: State of the art and future directions. CA Cancer J Clin. 2020;70:299-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 1209] [Article Influence: 241.8] [Reference Citation Analysis (0)] |
| 12. | Hotchkiss KM, Sampson JH. Temozolomide treatment outcomes and immunotherapy efficacy in brain tumor. J Neurooncol. 2021;151:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | McAbee JH, Rath BH, Valdez K, Young DL, Wu X, Shankavaram UT, Camphausen K, Tofilon PJ. Radiation Drives the Evolution of Orthotopic Xenografts Initiated from Glioblastoma Stem-like Cells. Cancer Res. 2019;79:6032-6043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Draaisma K, Chatzipli A, Taphoorn M, Kerkhof M, Weyerbrock A, Sanson M, Hoeben A, Lukacova S, Lombardi G, Leenstra S, Hanse M, Fleischeuer R, Watts C, McAbee J, Angelopoulos N, Gorlia T, Golfinopoulos V, Kros JM, Verhaak RGW, Bours V, van den Bent MJ, McDermott U, Robe PA, French PJ. Molecular Evolution of IDH Wild-Type Glioblastomas Treated With Standard of Care Affects Survival and Design of Precision Medicine Trials: A Report From the EORTC 1542 Study. J Clin Oncol. 2020;38:81-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |