Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10293
Peer-review started: July 7, 2021
First decision: July 15, 2021
Revised: July 27, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: November 26, 2021
Processing time: 138 Days and 0.3 Hours
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and highly aggressive hematopoietic malignancy. BPDCN is difficult to diagnose because of the overlap in morphologic and immunophenotypic features with various cutaneous lymphatic hematopoietic tumors.
We report on three BPDCN cases, all characterized by skin nodules and examined by histology, immunohistochemical detection, in situ hybridization for Epstein-Barr virus, and follow-up. We also review the relevant literature. All patients were positive for CD56 and negative for Epstein-Barr encoded small RNA. Two patients had bone marrow involvement. Chemotherapy is the main treatment for BPDCN, but case 1 showed bone marrow suppression and case 2 developed recurrence after chemotherapy. Case 1 survived for 7 mo, case 2 for 17 mo, and case 3 for 9 mo.
An accurate pathological diagnosis is a precondition for treatment, and the diagnosis of BPDCN should be based on a combination of clinical symptoms, pathological characteristics, immunophenotype, and other auxiliary examinations. It is necessary to clarify the clinicopathological features and biological behavior of BPDCN to improve its understanding by both clinicians and pathologists. Case 2 survived significantly longer than the other two cases, suggesting that the treatment received by case 2 was more effective.
Core Tip: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is difficult to diagnose because of the overlap in morphologic and immunophenotypic features with various cutaneous lymphatic hematopoietic tumors. We report the clinical symptoms, pathological characteristics, immunophenotype, treatment, and follow-up (from diagnosis until death) for three patients with BPDCN. It is necessary to clarify the clinicopathological features and biological behavior of BPDCN to improve the understanding of the disease by both clinicians and pathologists. The survival time of case 2 was significantly longer than usual, suggesting that the treatment received by this case was suitable for clinical application.
- Citation: Guo JH, Zhang HW, Wang L, Bai W, Wang JF. Blastic plasmacytoid dendritic cell neoplasm with skin and bone marrow involvement: Report of three cases. World J Clin Cases 2021; 9(33): 10293-10299
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10293.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10293
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a highly aggressive lymphoid hematopoietic system tumor derived from precursors of plasmacytoid dendritic cells, with no racial or ethnic predisposition. BPDCN is difficult to diagnose because of the overlap in morphologic and immunophenotypic features with various cutaneous lymphatic hematopoietic tumors. We retrospectively report the clinicopathological data, histological and morphological characteristics, immunophenotype, and differential diagnosis in three cases of BPDCN, in order to raise awareness of the condition and provide evidence for its clinical treatment and prognosis. In addition, we briefly review previous cases of BPDCN.
Case 1 was a 59-year-old man who was admitted to the Department of Hematology of Shanxi Cancer Hospital in February 2013, with a complaint of skin nodules all over his body.
Case 2 was a 15-year-old girl who was admitted to the Department of Hematology of Shanxi Cancer Hospital in July 2016, with a complaint of nodules on her back.
Case 3 was a 70-year-old woman who was hospitalized in another hospital, but she came to our hospital for consultation and examination of pathological samples.
Case 1 found a nodule in his right inner thigh in November 2012, and unequal nodules gradually appeared on his trunk and limbs, followed by multiple tender enlarged lymph nodes in his neck, armpits, and groin. The tumors grew rapidly.
Case 2 inadvertently found scattered hard subcutaneous nodules on her back in May 2016.
Case 1 had a 3-year history of hypertension, but his blood pressure was controlled with oral nifedipine sustained-release tablets.
Cases 2 and 3 had no relevant medical histories.
All the three cases had no relevant medical histories.
Case 1 had a temperature of 36.3 °C, heart rate of 81 bpm, respiratory rate of 20 breaths per minute, and blood pressure of 153/89 mmHg. Dense reddish-brown, slightly protruding, macular papules were scattered over his whole body, accompanied by ulceration. Multiple tender lymph nodes measuring about 1-5 cm in diameter were palpable in the bilateral neck, armpit, and groin regions. The clinical consideration was lymphoma.
The temperature of case 2 was 36.3 °C, her heart rate was 116 bpm, respiratory rate was 20 breaths per minute, and blood pressure was 107/63 mmHg. Multiple subcutaneous dark red, tender nodules were present under the skin, with the largest being about 4 cm in diameter. The clinical consideration was lymphoma.
All patients underwent skin biopsy. The biopsy tissues were stained with hematoxylin and eosin, immunohistochemically stained for antigens including CD56, CD4, CD123, and CD68, and used for in situ hybridization to detect Epstein-Barr virus [EBV; Epstein-Barr encoded small RNA (EBER)].
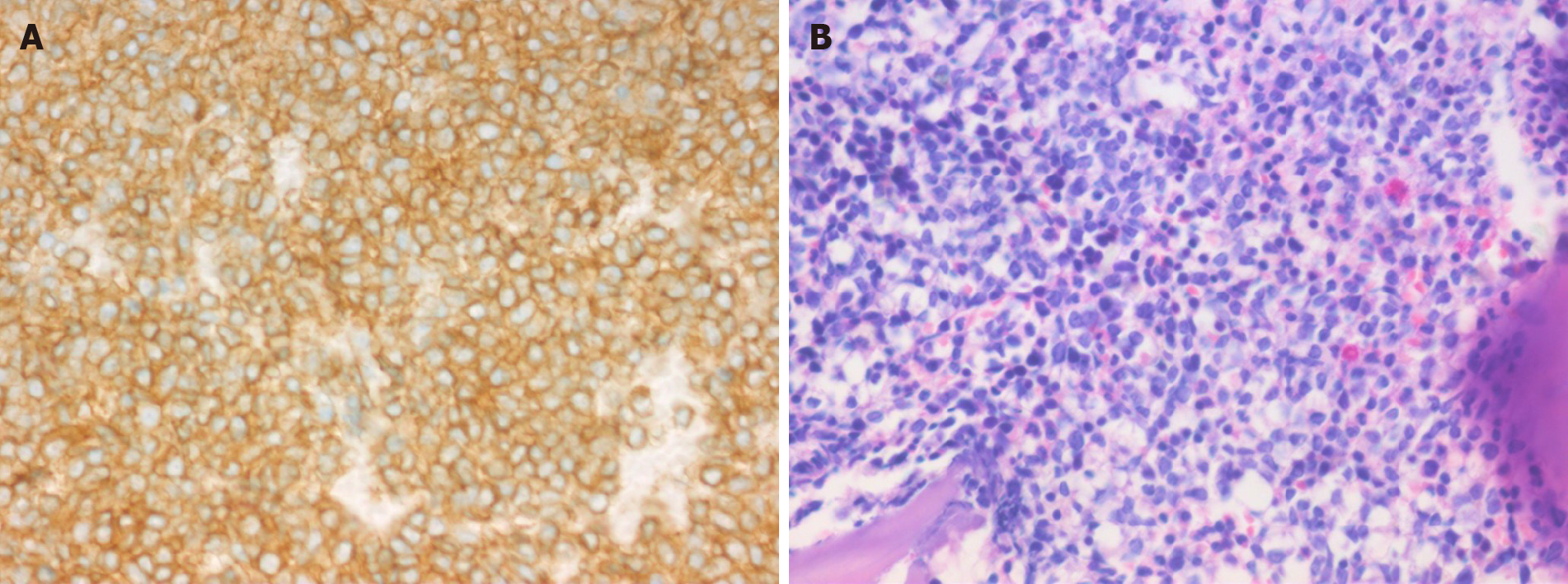
Case 1: Lactate dehydrogenase (LDH) level was normal (156 U/L). A 2.0-cm lymph node biopsy from the right upper arm showed the absence of normal lymph node structure, and medium-sized diffuse lymphoid cells. Immunohistochemically, lymphocytes were positive for CD56, CD123 (Figure 1A), CD38, LCA, CD43, CD99, and Ki67 (60%), and negative for CD4, CD68, myeloperoxidase (MPO), CD3, CD20, CD21, CD10, CD5, CyclinD1, CD23, CD15, CD30, CD138, S-100, Pax-5, MUM1, CD34, Granzyme B, TIA-1, and TdT. The EBER test was negative. Bone marrow biopsy revealed diffuse lymphoid cells between the bone trabeculae, with medium-sized heterotypic nuclei (Figure 1B). Immunohistochemical analysis of lymphocytes indicated positivity for CD56, CD123, CD43, and Ki67 (30%), and negativity for CD4, CD68, MPO, CD3, CD20, TIA-1, and TdT.
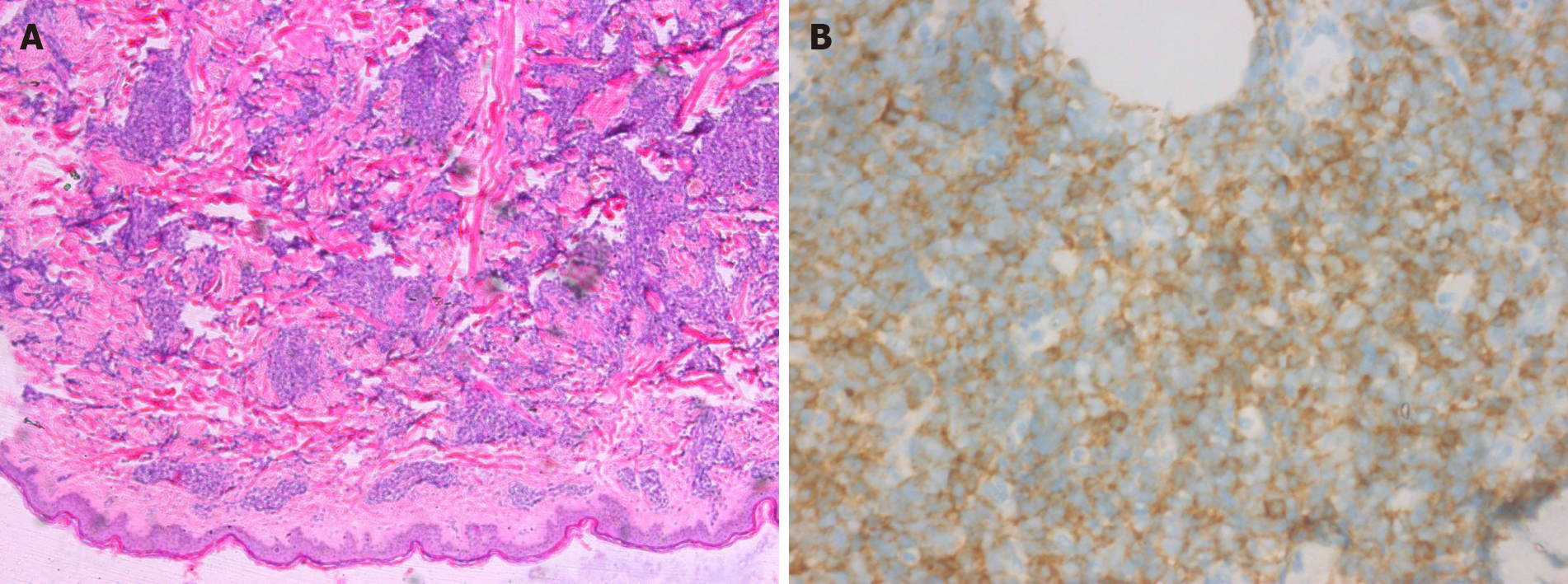
Case 2: LDH level increased to 348 U/L. Biopsy showed dense heteromorphic lymphoid cells throughout the dermis (Figure 2A). Immunohistochemically, the lymphocytes were positive for CD56, CD4 (Figure 2B), CD123, Bcl-2, and Ki67 (80%), but negative for CD68, MPO, CD3, CD20, CD30, CD43, CD5, MUM-1, CD34, TIA-1, Granzyme B, and CD10. An EBER test was negative. Posterior iliac puncture revealed diffuse lymphoid cells between the bone trabeculae and bone marrow, with medium-sized heterotypic nuclei, and bone marrow infiltration. Immunohistochemistry revealed that the lymphocytes were positive for CD56, CD4, CD123, and Ki67 (20%-30%), and negative for CD68, MPO, CD3, CD20, and CD38.
Case 3: Immunohistochemical and in situ hybridization examinations were performed in the Department of Pathology of Shanxi Cancer Hospital. A biopsy from her inner left thigh demonstrated diffuse infiltrating plasmacytoid cells with nuclear deviation. Immunohistochemically, the lymphocytes were positive for CD56, CD68, and Ki67 (10%), and negative for CD4, CD123, CD3, CD20, CD10, MUM1, AE1/AE3, Desmin, S-100, and CD30. The main test results are shown in Table 1.
| Case | Site | CD56 | CD4 | CD123 | CD68 | MPO | CD3 | CD20 | Ki67 | EBER | LDH | Survival |
| 1 | Skin | + | - | + | - | - | - | - | 60% | - | 156 U/L | 7 mo |
| Bone marrow | + | - | + | - | - | - | - | 30% | - | |||
| 2 | Skin | + | + | + | - | - | - | - | 80% | - | 348 U/L ↑ | 17 mo |
| Bone marrow | + | + | + | - | - | - | - | 20%-30% | - | |||
| 3 | Skin | + | - | - | + | * | - | - | 10% | - | Not checked | 9 mo |
Case 1: Computed tomography (CT) revealed multiple lymph nodes in bilateral areas I and IV of his neck, bilateral axilla, anterior inferior mediastinum, and bilateral iliac and inguinal regions. The largest lymph node was about 2.6 cm × 2.2 cm. A 1.25-cm pleural nodule in the right lower lung was considered as an inflammatory lesion, and most of the lymph nodes were solid.
Case 2: CT scans revealed multiple lymph nodes in the perivascular spaces in her neck, mediastinum, bilateral axilla, abdominal aorta, and bilateral iliac and inguinal regions, and a 0.9-cm subcutaneous nodule on her right lower back.
The final diagnosis in all three cases was BPDCN.
Chemotherapy was administered as the main treatment for BPDCN, but the patient developed bone marrow suppression after treatment with the VDLD regimen (vincristine 2 mg, intravenous injection, days 1, 8, 15, and 22 [1.4 mg/m2, ≤ 2 mg each time]; daunorubicin 40 mg/m2, intravenous drip, days 1-3 and 15-16; L-asparaginase 6000 IU/m2, intravenous drip, days 11, 14, 17, 20, 23, and 26; dexamethasone 1 mg/kg/d, orally, for 14 consecutive days, reduced by 1/3 on days 15-28). One month later, he was treated with the CAM regimen (cytoxan 750 mg/m2, intravenous drip, days 1 and 8 [uromitexan rescue]; cytosine arabinoside 100 mg/m2/d, intravenous drip, days 1-3 and 8-10; 6-mercaptopurine 60 mg/m2/d, oral, days 1-7), and again showed bone marrow suppression.
The subcutaneous nodules subsided after anti-infective treatment but subsequently reappeared and became more severe, gradually involving the limbs and body. She was treated with the CHOP (cytoxan 750 mg/m2, vincristine 1.4 mg/m2, doxorubicin 50 mg/m2, all by intravenous drip on day 2, prednisone 100 mg, oral, days 2-6; repeated every 21 d) and VDCLP (vincristine 2 mg, intravenous injection, days 1, 8, 15, and 22 [1.4 mg/m2, ≤ 2 mg each time]; daunorubicin 40 mg/m2, intravenous drip, days 1-3 and 15-16; cytoxan 750 mg/m2, intravenous drip, days 1 and 15 [uromitexan rescue]; L-asparaginase 6000 IU/m2, intravenous drip, days 11, 14, 17, 20, 23, and 26; prednisone 1 mg/kg/d, oral, for 14 consecutive days, reduced by 1/3 on days 15-28) regimens for BPDCN, with improvement of the subcutaneous nodules and a complete bone marrow response.
Case 3 was treated in another hospital. She knew that she had received chemotherapy but did not know the chemotherapy regimen.
Case 1 subsequently developed pulmonary infection in May 2013 and central infiltration on August 8, and finally died 7 mo after his diagnosis.
Case 2 was followed for 17 mo from diagnosis to death, after stopping treatment for economic reasons.
Case 3 died 9 mo after her diagnosis.
BPDCN accounts for only 0.7% of primary lymphatic hematopoietic tumors of the skin[1]. It can occur in any age group[2,3], but is most common in the elderly. The male:female ratio is 3.3:1. Jegalian et al[2] reported that children and young patients had a relatively good prognosis. About 76%-85% of cases[4] have skin involvement, with asymptomatic isolated or multiple nodules, plaques, or bruises. In some cases only the skin is involved, but multiple parts may be affected. The current cases of BPDCN included one man and two women, and although two were elderly, case 2 was unusually only 15 years old, and she had a relatively good prognosis (17 mo). In the present study, all three cases were characterized by skin nodules.
The diagnosis of BPDCN is based on pathological biopsy. Kerr et al[5] showed tumor cells invading the dermis and adipose tissue of the skin, with no tumor cells in the epidermis. When the lesion involves the bone marrow, it may show as an interstitial infiltration or as a mass of tumor cells, like infiltrating leukemia, often accompanied by hematopoietic tissue dysplasia[6]. In the current study, the pathological features of all three patients showed no tumor cells invading the epidermis, and a morphology consistent with that reported in the literature. In addition, the bone marrow was involved in cases 1 and 2, and numbers of diffuse lymphoid cells could be observed.
The immunological phenotype of BPDCN is important. Julia et al[7] proposed five specific immunological markers for BPDCN: CD56, CD4, CD123, TCL1, and CD303, and suggested that positivity for at least four of these indicated a diagnosis of BPDCN. Overexpression of CD123 or interleukin-3 receptor subunit alpha occurs in essentially all cases of BPDCN[8-10]; however, a few atypical cases have been reported which did not express CD4 or CD56[11], and individual CD123-negative cases of BPDCN have also been reported[12]. CD68 is expressed in 50% of tumor cells, and such patients are prone to leukemic transformation[2]. In contrast, the cytotoxic markers CD23, CD30, and CD138 are negative. In this study, tumor cells expressed CD56 in all three cases, CD4, CD123, and CD68 were positive in one, two, and one case, respectively. Tumor cells were positive for CD68 expression in case 3, but she did not develop acute leukemia. Laboratory examinations are also essential for diagnosing BPDCN, and LDH was significantly elevated in case 2.
Clinicopathologically, the differential diagnosis of BPDCN includes myeloid sarcoma or leukemia, T-lymphoblastic lymphoma, and natural killer (NK)/T cell lymphoma. Both myeloid sarcoma or leukemia and BPDCN can show skin, lymph node, and bone marrow involvement. However, applying a series of markers (such as CD56, CD4, CD123, and TCL-1) can better distinguish these diseases[12]. MPO is a specific immunological marker of acute myeloid leukemia (AML). In the current study, MPO was negative in cases 1 and 2, and BPDCN could be confirmed by a combination of morphology and immunological positivity for CD56, CD4, and CD123 and MPO negativity. Compared with BPDCN, T-lymphoblastic lymphoma usually occurs in adolescents or young adults, with more frequent mediastinal involvement. It is important to note that patients with BPDCN without skin involvement may have a younger onset age, and may have mediastinal involvement and CD34-positive tumor cells, which may be more easily confused with T-lymphoblastic lymphoma. Although case 2 was an adolescent, all three patients had skin involvement and no mediastinal involvement; furthermore, CD34 was negative in cases 1 and 2, and T-lymphoblastic lymphoma could thus be excluded. BPDCN and NK/T cell lymphoma can be distinguished by morphology, immunohistochemistry, and EBER testing. Tumor cells in NK/T cell lymphoma have various forms, often invade the vascular wall and are accompanied by necrosis, and cytotoxic markers such as TIA-1 and Granzyme B are often positive. Furthermore, NK/T cell lymphoma is associated with EBV infection, and the EBER test is thus positive in patients with NK/T cell lymphoma. Although CD56 was expressed in BPDCN, serum EBV and in situ hybridization for detection of EBER were negative, indicating that BPDCN was not related to EBV infection[1]. In this study, TIA-1 and Granzyme B were negative in cases 1 and 2, and all three patients were EBER-negative, thus excluding a diagnosis of NK/T cell lymphoma.
Given the lack of consensus, BPDCN has been treated with regimens used for other acute leukemias. Most case reports indicated that the majority of patients who received initial treatment with acute lymphoblastic leukemia, AML, or lymphoma CHOP chemotherapy achieved complete remission but had a high recurrence rate[13,14]. In this study, case 1 received successive VDLD treatment and CAM chemotherapy, but both caused significant bone marrow suppression. VDCLP has since been used to replace VDLD. Notably, CHOP and VDCLP resulted in improvement of the sub
In summary, BPDCN is a rare and highly aggressive hematopoietic malignancy. Skin involvement is the most common initial clinical manifestation, but it is not specific, and the bone marrow can also be affected. CD56, CD4, and CD123 are important diagnostic makers for BPDCN. The diagnosis of BPDCN should thus be based on a combination of clinical symptoms, pathological characteristics, and other auxiliary examinations, to minimize the risk of a missed or delayed diagnosis. These cases highlight the need to improve the understanding of BPDCN by both clinicians and pathologists.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imai Y S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Petrella T, Bagot M, Willemze R, Beylot-Barry M, Vergier B, Delaunay M, Meijer CJ, Courville P, Joly P, Grange F, De Muret A, Machet L, Dompmartin A, Bosq J, Durlach A, Bernard P, Dalac S, Dechelotte P, D'Incan M, Wechsler J, Teitell MA. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123:662-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Jegalian AG, Buxbaum NP, Facchetti F, Raffeld M, Pittaluga S, Wayne AS, Jaffe ES. Blastic plasmacytoid dendritic cell neoplasm in children: diagnostic features and clinical implications. Haematologica. 2010;95:1873-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Yang CS, Wang J, Chang TK. Congenital blastic plasmacytoid dendritic cell neoplasm. Pediatr Blood Cancer. 2012;58:109-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Tsagarakis NJ, Kentrou NA, Papadimitriou KA, Pagoni M, Kokkini G, Papadaki H, Pappa V, Marinakis T, Anagnostopoulos NI, Vadikolia C, Anagnostopoulos A, Angelopoulou MK, Terpos E, Poziopoulos C, Anargyrou K, Rontogianni D, Papadaki T, Psarra A, Kontopidou FN, Skoumi D, Papadhimitriou SI, Paterakis G; Hellenic Dendritic Cell Leukemia Study Group. Acute lymphoplasmacytoid dendritic cell (DC2) leukemia: results from the Hellenic Dendritic Cell Leukemia Study Group. Leuk Res. 2010;34:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Kerr D 2nd, Zhang L, Sokol L. Blastic Plasmacytoid Dendritic Cell Neoplasm. Curr Treat Options Oncol. 2019;20:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Cota C, Vale E, Viana I, Requena L, Ferrara G, Anemona L, Metze D, Fink-Puches R, Wiesner T, Cerroni L. Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm-morphologic and phenotypic variability in a series of 33 patients. Am J Surg Pathol. 2010;34:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Julia F, Dalle S, Duru G, Balme B, Vergier B, Ortonne N, Vignon-Pennamen MD, Costes-Martineau V, Lamant L, Dalac S, Delattre C, Déchelotte P, Courville P, Carlotti A, De Muret A, Fraitag S, Levy A, Mitchell A, Petrella T. Blastic plasmacytoid dendritic cell neoplasms: clinico-immunohistochemical correlations in a series of 91 patients. Am J Surg Pathol. 2014;38:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, Luger SM, Phillips GL. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 620] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 9. | Han L, Qiu P, Zeng Z, Jorgensen JL, Mak DH, Burks JK, Schober W, McQueen TJ, Cortes J, Tanner SD, Roboz GJ, Kantarjian HM, Kornblau SM, Guzman ML, Andreeff M, Konopleva M. Single-cell mass cytometry reveals intracellular survival/proliferative signaling in FLT3-ITD-mutated AML stem/progenitor cells. Cytometry A. 2015;87:346-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Testa U, Pelosi E, Frankel A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark Res. 2014;2:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 11. | Kim MJ, Nasr A, Kabir B, de Nanassy J, Tang K, Menzies-Toman D, Johnston D, El Demellawy D. Pediatric Blastic Plasmacytoid Dendritic Cell Neoplasm: A Systematic Literature Review. J Pediatr Hematol Oncol. 2017;39:528-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Cronin DM, George TI, Reichard KK, Sundram UN. Immunophenotypic analysis of myeloperoxidase-negative leukemia cutis and blastic plasmacytoid dendritic cell neoplasm. Am J Clin Pathol. 2012;137:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Martín-Martín L, López A, Vidriales B, Caballero MD, Rodrigues AS, Ferreira SI, Lima M, Almeida S, Valverde B, Martínez P, Ferrer A, Candeias J, Ruíz-Cabello F, Buadesa JM, Sempere A, Villamor N, Orfao A, Almeida J. Classification and clinical behavior of blastic plasmacytoid dendritic cell neoplasms according to their maturation-associated immunophenotypic profile. Oncotarget. 2015;6:19204-19216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 14. | Reimer P, Rüdiger T, Kraemer D, Kunzmann V, Weissinger F, Zettl A, Konrad Müller-Hermelink H, Wilhelm M. What is CD4+CD56+ malignancy and how should it be treated? Bone Marrow Transplant. 2003;32:637-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Syed YY. Tagraxofusp: First Global Approval. Drugs. 2019;79:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |