Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10273
Peer-review started: July 6, 2021
First decision: July 26, 2021
Revised: August 5, 2021
Accepted: August 18, 2021
Article in press: August 18, 2021
Published online: November 26, 2021
Processing time: 139 Days and 1.3 Hours
Capillary leak syndrome (CLS) is characterized by the leakage of large amounts of fluid and plasma proteins into the interstitial space, resulting in hypoalbuminemia, hypovolemic shock, elevated blood concentration, systemic progressive edema, and multiple serosal cavity effusion. Clinical syndromes such as cavity effusion pose a grave threat to the life and health of the patient.
A 58-year-old female patient was admitted to the hospital after being in a coma for 6 h following accidental ingestion of a pesticide. She was treated with phencyclidine hydrochloride and pralidoxime iodide for detoxification, mechanical ventilation to maintain oxygen supply, continuous renal replacement therapy to maintain the internal environment, and hemoperfusion to promote the excretion of toxins. She also received a transfusion of red blood cells and massive fluid resuscitation. However, her blood pressure was not maintained. The patient was diagnosed with CLS due to pesticide poisoning. Oxygenation was difficult to maintain under full ventilator support; therefore, veno-venous-extracorporeal membrane oxygenation (VV-ECMO) treatment was given 13 h after admission. Her oxygenation level improved, but a large amount of ascites and pleural effusion soon became apparent. We continued drainage with an indwelling drainage tube, and the ECMO flow stabilized. The leakage gradually decreased, and ECMO was discontinued 3 d later. On the 6th day, the patient recovered from unconsciousness, but on gastroscopic evaluation, severe erosions were found in her entire stomach. With the family’s consent, treatment was stopped, and the patient was discharged from the hospital on the 7th day.
ECMO, liquid resuscitation and management, and improvement in plasma colloidal osmotic pressure, circulation, and tissue oxygen supply are crucial in treating CLS.
Core Tip: The case of a patient with severe capillary leak syndrome who was successfully treated by veno-venous-extracorporeal membrane oxygenation (VV-ECMO) is reported, which reflects the positive role of VV-ECMO in the treatment of capillary leak syndrome (CLS), and reflects the key elements of clinical treatment of CLS, such as increasing plasma colloidal osmotic pressure, improving circulation, and ensuring tissue oxygen supply.
- Citation: Nong WX, Lv QJ, Lu YS. Veno-venous-extracorporeal membrane oxygenation treatment for severe capillary leakage syndrome: A case report. World J Clin Cases 2021; 9(33): 10273-10278
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10273.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10273
Capillary leakage syndrome (CLS) refers to a group of clinical syndromes such as hypoproteinemia, hypovolemic shock, blood concentration, systemic progressive edema, and multiple serosal cavity effusion caused by leakage of a large amount of fluid and plasma proteins into the interstitial space resulting from injury of capillary endothelial cells and increased vascular permeability[1-4].
CLS is most likely to cause harm to the alveoli and limit gas exchange, leading to tissue hypoxia that aggravates the capillary injury, resulting in respiratory and circulatory failure. Subsequently, it leads to systemic multiple organ dysfunction syndrome and increases mortality[5]. Since CLS is difficult to treat effectively, it can easily develop into multiple organ dysfunction syndrome. Hence, early recognition and active treatment (including control of the primary disease, liquid resuscitation and management, increasing the plasma colloid osmotic pressure, improving circulation, and enhancing oxygen supply in the tissues) and prevention of deterioration are the crucial elements in the clinical treatment of critical illnesses resulting from CLS. We herein present a case of severe CLS that was successfully treated by veno-venous-extracorporeal membrane oxygenation (VV-ECMO) at our department.
A 58-year-old female patient was admitted to the hospital after being unconscious for 6 h following accidental ingestion of a pesticide.
The patient was unconscious for 6 h.
The patient had a prolonged history of schizophrenia.
The patient received endotracheal intubation and gastric lavage at a local health center and was then referred to the electronic intensive care unit of our hospital.
Physical examination revealed the following: Temperature, 35 °C; pulse, 150 beats/min; heart rate, 30 beats/min; blood pressure, 75/50 mmHg; shallow coma; bilateral pupil diameter, 3 mm; light reflection disappearance; wet and cold skin; moist rales in both lungs; soft abdomen; disappearance of intestinal sounds. The Acute Physiology and Chronic Health Evaluation II score was 22 points, and the Sequential Organ Failure Assessment score was 11 points.
Arterial blood gas analysis revealed the following: pH, 7.28; blood partial pressure of carbon dioxide, 24 mmHg; partial pressure of blood oxygen (PaO2), 57 mmHg; oxygenation index (PaO2/FiO2), 145 mmHg; lactic acid, 6.7 mmol/L; HCO2, 18.2 mmol/L; cholinesterase (urgent check), 183 U/L. Specific information on the ingested pesticide was not obtained.
The final diagnosis included acute organophosphorus pesticide poisoning, acute respiratory failure, aspiration pneumonia, and schizophrenia.
After admission, the patient was treated with phencyclidine hydrochloride and pralidoxime iodide to detoxify, ventilator support to maintain oxygen supply, continuous renal replacement therapy to maintain the internal environment, hemoperfusion to promote the excretion of toxins, transfusion of red blood cells, and massive fluid resuscitation. However, her blood pressure could hardly be stabilized despite a large dose of hypertensive drugs. Subsequently, ultrasonography combined with pulse contour cardiac output (PICCO) monitoring was carried out to check the hemodynamics. Cardiopulmonary ultrasonography showed the following: Ejection fraction, 45%; good performance of both lungs; PICCO cardiac index, 2.9 L/min/m2; global end-diastolic volume index, 439 mL/m2; systemic vascular resistance index, 1458 dyn.s.cm-5.m2; increased extravascular lung water index (EVLWI), 16 mL/kg; pulmonary vascular permeability index (PVPI), 4.3; central venous pressure, 16 mmHg. The findings suggested severe capacity loss and fluid reactivity in continuous expansion. Moreover, the intravascular volume was being lost rapidly, the patient gradually developed systemic edema, and the findings deteriorated further: PICCO EVLWI, 22 mL/kg; PVPI, 8.7; and bladder pressure, 30 mmHg. The patient was diagnosed with CLS caused by pesticide poisoning.
Considering the pathophysiology of CLS, colloid fluid, plasma, and albumin were selected for expansion. However, the patient's leakage continued to worsen, with difficulties in maintaining oxygenation under full ventilator support (Positive end-expiratory pressure, 15 cm H2O; PPLAT, 35 cm H2O; PICCO, EVLWI, 32; oxygenation index, 54 mmHg). Hence, the patient received VV-ECMO treatment at the 13th hour after admission with the consent of the family members. After ECMO, the patient’s oxygenation level improved, but soon a low-flow alarm appeared. On performing bedside ultrasonography, the patient was found to have a large amount of ascites and pleural effusion. Abdominal puncture and pleural puncture were performed, and the protein level in pleural hydroperitoneal fluid was found to be significantly high. An indwelling drainage tube was placed for continuous drainage, and the ECMO flow recovered to a stable level.
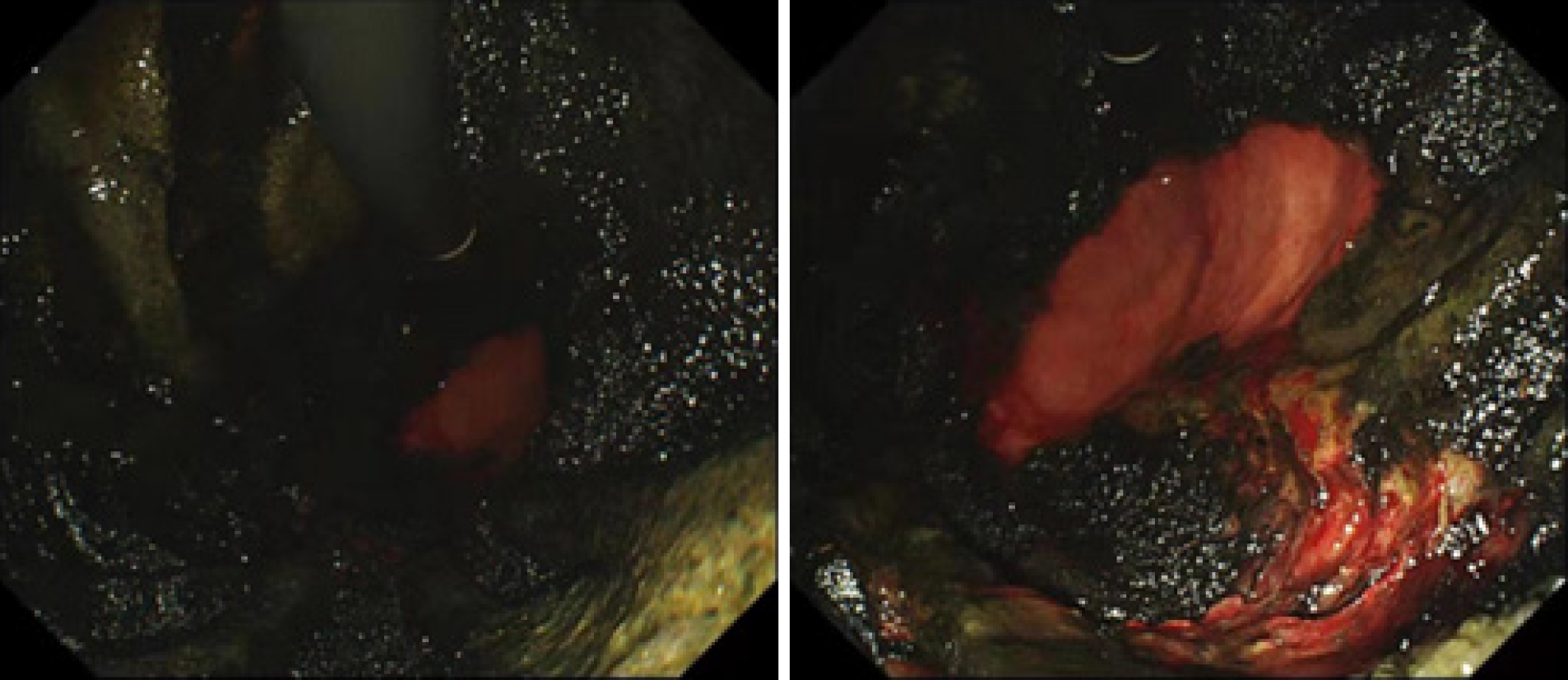
After the above active treatments, the leakage of the patient gradually decreased. The cholinesterase level had recovered to 4917 U/L, and the patient was taken off the machine successfully after 3 d of ECMO. Under ventilator support, the oxygenation index was 180 mmHg, and the bladder pressure was 12 mmHg. On day 6, the patient became lucid, and her muscle strength was at grade 2. A jejunal tube was placed under endoscopic visualization for enteral nutrition, but the whole stomach was found to be severely eroded; the mucosa in the gastric bulb and descending part covered a large area of coke, with a small amount of bleeding, and a very small amount of normal tissue was functional (Figure 1). The prognosis was very poor, and after the family members learned about the condition, they consented to stop the treatment, and the patient was discharged on the 7th day.
CLS is a rare condition[6,7] associated with increased capillary permeability due to endothelial damage, resulting in leakage of plasma and protein into interstitial spaces[2,8,9]. It is characterized by rapidly developing edema, hypotension, and hypoproteinemia[10,11]. CLS has been observed in a variety of diseases[12,13], with the most common being sepsis. The pathogenesis of CLS is complex and has not been fully elucidated. Some scholars believe that the pathophysiology of CLS includes capillary endothelial injury from the influence of inflammatory mediators. The capillary semi-permeability barrier is composed of endothelial cells and the basement membrane. Under normal physiological conditions, only small molecules such as water and electrolytes are allowed to pass through and enter the tissue gap to complete substance exchange. Meanwhile, the passage of large molecules such as toxins, metabolites, and albumin is restricted. When the body is stimulated by a variety of pathogens and stressors, endothelial cells become injured, necrosis or cell gaps increase, and the capillary semi-permeability barrier is seriously damaged. This reduces the capillary filtration rate of macromolecular substances and increases vascular permeability, leading to CLS. Under the stimulation of various pathogenic factors, monocytes and macrophages are activated to release various proinflammatory cytokines. This further activates effector cells such as granulocytes and endothelial cells, and accelerates the metabolism of arachidonic acid and the release of free radicals, proteases, thrombotin A2, prostaglandins, and other inflammatory mediators, forming the "inflammatory cascade effect" that mediates the immune response and causing systemic inflammatory response syndrome and immune disorders[14]. The release of a large number of inflammatory mediators can directly damage capillary endothelial cells, leading to vascular endothelial cell injury, apoptosis, deformation, cell connection separation, or even fracture and resulting in endothelial cell dysfunction and cell membrane destruction. Further, cadherin deposition in microvascular endothelial cells leads to the formation of actin stress fibers, ultimately leading to increased capillary permeability[15]. In addition, the paracrine release of histamine and bradykinin from the mast cells in damaged tissues may further promote the occurrence of CLS[16].
Currently, there are many studies on the treatment of CLS[17]. However, there is still a lack of specific treatment measures, and the treatment of the primary disease is time-consuming[18]. In the leakage stage of CLS, small molecular proteins in the plasma leak out of the blood vessels, the plasma colloid osmotic pressure decreases, the effective circulating blood volume decreases, and the blood pressure drops progressively. The key to treatment is to maintain the effective circulating blood volume and to prevent or treat shock. Whether crystalloid or colloid treatment should be provided for CLS is still controversial[19]. Due to the high capillary permeability and low molecular weight of the crystal liquid phase, most of the crystal liquid is exudated to the tissue space, leading to increased systemic edema after infusion. Therefore, the amount of crystal liquid should be limited for ensuring an effective circulating blood volume to avoid inter-tissue edema and cell edema. Thus, colloidal fluids were provided during fluid and albumin resuscitation (Table 1). Despite this, the fluid infusion inevitably aggravated leakage, and edema occurred in all organs. A large amount of fluid leakage from capillaries into the interstitium of the lung led to decreased pulmonary compliance, increased airway resistance, and decreased ventilation function. Severe hypoxemia occurred, and oxygenation was hardly maintained despite full ventilator support[20]. Therefore, VV-ECMO was used for oxygenation. The patient also presented with abdominal space syndrome; therefore, we relieved the compression symptoms of internal edema using an external minimally invasive drainage, such as puncture, to maintain oxygen supply and to obtain time for the treatment of the primary disease. The pathophysiological process of CLS can be observed from the intake and output volume of patients, as shown in Table 1.
| Time | Intake | Output | ||||||||
| Crystalloid (mL) | Colloid (mL) | Albumin (g) | Total volume (mL) | Urine volume (mL) | Stomach tube (mL) | Hydrothorax (mL) | Ascites (mL) | CRRT (mL) | Total volume (mL) | |
| 1st day | 8330 | 6400 | 150 | 15480 | 4470 | 1100 | 0 | 0 | 0 | 5570 |
| 2nd day | 4840 | 4500 | 270 | 10690 | 4110 | 950 | 700 | 3650 | 1500 | 10910 |
| 3rd day | 3736 | 2100 | 210 | 6886 | 4970 | 1740 | 470 | 1810 | 660 | 9650 |
| 4th day | 2740 | 1550 | 40 | 4490 | 2180 | 700 | 350 | 950 | 460 | 4290 |
| 5th day | 3015 | 1100 | 110 | 4665 | 2720 | 700 | 220 | 790 | 890 | 5320 |
| 6th day | 2590 | 0 | 20 | 2690 | 4700 | 200 | 60 | 350 | 0 | 5310 |
In summary, CLS has a complex etiology with a quick onset. Specific treatment methods are often lacking, and it can easily develop into multiple organ dysfunction syndrome. In this regard, early identification, active treatment of primary disease, fluid resuscitation and management, increasing plasma colloidal osmotic pressure, improving circulation, and ensuring tissue oxygen supply are key elements in the clinical treatment of critical diseases due to CLS. ECMO plays an important and positive role in the treatment of CLS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Choi SQ S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Suzuki G, Ichibayashi R, Yamamoto S, Serizawa H, Nakamichi Y, Watanabe M, Honda M. Plasma filtration with dialysis for the treatment of capillary leak syndrome occurring secondary to surgery for colon cancer-related perforating peritonitis. Clin Case Rep. 2021;9:1490-1493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Massafra M, Passalacqua MI, Lupo G, Altavilla G, Santarpia M. Capillary leak syndrome induced by neoadjuvant cisplatin and gemcitabine in a patient with bladder cancer. Urol Case Rep. 2021;34:101461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Kasugai D, Tajima K, Jingushi N, Uenishi N, Hirakawa A. Multiple limb compartment syndrome as a manifestation of capillary leak syndrome secondary to metformin and dipeptidyl peptidase IV inhibitor overdose: A case report. Medicine (Baltimore). 2020;99:e21202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Khan HR, Khan S, Srikanth A, Smith WHT. A case report of capillary leak syndrome with recurrent pericardial and pleural effusions. Eur Heart J Case Rep. 2020;4:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Lee YS, Kim SY, Kwon CW, Song HG, Lee YK, Kim HJ, Zang DY. Two cases of systemic capillary leak syndrome that were treated with pentastarch. Korean J Intern Med. 2007;22:130-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Zhang YT, Zhang L, Yao YM, Zhong XD, Chang J. Unexpected vincristine-induced systemic capillary leak syndrome in patients with Wilm's tumor: A single institution experience. Pediatr Hematol Oncol. 2020;37:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Jeong GH, Lee KH, Lee IR, Oh JH, Kim DW, Shin JW, Kronbichler A, Eisenhut M, van der Vliet HJ, Abdel-Rahman O, Stubbs B, Solmi M, Veronese N, Dragioti E, Koyanagi A, Radua J, Shin JI. Incidence of Capillary Leak Syndrome as an Adverse Effect of Drugs in Cancer Patients: A Systematic Review and Meta-Analysis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Pérez-Moyano S, Rodríguez-Bolaños S, Ortega-Gálvez I, Borrego-García E, Benavente-Fernández A. Capillary leak syndrome: often forgotten in differential diagnosis. Emergencias. 2020;32:220. [PubMed] |
| 9. | Morin J, Simon K, Chadelaud F, Delarbre D, Druelle A, Blatteau JE. [Capillary leak syndrome secondary to decompression sickness: A case report]. Rev Med Interne. 2019;40:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Vedala K, Desikan SP, McClain C 3rd, Jacob D, Desikan R. Capillary Leak Syndrome From Rituximab Therapy of Lymphoma. J Investig Med High Impact Case Rep. 2020;8:2324709620942372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Case R, Ramaniuk A, Martin P, Simpson PJ, Harden C, Ataya A. Systemic Capillary Leak Syndrome Secondary to Coronavirus Disease 2019. Chest. 2020;158:e267-e268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Wan H, Du M, Deng H, Fu J, Zhang Y, Wang X, Liu R. Capillary leak syndrome and aseptic meningitis in a patient with Kawasaki disease: A case report. Medicine (Baltimore). 2018;97:e10716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Kendre PP, Jose MP, Varghese AM, Menon JC, Joseph JK. Capillary leak syndrome in Daboia russelii bite-a complication associated with poor outcome. Trans R Soc Trop Med Hyg. 2018;112:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Oberholzer A, Oberholzer C, Moldawer LL. Cytokine signaling--regulation of the immune response in normal and critically ill states. Crit Care Med. 2000;28:N3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 173] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Xie Z, Ghosh CC, Patel R, Iwaki S, Gaskins D, Nelson C, Jones N, Greipp PR, Parikh SM, Druey KM. Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome). Blood. 2012;119:4321-4332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 420] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 17. | Darvishi B, Farahmand L, Jalili N, Majidzadeh-A K. Probable Mechanisms Involved in Immunotoxin Mediated Capillary Leak Syndrome (CLS) and Recently Developed Countering Strategies. Curr Mol Med. 2018;18:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Watanabe K, Murakami S, Misago M, Yoshikawa M, Tamai D, Nakao S, Ueoka T, Ito M, Shinomura Y, Kajiwara N. Sjögren's syndrome concurrent with protein-losing gastroenteropathy with secondary systemic capillary leak syndrome : A case report. Clin Case Rep. 2018;6:1829-1833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Marx G. Fluid therapy in sepsis with capillary leakage. Eur J Anaesthesiol. 2003;20:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Ortega F, Carboni Bisso I, Fernandez Ceballos I, Montserrat Rivera A, Tisminetzky M, Dianti J, San Román E, Villarroel S, Di Stefano S, Las Heras M. [Use of Extracorporeal Membrane Oxygenation in idiopathic capillary leak syndrome: a case report]. Rev Fac Cien Med Univ Nac Cordoba. 2020;77:208-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |