Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10265
Peer-review started: April 21, 2021
First decision: June 23, 2021
Revised: June 23, 2021
Accepted: August 17, 2021
Article in press: August 17, 2021
Published online: November 26, 2021
Processing time: 214 Days and 23.5 Hours
Metastasis of pancreatic cancer to the colon is rare and the features need to be further elucidated. Herein, we report a rare case of pancreatic cancer with simultaneous liver and colon metastases.
A 48-year-old man with intrahepatic space-occupying lesions based on a computed tomography scan was admitted to our hospital for further treatment. Abdominal magnetic resonance imaging revealed a 6.4 cm × 4.2 cm mass in the tail of the pancreas and multiple low-density masses in the liver parenchyma. In addition, a mass of 2.2 cm × 1.6 cm with surface congestive erosions in the sigmoid colon was detected by colonoscopy. Histopathological examination of biopsies from both the liver and colon lesions revealed a moderately to poorly differentiated adenocarcinoma. Immunohistochemical staining of the colon tumor was positive for cytokeratin (CK) 7 and CK, but negative for colorectal adenocarcinoma-related markers CK 20, CDX2, and SATB2, thus indicating that the metastasis originated from the pancreas. Next-generation sequencing for genomic profiling of the liver and colon metastases both found mutations in KRAS (p.G12D) and TP53 (c.376-1delG), with microsatellite stable and low tumor mutational burden without actionable or cancer-predisposing gene mutations detected. The patient was subsequently treated with 12 cycles of FOLFIRINOX which led to a sustainable response, followed by ongoing maintenance treatment with irinotecan plus fluorouracil.
For this rare case, careful evaluation of histopathological and immunohistochemical staining results are required. The genomic profiling of colon lesions was revealed for the first time, and FOLFIRINOX showed good treatment efficacy in this patient.
Core Tip: Metastasis of pancreatic cancer to the colon is rare. Herein, we present a rare case of pancreatic cancer with simultaneous liver and colon metastases. Histopathological examination and immunohistochemical staining of the colon tumor confirmed that the metastasis originated from the pancreas. Next-generation sequencing for genomic profiling of the liver and colon metastases from the primary pancreatic carcinoma were revealed for the first time, with no cancer-predisposing or actionable gene mutations detected. The patient was treated with 12 cycles of FOLFIRINOX, which yielded a sustainable response.
- Citation: Dong YM, Sun HN, Sun DC, Deng MH, Peng YG, Zhu YY. Pancreatic cancer with synchronous liver and colon metastases: A case report. World J Clin Cases 2021; 9(33): 10265-10272
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10265.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10265
Pancreatic cancer is reported to be the fourth most common cause of cancer-related death in the United States, with a low 5-year survival rate of 9%[1]. Given the lack of early signs or symptoms of pancreatic cancer, the majority of patients are diagnosed at advanced stages (53%) with metastases to the liver, lungs, or peritoneum, and their 5-year survival is only 3%[2]. However, colon metastasis from pancreatic cancer is extremely rare, with several cases reported in literature and the majority being metachronous[3,4]. Herein, we report a case with synchronous liver and colon metastases of pancreatic cancer and review the literature regarding colon metastases of pancreatic cancer.
A 48-year-old man with intrahepatic space occupying lesions, as shown by abdominal computed tomography, was admitted to our hospital for further treatment in June 2020.
The patient visited a local hospital due to high blood sugar level and loss of appetite in October 2019. The local doctor administered metformin symptomatic treatment, but after three months of treatment, the patient lost four kilograms of weight and had poor blood sugar regulation. Subsequently, acarbose was administered and the blood sugar level was normalized. However, in April 2020, the patient developed anorexia and heartburn and continued to lose weight and this was followed by back pain and abdominal distension.
The patient had no previous medical history.
The patient had a history of drinking, but had no family history of malignant tumors.
Upon arrival, physical examination of the patient revealed a body temperature of 36.3℃, blood pressure of 129/77 mmHg, heart rate of 78 beats/min, and respiratory rate of 20 breaths/min. No jaundice or palpable masses were observed. The patient's Karn
Complete blood count of the patient showed a slight reduction in hemoglobin (116 g/L; normal range: 137-179 g/L) and red blood cells (3.72 × 1012/L; normal range: 4.3-5.9 × 1012/L). Blood chemistry tests showed an increase in total bilirubin (32.3 μmol/L; normal range: 0-21.0 μmol/L), direct bilirubin (19.9 μmol/L; normal range: 0-8.6 μmol/L), γ-glutamyltransferase (785.1 U/L; normal range: 0-50 U/L), alkaline phosphatase (380.7 U/L; normal range: 45-125 U/L) and lactate dehydrogenase (481.2 U/L; normal range: 40-250 U/L), but demonstrated normal values for alanine aminotransferase (20.6 U/L; normal range: 0-40 U/L), and aspartate aminotransferase (23.5 U/L; normal range: 0-40 U/L). The level of serum tumor marker was significantly elevated for carcinoembryonic antigen (CEA) (198 ng/mL; normal range: 0-5.0 ng/mL), CA125 (204.2 U/mL; normal range: 0.1-35 U/mL), CA15-3 (285.5 U/mL; normal range: 0.1-30 U/mL), CA72-4 (65.69 U/mL; normal range: 0.1-10 U/mL), CYFRA21-1 (18.35 ng/mL; normal range: 0.1-4.0 ng/mL), NSE (50.39 ng/mL; normal range: 0-24 ng/mL), and SCC (4.0 ng/mL; normal range: < 1.8 ng/mL). However, the values for CA19-9 (21.25 U/mL) and alpha fetoprotein (2.08 ng/mL) were normal (normal range: 0.1-37 U/mL and 0-20 ng/mL, respectively).
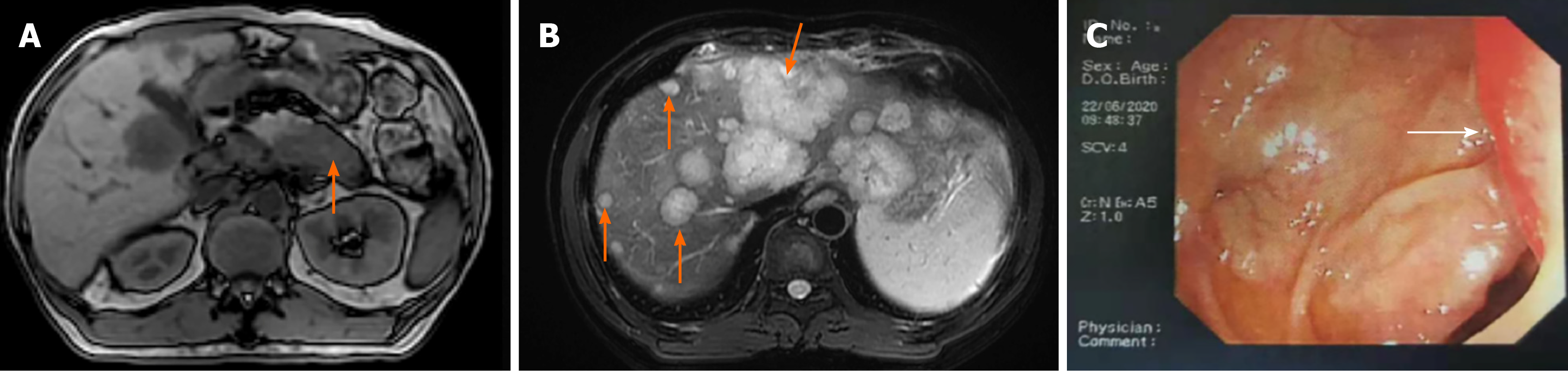
Abdominal magnetic resonance imaging (MRI) scan revealed a hypovascular lesion in the tail of the pancreas (6.4 cm × 4.2 cm in size) and multiple hypovascular nodules in the liver parenchyma (Figure 1A and B). Colonoscopy was performed due to the high CEA level and a mass 2.2 cm × 1.6 cm in size with surface congestive erosions in the sigmoid colon was found, which occupied a quarter of the intestinal cavity and was 33 cm from the anus (Figure 1C).
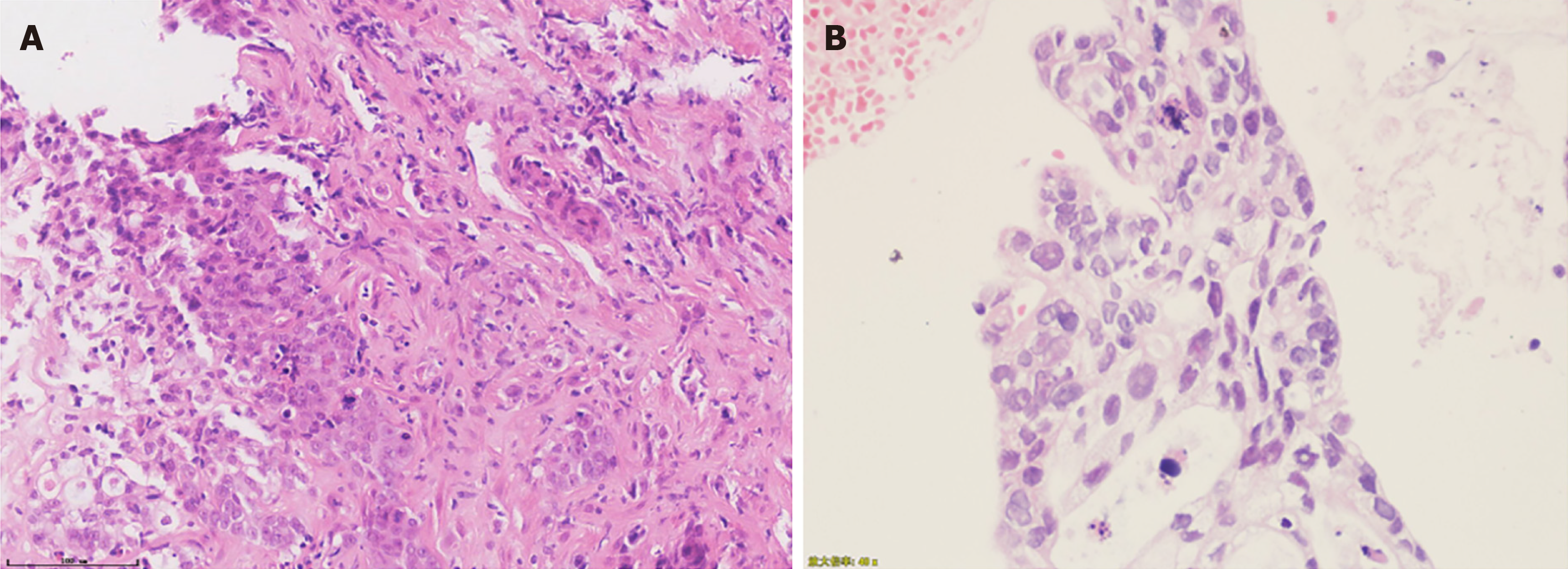
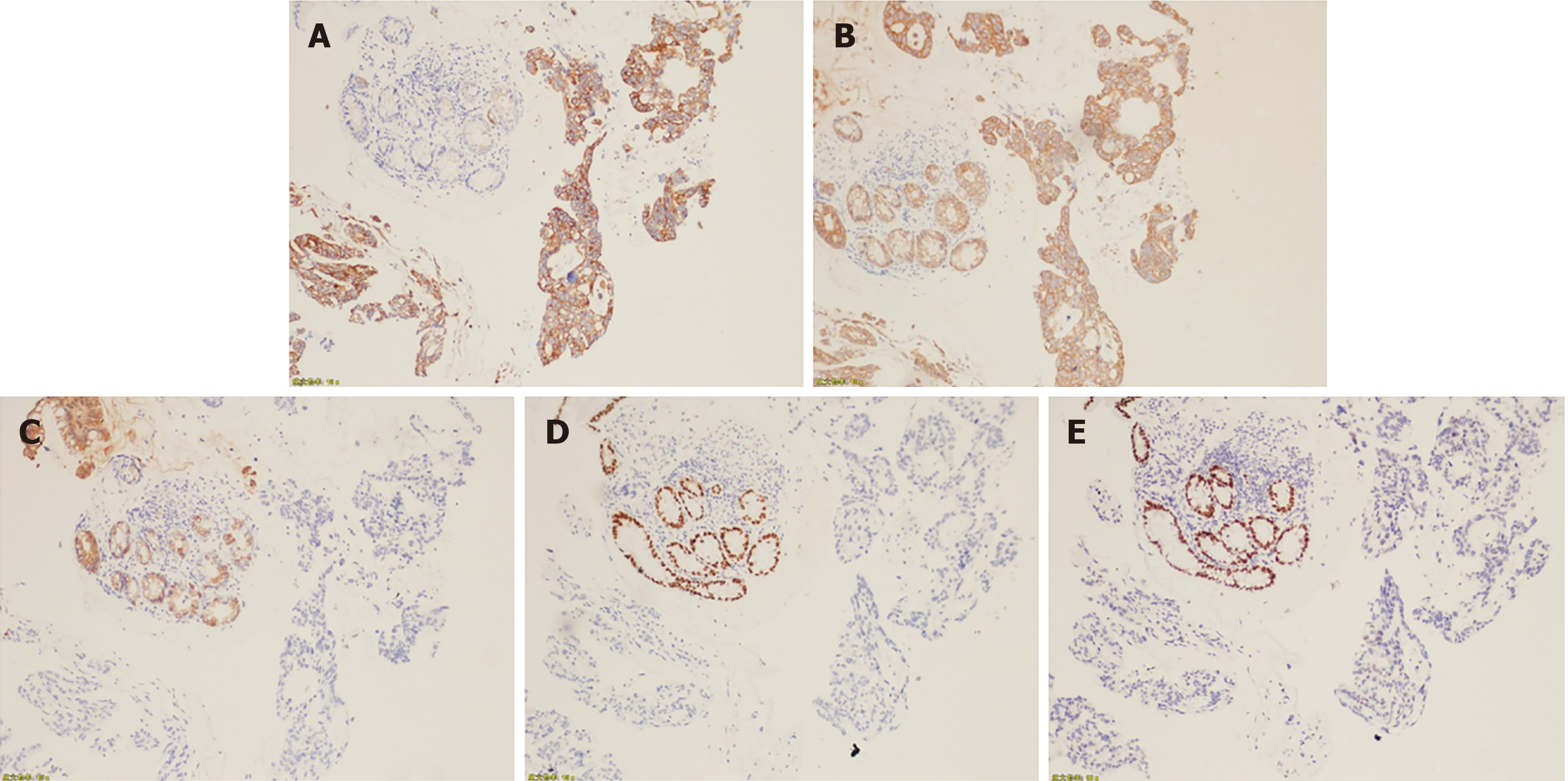
Given the difficulty in performing endoscopic ultrasound-guided fine-needle aspiration of the pancreas mass, biopsy of the left lobe of the liver was obtained and pathologically presented as moderately to poorly differentiated degenerative adenocarcinoma within large areas of necrosis (Figure 2A). Histopathological examination of biopsies from the colon mucosal lesions revealed moderately to poorly differentiated adenocarcinoma, which was compatible with liver metastasis from the primary pancreas (Figure 2B). Immunohistochemical staining of the colon tumor was positive for cytokeratin (CK) 7 and CK, which were expressed particularly in epithelial cells, but negative for colorectal adenocarcinoma-related markers, such as CK 20, CDX2, and SATB2 (Figure 3). A targeted comprehensive genomic profiling assay was performed on the liver and colon metastases using a next-generation sequencing (NGS) panel containing 654 cancer-related genes (Berryoncology, Beijing, China), which detected KRAS p.G12D (27.04%), TP53 c.376-1delG (18.07%), EP300 p.R1462* (5.13%), and CD244 p.M299Sfs*17 (5.51%) in the liver lesion and KRAS p.G12D (6.49%), and TP53 c.376-1delG (5.91%) in the colon biopsy; In addition, microsatellite stable (MSS) and low tumor mutational burden (TMB) were seen in both liver and colon metastases.
The final diagnosis in this case was pancreatic cancer with synchronous liver and colon metastases.
After comprehensive diagnostic evaluations, the patient was administered 12 cycles of FOLFIRINOX and subsequently maintained with ongoing irinotecan plus fluorouracil.
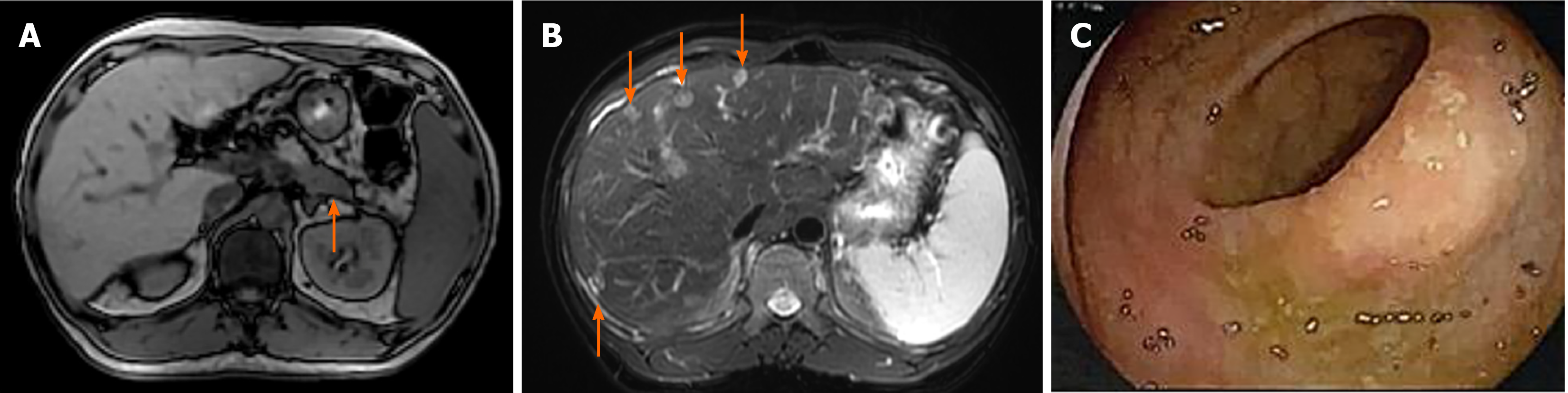
Abdominal MRI showed partial response in the pancreas and liver lesion after treatment with three cycles of FOLFIRINOX, thus the patient continued to receive this chemotherapy. Abdominal MRI scans showed that the tumor had shrunk in the tail of the pancreas (Figure 4A) and liver (Figure 4B) after receiving 12 cycles of FOL
Metastasis of pancreatic cancer to the colon is extremely rare, with less than ten cases reported in the literature (Table 1), to the best of our knowledge. The median age of these cases was 70 years (range: 45-91 years; male: 4, female: 3). Lesions in the pancreas and colon were identified simultaneously in four of these cases, but only one pancreatic cancer patient presented with synchronous colon and liver metastases[5-8]. However, our case is the only one reported with complete pathology, treatment, and follow-up data.
| Ref. | Age, yr | Sex | Metastatic site | Timing of metastasis | Immunohistochemical staining | Treatment | OS from CM Dx |
| Charles et al[21] | 45 | Male | Colon | NA | CA19-9 (+), CK (+), EMA (+), CEA (+), CDX2 (-), CK20 (-), CK7 (-), CD10 (-), vimentin (-), TTF-1) (-) | NA | NA |
| Kentaro et al[4] | 62 | Male | Colon | Metachronous | CK7 (+), CK20 (-) | Hemicolectomy | NA |
| Woogyeong et al[3] | 64 | Male | Colon | Metachronous | CK7 (+), CK20 (-), CK19 (+) | Hemicolectomy + gemcitabine | 6+ |
| Giuseppe et al[5] | 70 | Female | Liver, colon | Synchronous | CK7 (+), CK20 (-) | NA | NA |
| Ryan et al[6] | 91 | Female | Colon | Synchronous | CK7 (+), CK20 (-), CDX2 (-) | Palliative care | NA |
| Deborah et al[7] | 73 | Female | Colon | Synchronous | CK7 (+), CK20 (-), CDX2 (-), SATB2 (-) | Gemcitabine + nab-paclitaxel | 7 mo |
| Rohan et al[8] | 71 | Male | Colon | Synchronous | CK7 (+), CK20 (-) | Gemcitabine + nab-paclitaxel | NA |
As the majority of metastases from pancreatic cancer occurs in the liver, lung, abdomen, regional lymph nodes, and peritoneum, such cases are easily misdiagnosed as primary colon cancer, which influences treatment decision-making. The presence of masses in both the colon and pancreas could be a result of metastasis from the pancreas to the colon, metastasis from the colon to the pancreas, or synchronous primary cancers. The majority of previously reported cases were identified by histological examination and immunohistochemical staining of colon biopsies, most of which were based on CK 7 and CK 20 expression (Table 1). Cytokeratins are proteins of keratin-containing intermediate filaments, which are found in epithelial tissues. The expression of CK 7 is observed in the majority of cases of carcinoma, except in those carcinomas derived from the colon, prostate, kidney, and thymus. Positive CK 20 was seen in virtually all cases of colorectal carcinomas and Merkel cell tumors; CK 20-positive staining has also been seen in some cases of pancreatic carcinomas (62%)[9]. Besides these two tumor markers, CDX2 expression was demonstrated to be an exquisitely sensitive marker, but incompletely specific for intestinal adenocarcinomas[10]. SATB2 was shown to be highly expressed in the epithelium of the lower gastrointestinal tract[11] and CK was also included by us to further improve the diagnostic accuracy of the colon lesion. In this case, immunohistochemical staining of the colon tumor was positive for CK 7 and CK, but negative for CK 20, CDX2, and SATB2, thus suggesting that the lesion originated from the pancreas. Interestingly, KRAS p.G12D and TP53 c.376-1delG were detected in both the liver and colon lesions, thus indicating the same histopathological origin, which was typical of an advanced stage of pancreatic cancer[12]. How the pancreatic cancer in the present case metastasized to the colon remains unclear. Since the lymph nodes near the colon lesion were negative, cancer cells from the pancreas may have traveled to the colon through the bloodstream and this was presumed to be the most probable pathway.
It has been reported that 5%-10% of all pancreatic cancers are estimated to be attributable to inherited risk factors and some patients who had no family history of this cancer harbor at least one known inherited pancreatic cancer-predisposing genetic alteration[13,14]. Therefore, the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend that all patients diagnosed with pancreatic cancer should consider germline testing[14,15]. Genomic profiling of our patient was performed using a 654 gene panel containing 102 cancer-susceptibility genes, and pathogenic or likely pathogenic gene mutations associated with increased risk for pancreatic cancer were not found, which was consistent with the fact that the pancreatic cancer patient had no family history of this cancer.
Furthermore, efforts to translate the latest advances in the molecular characterization of pancreatic cancer into targeted therapeutics are in progress. The Know Your Tumor program is a collaboration between industry and academia to determine whether targeted therapy based on actionable mutations can improve outcomes in pancreatic cancer patients[16,17]. In addition to target therapy, immunotherapy has emerged as an exciting treatment alternative for patients with TMB-high or MSI-high tumors. Unfortunately, actionable mutations were not observed in the patient, which was accompanied by low TMB and MSS; thus, systemic chemotherapy was consi
Herein, we present a rare case of primary pancreatic cancer with synchronous liver and colon metastases. Immunohistochemical staining of CK, CK7, CK20, CDX2, and SATB2 on the colon biopsy distinguished the metastatic and primary tumors. Comprehensive NGS profiling of the liver and colon lesions at diagnosis was performed to identify cancer susceptibility gene variants and therapies. FOLFIRINOX, which was administered as first-line systemic therapy, improved the patient’s outcome. To the best of our knowledge, this is the first case report that reveals the genomic profiles of pancreatic cancer with colon metastasis using a multigene NGS panel, which is a step forward for clinical pathology.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish ME S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Yu HG
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15271] [Article Influence: 3054.2] [Reference Citation Analysis (4)] |
| 2. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1250] [Article Influence: 178.6] [Reference Citation Analysis (37)] |
| 3. | Kim W, Lee Y. Metachronous colonic metastasis from pancreatic cancer presenting as mechanical obstruction: a case report. Clin Imaging. 2015;39:699-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Inada K, Shida D, Noda K, Inoue S, Warabi M, Umekita N. Metachronous colonic metastasis from pancreatic cancer seven years post-pancreatoduodenectomy. World J Gastroenterol. 2013;19:1665-1668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Gizzi G, Santini D, Golfieri R, Fuccio L. Diffuse colonic metastases from primary pancreatic cancer. Gastrointest Endosc. 2017;85:678-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Kahl R, George K, Patel K, Stawick L. Pancreatic Adenocarcinoma With Rare Sigmoid Colon Metastasis. ACG Case Rep J. 2019;6:e00132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Park DY, Krishnamurthi S, Chahal P, Downs-Kelly E, Morris-Stiff G. Pancreatic metastases to the colon: an unusual cause of colonic obstruction. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Yewale R, Ramakrishna B, Vijaykumar K, Balasundaram P, Arulprakash S, Radhakrishna P, Ramakrishna BS. Pancreatic Adenocarcinoma With Synchronous Colonic Metastases. ACG Case Rep J. 2020;7:e00299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 637] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 10. | Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 502] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 11. | Berg KB, Schaeffer DF. SATB2 as an Immunohistochemical Marker for Colorectal Adenocarcinoma: A Concise Review of Benefits and Pitfalls. Arch Pathol Lab Med. 2017;141:1428-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Wood LD, Yurgelun MB, Goggins MG. Genetics of Familial and Sporadic Pancreatic Cancer. Gastroenterology. 2019;156:2041-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Solomon S, Das S, Brand R, Whitcomb DC. Inherited pancreatic cancer syndromes. Cancer J. 2012;18:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Stoffel EM, McKernin SE, Brand R, Canto M, Goggins M, Moravek C, Nagarajan A, Petersen GM, Simeone DM, Yurgelun M, Khorana AA. Evaluating Susceptibility to Pancreatic Cancer: ASCO Provisional Clinical Opinion. J Clin Oncol. 2019;37:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Tempero MA. NCCN Guidelines Updates: Pancreatic Cancer. J Natl Compr Canc Netw. 2019;17:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 121] [Reference Citation Analysis (0)] |
| 16. | Pishvaian MJ, Bender RJ, Halverson D, Rahib L, Hendifar AE, Mikhail S, Chung V, Picozzi VJ, Sohal D, Blais EM, Mason K, Lyons EE, Matrisian LM, Brody JR, Madhavan S, Petricoin EF 3rd. Molecular Profiling of Patients with Pancreatic Cancer: Initial Results from the Know Your Tumor Initiative. Clin Cancer Res. 2018;24:5018-5027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 17. | Pishvaian MJ, Blais EM, Brody JR, Lyons E, DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, Bellakbira S, Thach D, Rahib L, Madhavan S, Matrisian LM, Petricoin EF 3rd. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 362] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 18. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5609] [Article Influence: 400.6] [Reference Citation Analysis (1)] |
| 19. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4857] [Article Influence: 404.8] [Reference Citation Analysis (0)] |
| 20. | Taieb J, Prager GW, Melisi D, Westphalen CB, D'Esquermes N, Ferreras A, Carrato A, Macarulla T. First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: a retrospective, observational chart review study. ESMO Open. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Bellows C, Gage T, Stark M, McCarty C, Haque S. Metastatic pancreatic carcinoma presenting as colon carcinoma. South Med J. 2009;102:748-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |