Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10249
Peer-review started: March 29, 2021
First decision: August 18, 2021
Revised: August 27, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: November 26, 2021
Processing time: 237 Days and 22.9 Hours
Autosomal dominant tubulointerstitial kidney disease (ADTKD) is a progressive chronic disease that is inherited in an autosomal dominant fashion. Symptoms include hyperuricemia, gout, interstitial nephritis, renal cysts, and progressive renal damage that can lead to end-stage renal disease. Mutations in the uromodulin gene (UMOD) characterize the ADTKD-UMOD clinical subtype of this disease. To date, > 100 UMOD mutations have been identified. Early diagnosis of ADTKD-UMOD is important to treat the disease, slow down disease progression, and facilitate the identification of potentially affected family members.
We report a 40-year-old man harboring a novel heterozygous missense mutation in UMOD (c.554G>T; p. Arg185Leu). The patient had hyperuricemia, gout, and chronic kidney disease. The same mutation was detected in his daughter, aunt and cousin.
A single nucleotide substitution in exon 3 of UMOD was responsible for the heterozygous missense mutation (c.554G>T, p.Arg185Leu).
Core Tip: Autosomal dominant tubulointerstitial kidney disease (ADTKD) is a progressive chronic disease that is inherited in an autosomal dominant fashion. It can cause multiple organ damage and even end-stage renal disease. Mutations in the uromodulin gene (UMOD) characterize the ADTKD-UMOD clinical subtype of this disease. We report a novel heterozygous missense mutation in UMOD (c.554G>T; p. Arg185Leu). This mutation has not been previously reported, and it can help facilitate the presymptomatic diagnosis of this rare condition, in addition to helping guide genetic counseling and family planning for relatives of affected individuals.
- Citation: Zhang LL, Lin JR, Zhu TT, Liu Q, Zhang DM, Gan LW, Li Y, Ou ST. Autosomal dominant tubulointerstitial kidney disease with a novel heterozygous missense mutation in the uromodulin gene: A case report. World J Clin Cases 2021; 9(33): 10249-10256
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10249.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10249
Autosomal dominant tubulointerstitial kidney disease (ADTKD) is a set of heritable renal disorders that are characterized by autosomal dominant inheritance and clinical findings of tubulointerstitial disease in affected individuals. Most patients present with hyperuricemia, gout, arthritis, interstitial nephritis, and progressive renal damage, whereas some patients develop end-stage renal disease (ESRD) within 10–20 years[1]. Renal biopsy of patients with ADTKD often indicates interstitial fibrosis, tubular atrophy, and renal cysts[2]. Pathogenic mutations associated with ADTKD are present in the uromodulin gene (UMOD) in 40% of cases, whereas mutations in either renin or hepatocyte nuclear factor-1β genes are detected in 2.5% of cases[3,4]. UMOD mutations are considered under the clinical ADTKD-UMOD disease subtype, and most UMOD mutations are localized to exons 3–5 of this gene in the chromosome 16p11–p13 region[5-8].
Uromodulin is the most abundant protein in urine and is released from epithelial cells via proteolytic cleavage within the loop of Henle[9,10]. Missense mutations in this gene lead to misfolding of the protein and its accumulation within the endoplasmic reticulum of affected cells, thereby impairing urinary excretion[11]. These mutations also lead to disrupted trafficking of Na+-K+-2Cl− cotransporters in the luminal membrane of affected cells[12]. This ultimately leads to impaired urine concentration, increased rates of proximal tubular sodium and urate reabsorption, hyperuricemia, and gout, which are the clinical characteristics of ADTKD.
Owing to changes in diets and other factors, the rates of hyperuricemia are increasing annually and the age of ADTKD onset is decreasing continuously, similar to ADTKD-UMOD. However, the risk factors, pathogenesis and prognosis of these two disease isoforms differ considerably. Early diagnosis of ADTKD-UMOD is important to treat the disease, slow down disease progression, and facilitate the identification of potentially affected family members. Identification of potentially affected family members can facilitate decision-making about donors and family planning. In the present study, we report the case of a 40-year-old Chinese man harboring a novel heterozygous missense mutation in UMOD, which was also detected in his seven family members.
The patient was a 40-year-old man who was admitted to hospital because of increased pain in the metatarsal joints and renal impairment.
Around 15 years previously, the patient was diagnosed with gout.
The patient had a free previous medical history.
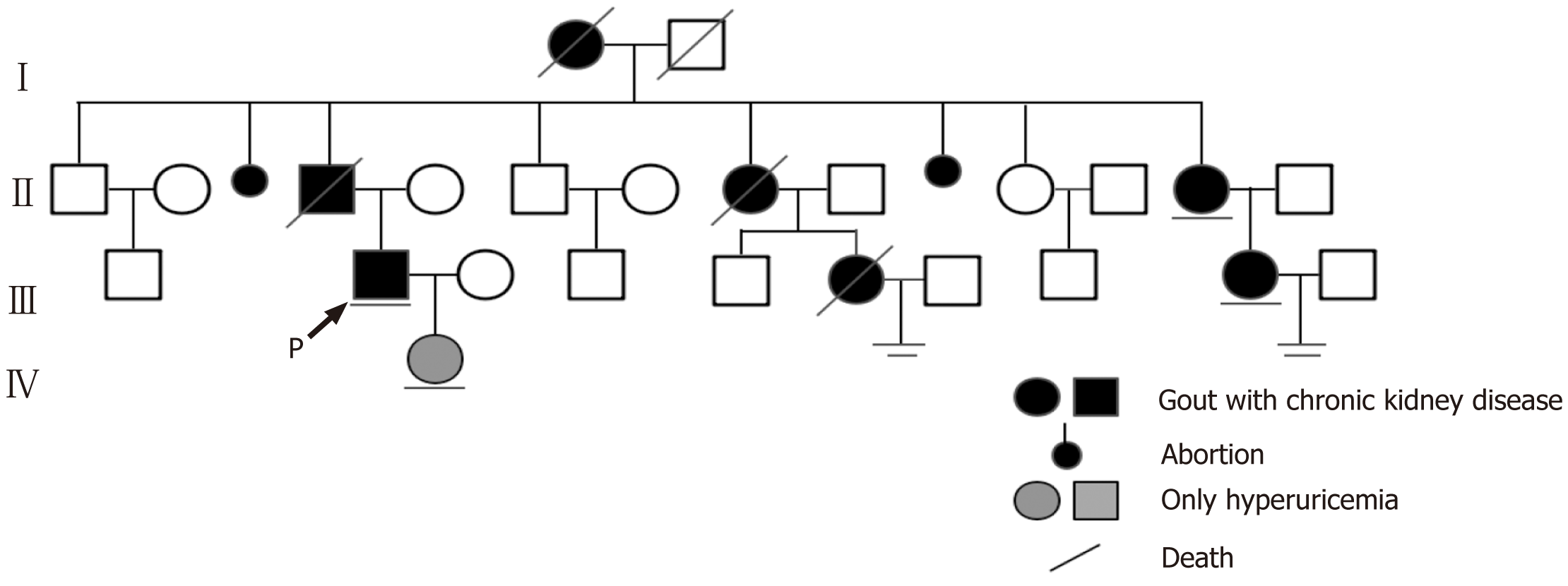
The proband had a family history of hyperuricemia as his grandmother, father, two of his aunts, and two of his female cousins were diagnosed with hyperuricemia and gout. The grandmother, father, one aunt, and one female cousin had been undergoing hemodialysis and died between the ages of 30 and 50 years. In addition, the patient’s 9-year-old daughter had also been diagnosed with hyperuricemia based on her 5.6 mg/dL serum uric acid level (normal range for children aged 1–10 years: < 5.3 mg/dL)[13] (Figure 1).
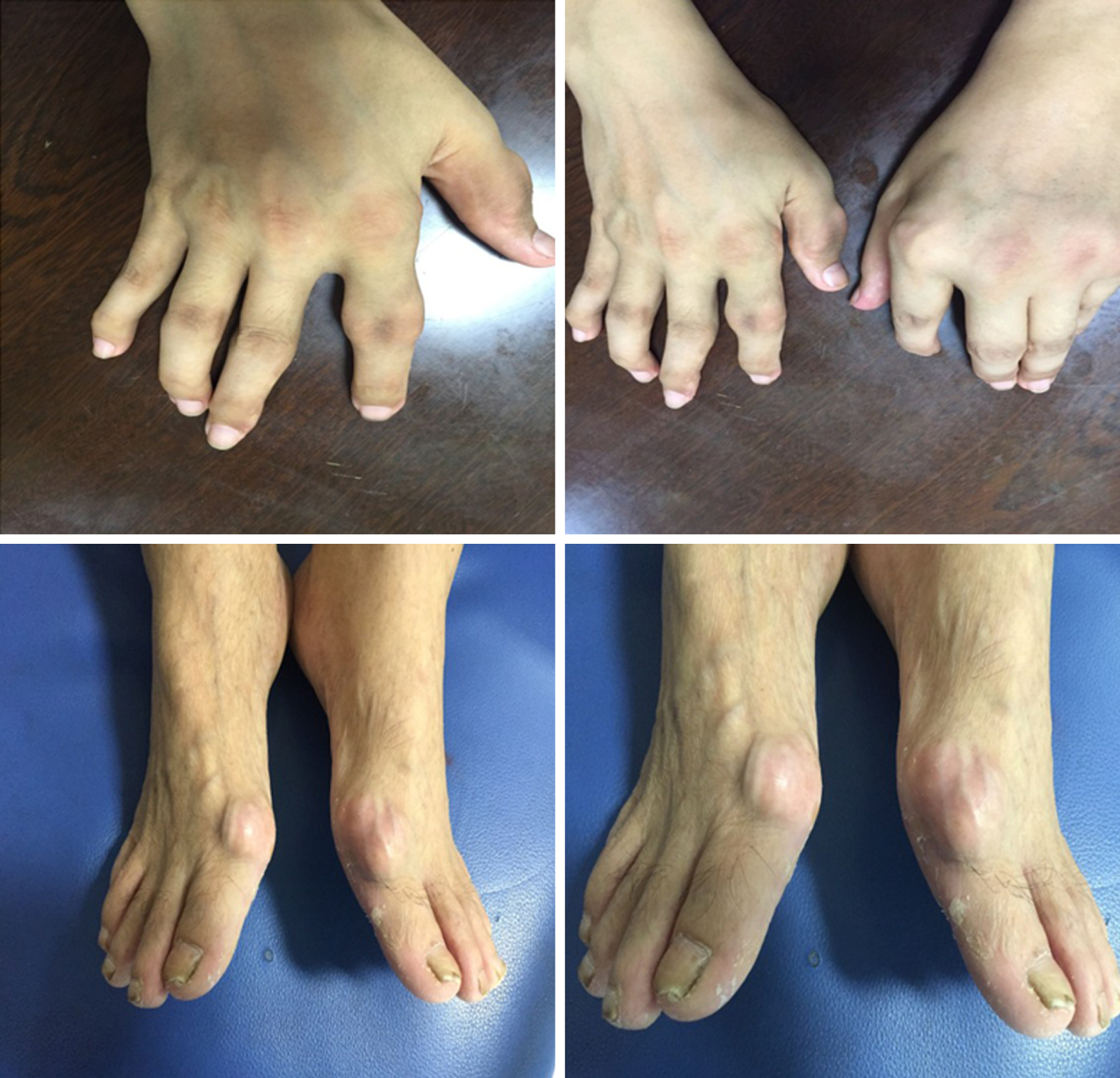
The patient’s temperature was 36.7 ℃, heart rate 88 bpm, respiratory rate 14 breaths/min, blood pressure 132/78 mmHg, and oxygen saturation in room air 100%. Physical examination indicated the presence of a mildly painful nodule behind the auricle, slight pain and swelling of the knee joints, serious pain and deformity of the interphalangeal joints, and gout stones on the 1 s metatarsal joints in the feet of the patient (Figure 2).
The patient had respective blood urea nitrogen and serum creatinine levels of 50.5 mg/dL and 6.2 mg/dL (normal ranges: 7.30–21.06 mg/dL and 0.46–0.82 mg/dL, respectively). The patient had a serum uric acid level of 13.2 mg/dL (normal range: 2.6–6.0 mg/dL), whereas fractional uric acid excretion was reduced by 3.43%. Other laboratory test results were within normal ranges.
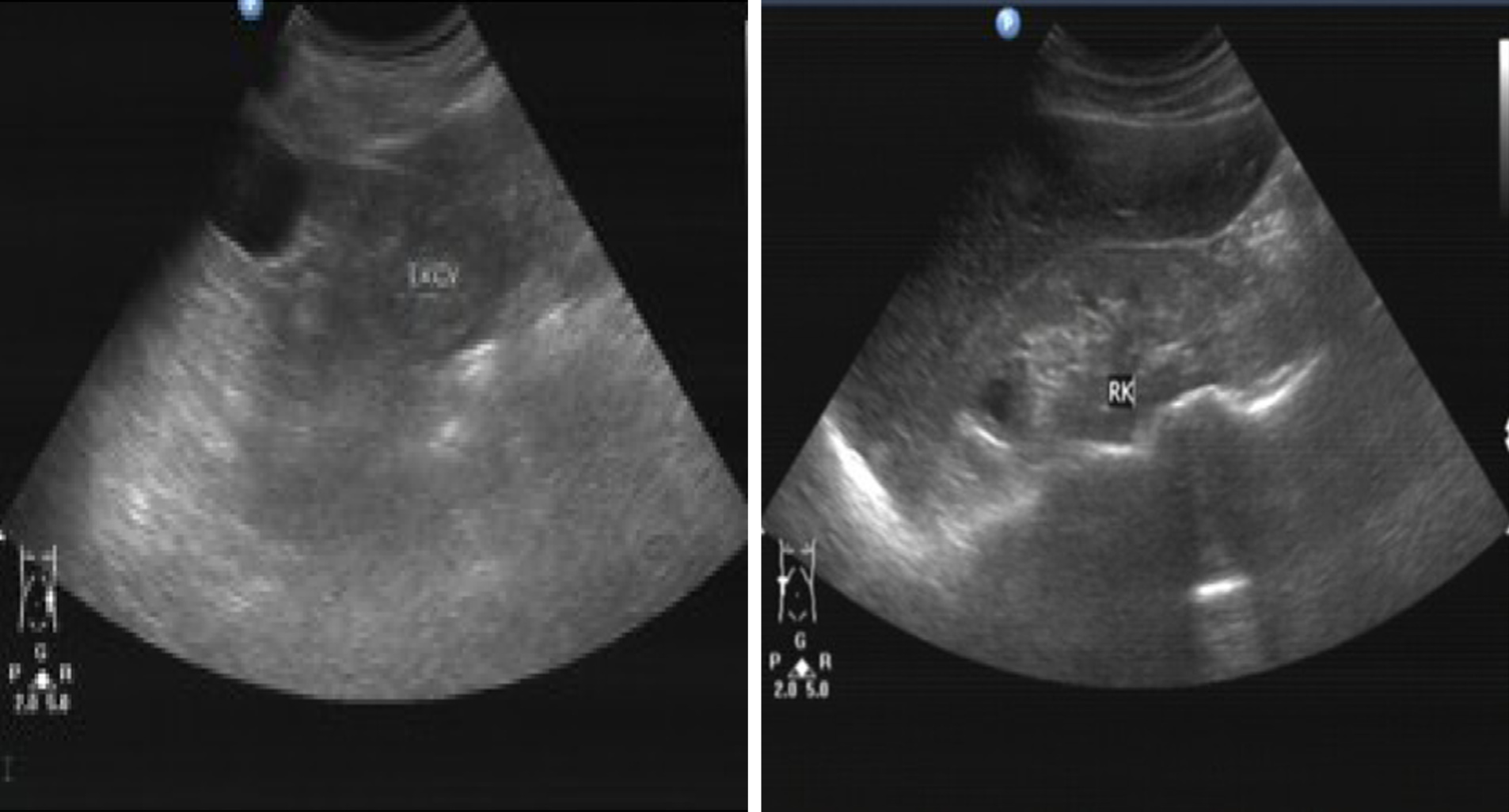
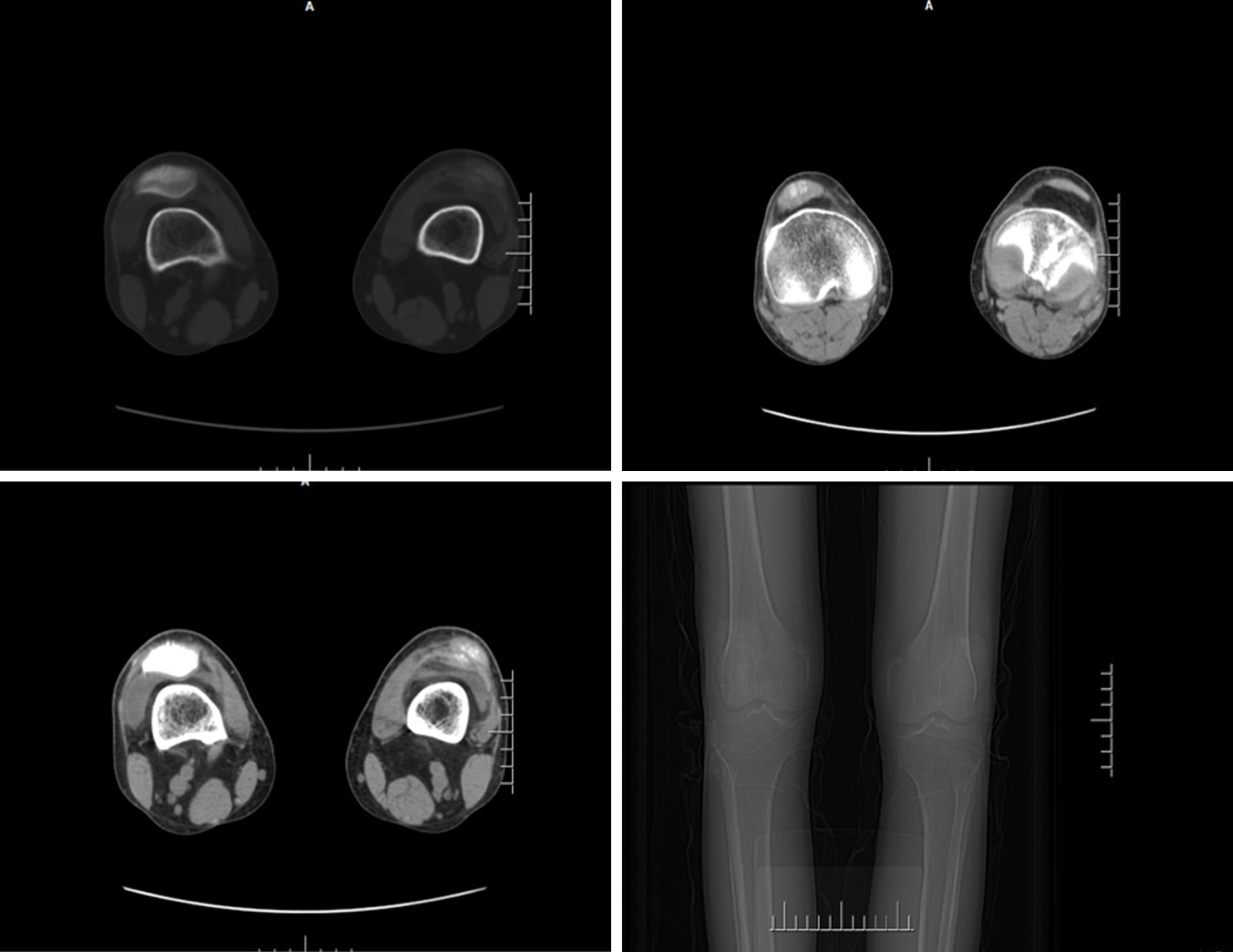
Renal ultrasonography showed that the patient’s kidneys were relatively atrophic (longitudinal image; 8.1 and 8.7 cm in the major axis of right and left kidneys), indicating the presence of cysts and suggestive of ESRD (Figure 3). Analysis of the knee joints by computed tomography showed high bone density, the presence of high-density shadows, narrowing of the joint space, and soft tissue swelling, which were consistent with the patient’s gout/arthritic symptoms (Figure 4).
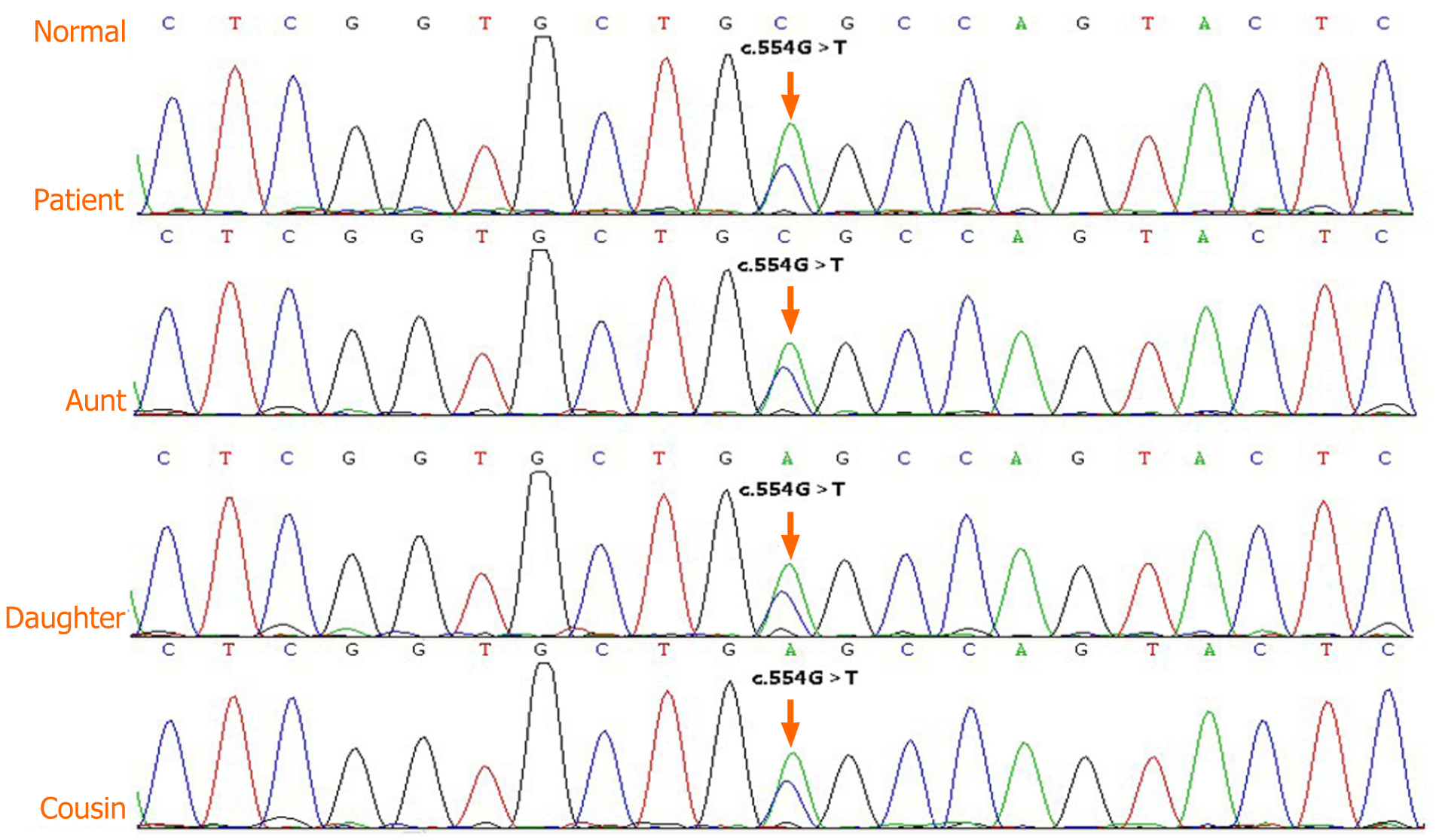
Considering the family history of kidney disease, juvenile-onset of hyperuricemia, symptoms of gout/arthritis, and progressive renal impairment beginning at an early age, ADTKD-UMOD was considered highly probable. After receiving written informed consent from the four affected living members of the patient’s family, DNA analyses, clinical data collection, and image publication were performed for these individuals. The peripheral blood was sent to CIPHER gene to perform the whole exome sequencing by Illumina HiSeq (the specific method can be consulted in the Supplementary Material). Genetic analyses revealed the presence of a novel heterozygous missense mutation in UMOD exon 3 of the patient, his daughter, aunt, and younger female cousin (Figure 5). The conclusion of the genetic test was variants of unknown clinical significance. According to the American College of Medical Genetics and Genomics genetic variation classification standards and guidelines, the variation site was heterozygous, and the zygote type could explain the patient’s disease. Furthermore, this missense mutation was the result of nucleotide exchange at position c.554 (c.554G>T), in which leucine was replaced by arginine at position 185 in the final protein (p.Arg185Leu). This resulted in abnormal folding of uromodulin protein, leading to its accumulation within the endoplasmic reticulum and impaired trafficking through the cell.
The final diagnosis of the present case was ADTKD with a mutation in UMOD.
Medication aimed at controlling uric acid levels was administered to the patient but was not efficacious in controlling the gradually increasing serum creatinine and uric acid levels. Recently, the glomerular filtration rate for this patient decreased to 6.3 mL/min/1.73 m2. Hence, we recommended arteriovenous fistula surgery for hemodialysis preparation. Moreover, his affected family members do not currently require dialysis but should maintain a healthy lifestyle and preventatively take uric acid medication to control the onset of hyperuricemia.
The patient received regular blood dialysis and medications, and gout was controlled.
ADTKD is a condition also referred to as medullary cystic kidney disease, familial juvenile hyperuricemic nephropathy, and UMOD-associated kidney disease. Recently, ADTKD has been proposed as a collective term to refer to the aforementioned progressive kidney diseases[3]. Most cases of ADTKD present with mutations in the UMOD, REN, MUC1, TCF2, or SEC51A1 genes[14,15]. ADTKD-UMOD disease subtype is the most common, and the clinical features of all disease subtypes differ based on the mutated gene.
Uromodulin is the most abundant protein in urine and is the primary component of urinary casts that are encoded by chromosome 16p11-p13[7]. Mutations in UMOD gene can result in defective sodium transport in the thick ascending limb, leading to natriuresis that results in secondary proximal tubular sodium and urate uptake. This abnormal sodium and urate uptake further leads to hyperuricemia and gout. In addition, misfolded uromodulin deposits accumulate in the endoplasmic reticulum of affected epithelial cells[11]. Owing to these molecular mechanisms, UMOD mutations can result in conditions such as progressive distal tubular dysfunction, hyperuricemia[11,16], hyperuricemic nephropathy[17], urinary tract stone formation[18], salt-sensitive hypertension, and kidney damage[19]. However, uromodulin excretion is reduced even in ADTKD patients without UMOD mutations[20], and UMOD-knockout mice do not have hyperuricemia[21]. Other recent studies have suggested that mutated uromodulin in the kidneys may elicit an immune response that is specific to this protein, ultimately leading to the observed tubular injury and interstitial fibrosis[22]. Hence, further work is needed to elucidate the underlying mechanisms of ADTKD-UMOD in detail.
ADTKD-UMOD can be diagnosed based on UMOD sequence analyses or immunostaining for misfolded uromodulin protein. However, misfolded uromodulin staining is not routinely performed in pathology laboratories, and only a limited number of institutions can perform this specialized test. In addition, many patients are not eligible for a kidney biopsy at the time of diagnosis, similar to the case reported here.
Currently, whether treatment of this condition with allopurinol or febuxostat can effectively reduce blood uric acid levels, relieve gout symptoms, and slow down progressive kidney impairment is unclear. The patient in the present report did not achieve disease remission after receiving medication aimed at controlling uric acid level, and eventually developed ESRD at an early age. However, whether dialysis or renal transplantation can help patients achieve long-term remission requires further study.
When a young adult individual presents with hyperuricemia and has a family history of hyperuricemia, ADTKD-UMOD should be considered and UMOD DNA analyses are necessary. Identification of the pathogenic mutations governing this condition can help facilitate the presymptomatic diagnosis of this rare condition, in addition to genetic counseling and family planning for relatives of affected individuals.
The authors are grateful to the patient and her relatives for allowing publication of this rare case report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barbosa OA, Cassell III AK, Sugihara Y, Yamaguchi K S-Editor: Fan JR L-Editor: Kerr C P-Editor: Zhang YL
| 1. | Utami SB, Mahati E, Li P, Maharani N, Ikeda N, Bahrudin U, Munemura C, Hosoyamada M, Yamamoto Y, Yoshida A, Nakayama Y, Higaki K, Nanba E, Ninomiya H, Shirayoshi Y, Ichida K, Yamamoto K, Hosoya T, Hisatome I. Apoptosis induced by an uromodulin mutant C112Y and its suppression by topiroxostat. Clin Exp Nephrol. 2015;19:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Lhotta K, Gehringer A, Jennings P, Kronenberg F, Brezinka C, Andersone I, Strazdins V. Familial juvenile hyperuricemic nephropathy: report on a new mutation and a pregnancy. Clin Nephrol. 2009;71:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Eckardt KU, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, Wiesener M, Wolf MT, Devuyst O; Kidney Disease: Improving Global Outcomes. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management--A KDIGO consensus report. Kidney Int. 2015;88:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 4. | Plumb LA, Marlais M, Bierzynska A, Martin H, Brugger K, Abbs S, Saleem MA. Unilateral hypoplastic kidney - a novel highly penetrant feature of familial juvenile hyperuricaemic nephropathy. BMC Nephrol. 2014;15:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Liu M, Chen Y, Liang Y, Liu Y, Wang S, Hou P, Zhang H, Zhao M. Novel UMOD mutations in familial juvenile hyperuricemic nephropathy lead to abnormal uromodulin intracellular trafficking. Gene. 2013;531:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Wei X, Xu R, Yang Z, Li Z, Liao Y, Johnson RJ, Yu X, Chen W. Novel uromodulin mutation in familial juvenile hyperuricemic nephropathy. Am J Nephrol. 2012;36:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Lee DH, Kim JK, Oh SE, Noh JW, Lee YK. A case of familial juvenile hyperuricemic nephropathy with novel uromodulin gene mutation, a novel heterozygous missense mutation in Korea. J Korean Med Sci. 2010;25:1680-1682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Carucci NS, Caridi G, Lugani F, Barone C, Conti G. A novel UMOD gene mutation associated with chronic kidney failure at a young age. Clin Nephrol. 2019;92:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Scolari F, Izzi C, Ghiggeri GM. Uromodulin: from monogenic to multifactorial diseases. Nephrol Dial Transplant. 2015;30:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Brunati M, Perucca S, Han L, Cattaneo A, Consolato F, Andolfo A, Schaeffer C, Olinger E, Peng J, Santambrogio S, Perrier R, Li S, Bokhove M, Bachi A, Hummler E, Devuyst O, Wu Q, Jovine L, Rampoldi L. The serine protease hepsin mediates urinary secretion and polymerisation of Zona Pellucida domain protein uromodulin. Elife. 2015;4:e08887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Rampoldi L, Caridi G, Santon D, Boaretto F, Bernascone I, Lamorte G, Tardanico R, Dagnino M, Colussi G, Scolari F, Ghiggeri GM, Amoroso A, Casari G. Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics. Hum Mol Genet. 2003;12:3369-3384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Mutig K, Kahl T, Saritas T, Godes M, Persson P, Bates J, Raffi H, Rampoldi L, Uchida S, Hille C, Dosche C, Kumar S, Castañeda-Bueno M, Gamba G, Bachmann S. Activation of the bumetanide-sensitive Na+,K+,2Cl- cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. J Biol Chem. 2011;286:30200-30210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Noone DG, Marks SD. Hyperuricemia is associated with hypertension, obesity, and albuminuria in children with chronic kidney disease. J Pediatr. 2013;162:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Bolar NA, Golzio C, Živná M, Hayot G, Van Hemelrijk C, Schepers D, Vandeweyer G, Hoischen A, Huyghe JR, Raes A, Matthys E, Sys E, Azou M, Gubler MC, Praet M, Van Camp G, McFadden K, Pediaditakis I, Přistoupilová A, Hodaňová K, Vyleťal P, Hartmannová H, Stránecký V, Hůlková H, Barešová V, Jedličková I, Sovová J, Hnízda A, Kidd K, Bleyer AJ, Spong RS, Vande Walle J, Mortier G, Brunner H, Van Laer L, Kmoch S, Katsanis N, Loeys BL. Heterozygous Loss-of-Function SEC61A1 Mutations Cause Autosomal-Dominant Tubulo-Interstitial and Glomerulocystic Kidney Disease with Anemia. Am J Hum Genet. 2016;99:174-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Venkat-Raman G, Gast C, Marinaki A, Fairbanks L. From juvenile hyperuricaemia to dysfunctional uromodulin: an ongoing metamorphosis. Pediatr Nephrol. 2016;31:2035-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM, Bleyer AJ. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39:882-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 346] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Tinschert S, Ruf N, Bernascone I, Sacherer K, Lamorte G, Neumayer HH, Nürnberg P, Luft FC, Rampoldi L. Functional consequences of a novel uromodulin mutation in a family with familial juvenile hyperuricaemic nephropathy. Nephrol Dial Transplant. 2004;19:3150-3154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int. 2004;66:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Graham LA, Padmanabhan S, Fraser NJ, Kumar S, Bates JM, Raffi HS, Welsh P, Beattie W, Hao S, Leh S, Hultstrom M, Ferreri NR, Dominiczak AF, Graham D, McBride MW. Validation of uromodulin as a candidate gene for human essential hypertension. Hypertension. 2014;63:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Vylet'al P, Kublová M, Kalbácová M, Hodanová K, Baresová V, Stibůrková B, Sikora J, Hůlková H, Zivný J, Majewski J, Simmonds A, Fryns JP, Venkat-Raman G, Elleder M, Kmoch S. Alterations of uromodulin biology: a common denominator of the genetically heterogeneous FJHN/MCKD syndrome. Kidney Int. 2006;70:1155-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Gersch M, Mutig K, Bachmann S, Kumar S, Ouyang X, Johnson R. Is salt-wasting the long awaited answer to the hyperuricaemia seen in uromodulin storage diseases? Nephrol Dial Transplant. 2006;21:2028-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Jennings P, Aydin S, Kotanko P, Lechner J, Lhotta K, Williams S, Thakker RV, Pfaller W. Membrane targeting and secretion of mutant uromodulin in familial juvenile hyperuricemic nephropathy. J Am Soc Nephrol. 2007;18:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |