Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10233
Peer-review started: November 13, 2020
First decision: July 8, 2021
Revised: August 4, 2021
Accepted: September 13, 2021
Article in press: September 13, 2021
Published online: November 26, 2021
Processing time: 373 Days and 16.5 Hours
Fiberoptic bronchoscopy has been widely used in the diagnosis and treatment of respiratory diseases. Numerous major and minor complications have been reported following this procedure. The incidence of major postoperative complications is approximately 0.5% and includes respiratory depression, pneumothorax, pulmonary edema, pneumonia, airway obstruction and cardiorespiratory arrest. Minor complications include vasovagal reactions, cardiac arrhythmias, hemo
A 70-year-old female patient presented with a history of rheumatic heart disease beginning at 10 years of age and an approximately 10-year history of hypertension. The patient was transferred from the cardiology department to the respiratory department due to recurrent coughing, pneumonia, and fever. She underwent fiberoptic bronchoscopy in the respiratory department. Approximately 2 h after completion of bronchoscopy, she complained of left arm numbness and weakness. Physical examination detected cyanosis of the left upper extremity, grade III weakened limb muscle strength, and undetectable left brachial artery pulsation. Auscultation indicated AF. B-mode ultrasound examination of the blood vessels showed hyperechoic material in the left subclavian, axillary and brachial arteries, and parallel veins. As our hospital has no vascular surgery capability, the patient was transferred to a specialized hospital for emergency thrombectomy that day. A tracking investigation found that the patient’s conditions improved after successful thrombectomy.
Thromboembolism following bronchoscopy is rare, and only a few cases of cerebral air embolism after bronchoscopy have been reported.
Core Tip: This case highlights the fact that although fiberoptic bronchoscopy is generally a well-known and safe procedure, serious complications, such as arterial thrombosis may occur. The risk of developing arterial thrombosis following bronchoscopy is higher in patients with atrial fibrillation with mitral stenosis, highlighting the need for a rigorous risk assessment in these patients.
- Citation: Yang CL, Zhou R, Jin ZX, Chen M, Zi BL, Li P, Zhou KH. Atrial fibrillation and concomitant left subclavian, axillary and brachial artery embolism after fiberoptic bronchoscopy: A case report. World J Clin Cases 2021; 9(33): 10233-10237
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10233.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10233
Fiberoptic bronchoscopy is a well-tolerated, minimally invasive surgical procedure, which has been widely used in the diagnosis and treatment of respiratory diseases[1]. For diagnostic purposes, bronchoscopy permits a visual inspection of the tracheobronchial tree and removal of tissue samples for biopsy. Therapeutically, bron
A 70-year-old female patient was admitted to the Department of Cardiology due to complaints of repeated dizziness over a 10-year period and shortness of breath and cough for 1 year. These symptoms progressed over a 1-mo period.
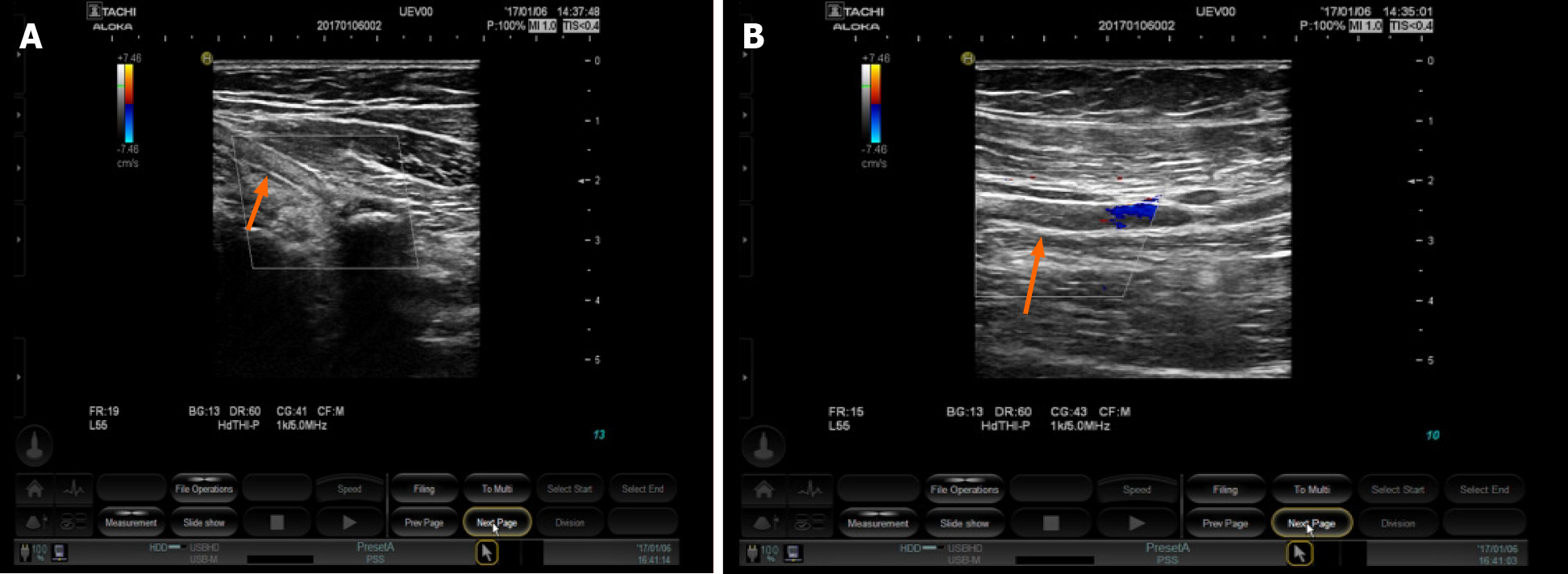
The patient was transferred from the Cardiology Department to the Respiratory Department due to recurrent cough, fever, and multiple lesions identified on computed tomography (CT) in both lungs. Physicians recommended fiberoptic bronchoscopy for further diagnosis. Two hours after completion of the operation, the patient complained of exacerbation of left arm numbness and weakness. The skin of the left upper extremity was found to be cyanotic, muscle strength decreased to grade 3, and the left brachial artery pulsation was not detectable. B-mode ultrasound examination of the blood vessels revealed hyperechoic material in the left subclavian artery, axillary artery, brachial artery, and parallel veins (Figure 1A, B). Based on the patient’s symptoms, history of rheumatic heart disease, and ultrasound images, we diagnosed embolism in the left upper extremity. As our hospital has no vascular surgery capability, the patient was transferred on the same day to a specialized hospital for an emergency thrombectomy. A tracking investigation found that the patient’s conditions improved after successful thrombectomy. The numbness in the left upper limb disappeared, and muscle strength and skin color returned to normal.
The patient had a history of rheumatic heart disease at age 10 years with hypertension as high as 160/110 mmHg, which was well controlled through oral amlodipine tablets.
The patient had no pertinent family history.
Vital signs of the patient appeared stable during bronchoscopy, except for minor coughing. Blood pressure was 128/80 mmHg, heart rate was 80–105 beats/min with signs of AF, peripheral capillary oxygen saturation (SpO2) was 95%–98%, respiratory rate was 20 breaths/min, and temperature was 36.5°C, 2 h after the examination. The patient complained of numbness in the left arm and difficulty stretching the fingers of her left hand. Her skin in the left upper extremity was cyanotic; muscle strength decreased to grade 3; and the left brachial artery pulsation was not detectable.
Five coagulation tests on the first day of admission revealed a prothrombin time (PT) of 13.6 s, an activated partial thromboplastin time (APTT) of 23.4 s, a thrombin time (TT) of 16.6 s, an international normalized ratio (INR) of 1.19, and fibrinogen (FIB) levels of 5.12 g/L. Emergency coagulation tests after bronchoscopy showed a PT of 12.3 s, an APTT of 22.7 s, a TT of 17.2 s, and an INR of 0.89. The FIB concentration was 6 g/L (reference range 2–4 g/L), D-dimer was 8.13 mg/L (reference range 0-0.55 g/L), te potassium level was 3.36 mmol/L (reference range 3.5–5.3 mmol/L), lactate dehydrogenase was 439 U/L (reference range 90–250 U/L), and troponin I was 0.216 g/L (reference range 0.006–0.06 g/L).
Color doppler ultrasound indicated the presence of rheumatic heart disease, moderate mitral valve stenosis, and moderate insufficiency, left atrium enlargement, decreased left ventricular diastolic and systolic function, moderate tricuspid valve regurgitation, severe pulmonary hypertension, and minor pericardial effusion. Chest CT detected multiple nodules and exudative lesions in both lungs, as well as local swelling of the left upper lung lobe. The bronchoscopy examination detected branch stenosis in the anterior segment of the right upper lobe, chronic inflammation in the right upper lobe, and inflammatory infiltration in the upper apicoposterior segment.
Based on the patients’ symptoms, history of rheumatic heart disease, and ultrasound images, the patient was finally diagnosed with AF with concomitant left subclavian artery, axillary artery, and brachial artery embolism after fiberoptic bronchoscopy.
Since our hospital is not equipped for vascular surgery, the patient was transferred on the same day to a specialized hospital for emergency thrombectomy.
A tracking investigation found that the patient’s conditions improved after successful thrombectomy. Left upper limb numbness disappeared, while muscle strength and skin color returned to normal.
The most likely cause behind the formation of an upper extremity embolism in the case presented was the detachment of an atrial embolus induced by AF combined with an accelerated heartbeat throughout the bronchoscopy procedure. Physical examination showed an AF rhythm after bronchoscopy. The patient had a long history of rheumatic heart disease, and echocardiography indicated moderate mitral stenosis with mild mitral regurgitation. Studies have shown that ~20% of patients with mitral stenosis had thromboembolism, and ~80% of patients with thrombosis had AF[7]. Thus, AF, mitral stenosis, and thromboembolism are closely related.
AF is one of the most common arrhythmias and is the main complication in thromboembolism. The 2019 AHA/ACC/HRS updated guidelines for the man
AF is not an absolute contraindication for fiberoptic bronchoscopy. According to the 2019 American AF guidelines, if a patient with AF or a mechanical heart valve requires temporary interruption of warfarin treatment during an elective invasive procedure or surgery, bridging therapy that includes unfractionated heparin or low molecular weight heparin is recommended. The decision on bridging therapy should balance the risks of stroke and bleeding.
This case highlights the importance of anticoagulation prior to fiberoptic bronchoscopy in patients with AF. Numerous guidelines recommend an oral anticoagulant, especially warfarin, as the first choice for patients with AF[9]. To achieve optimum results and to reduce the risks of bleeding and thromboembolism, the recommended INR for warfarin treatment is between 2.0 and 3.0. At this dose, the relative risk of stroke is reduced by 64%, and all-cause mortality may be significantly reduced by 26%. However, the utilization rate of oral anticoagulants in China is lower than in western countries[10]. The current utilization rate of warfarin-based antithrombotic therapy in Chinese patients with non-valvular AF is below 10%. Various reasons have been attributed to the low utilization rate of anticoagulation therapy in Chinese patients with AF, including the narrow safety window of warfarin, which results in poor compliance and extra coagulation monitoring and dose adjustments required due to the constant INR variations. GARFIELD global research has shown that the lack of effective long-term management and safety concerns of anticoagulant therapy results in approximately half of all eligible patients not receiving anticoagulant therapy[11]. Therefore, physicians have an important role in improving the acceptance and usage of anticoagulation therapy in patients with AF.
We thank the Ultrasound team of Kunming Municipal First People’s Hospital for providing the images for this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Patel VJ S-Editor: Gao CC L-Editor: Kerr C P-Editor: Ma YJ
| 1. | Costa ADS Jr, Scordamaglio PR, Suzuki I, Palomino ALM, Jacomelli M. Indications, clinical outcomes and complications of 1,949 flexible bronchoscopies. Einstein (Sao Paulo). 2018;16:eAO4380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Huang PM, Kao MW. Endobronchial foreign body removed by flexible bronchoscopy using the Trendelenburg position. Thorac Cardiovasc Surg. 2012;60:545-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Leiten EO, Martinsen EM, Bakke PS, Eagan TM, Grønseth R. Complications and discomfort of bronchoscopy: a systematic review. Eur Clin Respir J. 2016;3:33324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Maemura K, Kage H, Isago H, Takeshima H, Makita K, Amano Y, Takai D, Ohishi N, Nagase T. Cerebral Arterial Air Embolism after Diagnostic Flexible Fiberoptic Bronchoscopy: A Case Report and Review of the Literature. Case Rep Pulmonol. 2018;2018:7108215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Azzola A, von Garnier C, Chhajed PN, Schirp U, Tamm M. Fatal cerebral air embolism following uneventful flexible bronchoscopy. Respiration. 2010;80:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Toyota H, Kobayashi K, Matsukura S, Kawamura Y, Kanda T, Arai H, Nagase H, Yamaguchi M. A Case of Cerebral Air Embolism During Diagnostic Flexible Bronchoscopy. J Bronchology Interv Pulmonol. 2019;26:e58-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Giugliano RP, O'Gara PT. DOACs in Patients With Mitral Stenosis and Atrial Fibrillation: Time for a Randomized Clinical Trial. J Am Coll Cardiol. 2019;73:1132-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125-e151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1928] [Article Influence: 321.3] [Reference Citation Analysis (0)] |
| 9. | Katz DF, Maddox TM, Turakhia M, Gehi A, O'Brien EC, Lubitz SA, Turchin A, Doros G, Lei L, Varosy P, Marzec L, Hsu JC. Contemporary Trends in Oral Anticoagulant Prescription in Atrial Fibrillation Patients at Low to Moderate Risk of Stroke After Guideline-Recommended Change in Use of the CHADS2 to the CHA2DS2-VASc Score for Thromboembolic Risk Assessment: Analysis From the National Cardiovascular Data Registry's Outpatient Practice Innovation and Clinical Excellence Atrial Fibrillation Registry. Circ Cardiovasc Qual Outcomes. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Sun Y, Hu D; Chinese Investigators of GARFIELD; Chinese Investigators of GARFIELD. [Chinese subgroup analysis of the global anticoagulant registry in the FIELD (GARFIELD) registry in the patients with non-valvular atrial fibrillation]. Zhonghua Xin Xue Guan Bing Za Zhi. 2014;42:846-850. [PubMed] |
| 11. | Weitz JI, Haas S, Ageno W, Angchaisuksiri P, Bounameaux H, Nielsen JD, Goldhaber SZ, Goto S, Kayani G, Mantovani L, Prandoni P, Schellong S, Turpie AG, Kakkar AK. Global Anticoagulant Registry in the Field - Venous Thromboembolism (GARFIELD-VTE). Rationale and design. Thromb Haemost. 2016;116:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |