Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10161
Peer-review started: April 2, 2021
First decision: July 3, 2021
Revised: July 13, 2021
Accepted: September 6, 2021
Article in press: September 6, 2021
Published online: November 26, 2021
Processing time: 233 Days and 19.6 Hours
Dipeptidyl peptidase-4 (DPP4) is associated with cognitive dysfunction in patients with type 2 diabetes.
To assess a possible relationship between serum DPP4 and cognitive function in perinatal pregnant women with gestational diabetes mellitus (GDM).
The study subjects were divided into three groups: GDM group (n = 81), healthy pregnant (HP) group (n = 85), and control group (n = 51). The Montreal Cognitive Assessment (MoCA) was used to assess the cognitive status of each group. Venous blood samples were collected to measure blood lipids, glycated hemo
The MoCA scores in the GDM and HP groups were significantly different from those in the control group in terms of visuospatial/executive function and attention (P < 0.05); however, the scores were not significantly different between the GDM and HP groups (P > 0.05). In terms of language, the GDM group had significantly different scores from those in the other two groups (P < 0.05). In terms of memory, a significant difference was found between the HP and control groups (P < 0.05), as well as between the GDM and HP groups. The levels of DPP4, IL-6, and 8-iso-PGF2α in the GDM group were significantly higher than those in the HP and control groups (P < 0.05); however, the differences between these levels in the HP and control groups were not significant (P > 0.05). The level of BDNF in the GDM group was significantly lower than that in the HP and control groups (P < 0.05), although the difference in this level between the HP and control groups was not significant (P > 0.05).
Cognitive dysfunction in perinatal pregnant women with GDM mainly manifested as memory loss, which might be associated with elevated DPP4 levels.
Core Tip: It is generally believed that diabetes can cause cognitive impairment, and even is an important cause of Alzheimer’s disease. This study investigated whether gestational diabetes mellitus (GDM) induced cognitive decline in perinatal pregnant women and assessed a possible relationship between serum dipeptidyl peptidase-4 (DPP4) levels and maternal cognitive function in perinatal pregnant women with GDM, and detected DPP4 levels in cord blood. DPP4 may cause cognitive impairment by aggravating inflammatory response and oxidative stress response.
- Citation: Sana SRGL, Li EY, Deng XJ, Guo L. Association between plasma dipeptidyl peptidase-4 levels and cognitive function in perinatal pregnant women with gestational diabetes mellitus. World J Clin Cases 2021; 9(33): 10161-10171
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10161.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10161
With the advent of the two-child policy in China, the number of elderly parturient women is increasing, which has led to a rise in many pregnancy-related complications, the most common of which is gestational diabetes mellitus (GDM). The global incidence of DM during pregnancy is 15%, of which, GDM comprises 87.5% of cases[1]. GDM causes serious adverse effects to the mother and fetus, and increases the risk of eclampsia, maternal metabolic disorders, miscarriage, premature birth, obstructed labor, macrosomia, fetal distress, neonatal pulmonary immaturity, and neonatal hypoglycemia[2]. Although the pathogenesis of GDM is poorly understood, it might be associated with insulin resistance, and may share similarities with the pathogenesis of type 2 DM (T2DM). Most of the women with GDM regain normal glucose meta
Several recent studies have found that T2DM can lead to cognitive dysfunction[3], which mostly manifests as impairments in various abilities such as memory and orientation. Zheng et al[4] confirmed the correlation between cognitive dysfunction and serum dipeptidyl peptidase-4 (DPP4) activity in patients with T2DM; they also found that the underlying mechanism was related to DPP4-mediated activation of inflammatory responses and oxidative stress. Thus, they suggested that altered DPP4 activity could be a risk factor for cognitive dysfunction in T2DM. Other studies have also reported a decline in verbal memory, associative learning, reaction time, and verbal recall in healthy pregnant (HP) women[5-7]. However, only a few studies have examined the presence of altered cognitive function in patients with GDM. Therefore, this study aimed to determine whether having GDM for nearly 6 mo during the perinatal period, combined with the stress of anxiety during pregnancy, could cause mild cognitive impairment (MCI), and whether there are subsequent changes in serum DPP4 levels.
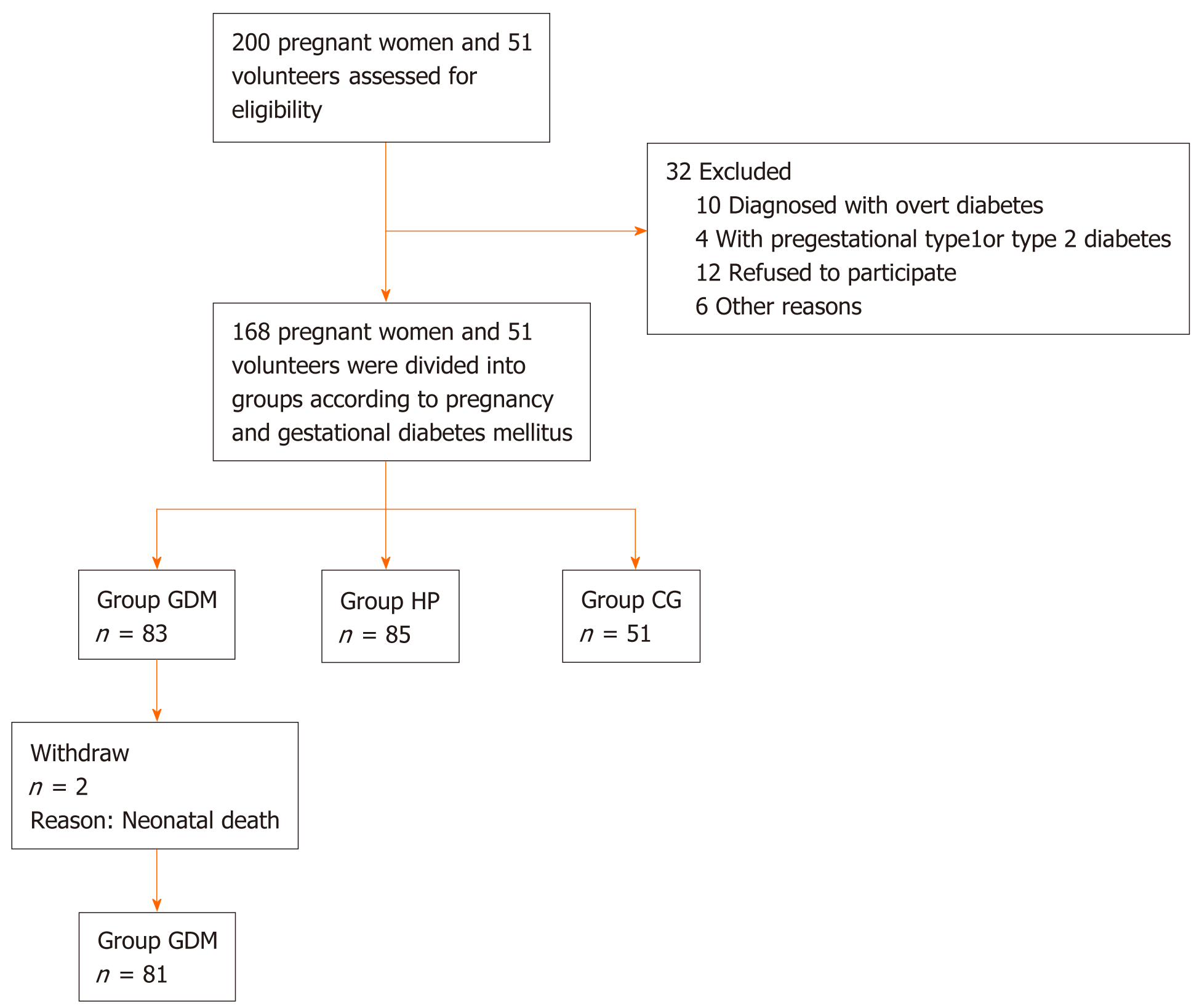
Patients aged 18–35 years, with American Society of Anesthesiologists physical status I/II, were included in this study. A total of 100 consecutive perinatal pregnant women with GDM who were diagnosed, followed up, and treated at the First Affiliated Hospital of Harbin Medical University were included in the study (GDM group). One hundred age-matched perinatal pregnant women without DM comprised the HP group. The healthy control group included 51 nonpregnant female volunteers of similar age with normal blood glucose levels. All patients and volunteers read and signed informed consent forms before enrolment in the study. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University, and was registered with the Chinese Clinical Trial Register (registration number: ChiCTR2000038703).
GDM was diagnosed with at least one abnormal result during the oral glucose tolerance test: plasma glucose during fasting, ≥ 92 mg/dL (5.1 mmol/L); at 1 h, ≥ 180 mg/dL (10.0 mmol/L); or at 2 h, ≥ 153 mg/dL (8.5 mmol/L). Women with fasting plasma glucose ≥ 126 mg/dL (7.0 mmol/L), glycated hemoglobin (HbA1c) ≥ 6.5%, or random plasma glucose ≥ 200 mg/dL (11.1 mmol/L) were diagnosed with overt diabetes and excluded. Subjects on medications that affect cognitive function, including corticosteroids, antidepressants, and antiepileptics, were also excluded. In addition, all patients with other chronic diseases were excluded[8]. The Hamilton Depression Rating Scale was used to assess the psychological status of pregnant women; those with a score > 7, who might have depression, were excluded[9].
On the survey date, all enrolled patients underwent routine medical history inquiries, physical examinations, and laboratory measurements. Clinical research coordinators used a standard questionnaire to collect information on demographic characteristics and medical history. All pregnant women were instructed to maintain their usual physical activity and diet for at least 3 d before the survey. After an overnight fast of ≥ 10 h, venous blood samples were collected to measure blood lipid, HbA1c and glucose levels. For each participant, a 3-mL blood sample was collected and centrifuged, and the serum was collected. Blood samples were stored at -80 ℃, and DPP4, interleukin-6 (IL-6), 8-iso-prostaglandinF2α (8-iso-PGF2α), and brain-derived neurotrophic factor (BDNF) were detected using ELISA (Mlbio, Shanghai, China). All measurements were performed within 6 mo of sample collection.
Umbilical cord blood was collected at the time of delivery from participants in the GDM and HP groups. Serum DPP4 level was measured using ELISA in these groups as well.
To assess cognitive function, the Montreal Cognitive Assessment (MoCA), which is a brief cognitive screen used in a variety of clinical settings to screen for MCI, was administered[10]. The cognitive assessment was conducted in a quiet room, without distractions, by a physical therapist trained in the administration of the MoCA. The maximum score for the MoCA is 30, and the assessment evaluates visuos
The data were analyzed using SPSS 19.0. All data were tested for normality and homogeneity of variance. Normally distributed data were expressed as mean ± SD. Normally distributed continuous variables were compared using Student’s t test, while those with non-normal distributions were compared using the Mann–Whitney U test; multiple comparisons between groups were performed using the least significant difference method. Categorical data were presented as numbers and percentages, and comparisons between groups were performed using the two-sided χ2 test. Correlations of DPP4 and BDNF levels with those of IL-6 and 8-iso-PGF2α, respectively, were performed using Pearson’s correlation coefficient, and P < 0.05 was considered statistically significant.
A total of 166 pregnant women and 51 nonpregnant healthy volunteers were enrolled in this study; they included 83 pregnant women with GDM in the GDM group, 85 HP women in the HP group, and 51 nonpregnant healthy volunteers in the control group (Figure 1). The weight, blood glucose level, and HbA1c level of the participants in the GDM group were significantly higher than those in the other two groups (P < 0.05) (Table 1).
| GDM | HP | CG | F | P value | |
| Sample | 81 | 85 | 51 | ||
| Age (yr) | 29.40 ± 4.06 | 29.86 ± 4.39 | 29.63 ± 4.33 | 0.24 | 0.79 |
| Height (cm) | 163.86 ± 4.79 | 164.11 ± 5.78 | 164.14 ± 5.05 | 0.06 | 0.94 |
| Weight (kg) | 78.96 ± 11.58 | 74.35 ± 9.57 | 58.60 ± 7.42 | 68.32 | < 0.001 |
| Glu | 4.90 ± 1.32 | 3.99 ± 0.71 | 4.83 ± 0.54 | 22.05 | < 0.001 |
| HBA1c (%) | 5.81 ± 0.61 | 4.73 ± 0.93 | 5.32 | < 0.001 | |
| Education, n (%) | 6.95 | 0.14 | |||
| Primary school | 8 (9.9) | 2 (2.4) | 1 (2.0) | ||
| High school | 22 (27.2) | 20 (23.5) | 14 (27.5) | ||
| University | 51 (63.0) | 63 (74.1) | 36 (70.6) |
The MoCA scores in the GDM and HP groups were significantly different from those in the control group in terms of visuospatial/executive function and attention (P < 0.05); however, the scores were not different between the GDM and HP groups (P > 0.05). In terms of language, the GDM group had significantly different scores from those in the other two groups (P < 0.05). In terms of memory, a significant difference was found between the HP and control groups (P < 0.05), as well as between the GDM and HP groups (P < 0.05) (Table 2).
| GDM | HP | CG | P value (GDM vs HP) | P value (GDM vs CG) | P value (HP vs CG) | |
| Sample | 81 | 85 | 51 | |||
| Visuospatial/executive | 4.52 ± 0.84 | 4.65 ± 0.63 | 4.94 ± 0.31 | 0.21 | 0.01 | 0.01 |
| Naming | 3.00 ± 0.00 | 3.00 ± 0.00 | 3.00 ± 0.00 | / | / | / |
| Attention | 5.53 ± 0.84 | 5.58 ± 0.70 | 5.92 ± 0.27 | 0.64 | 0.001 | 0.01 |
| Language | 2.65 ± 0.53 | 2.83 ± 0.40 | 2.96 ± 0.20 | 0.01 | < 0.001 | 0.08 |
| Abstraction | 1.98 ± 0.16 | 1.98 ± 0.14 | 2.00 ± 0.00 | / | / | / |
| Orientation | 6.00 ± 0.00 | 5.99 ± 0.10 | 6.00 ± 0.00 | / | / | / |
| Delayed recall | 2.80 ± 1.14 | 3.61 ± 1.20 | 4.16 ± 0.92 | < 0.001 | < 0.001 | 0.01 |
| Total | 26.99 ± 1.78 | 28.01 ± 1.80 | 29.00 ± 1.16 | < 0.001 | < 0.001 | < 0.001 |
| MoCA ≤ 25 | 20 (24.7) | 2 (2.4) | 0 | < 0.001 | / | / |
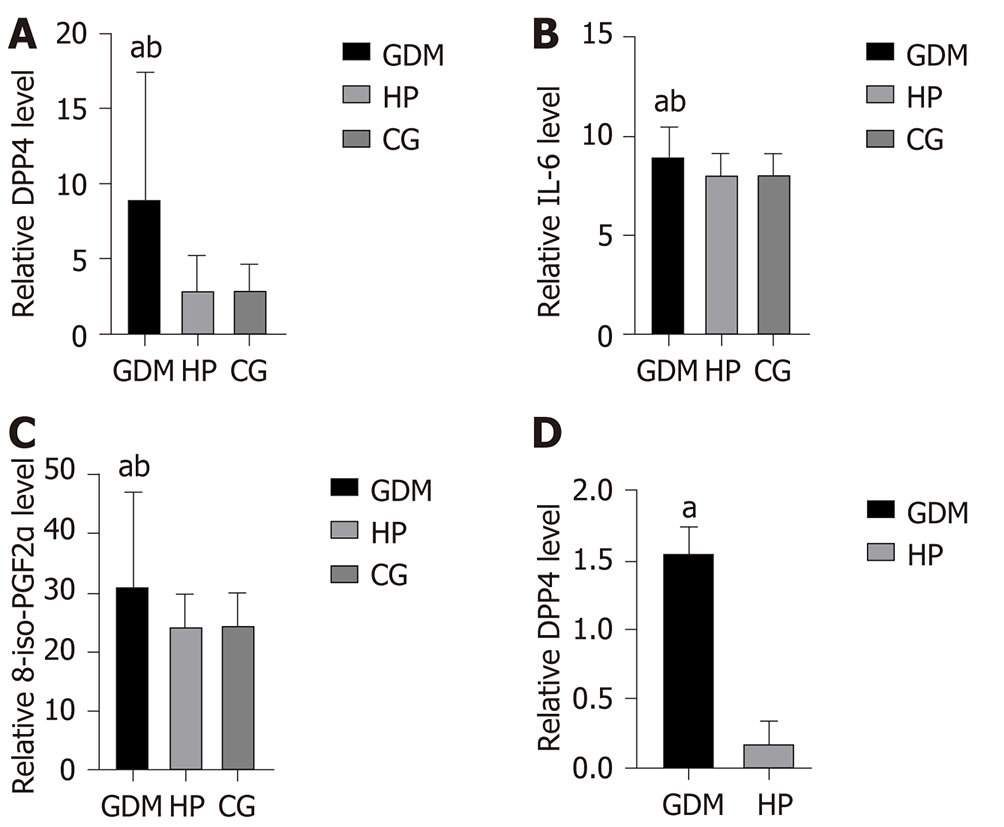
The levels of DPP4, IL-6, and 8-iso-PGF2α in the GDM group were significantly higher than those in the HP and control groups (P < 0.05); however, the differences between these levels in the HP and control groups were not significant (P > 0.05). The level of BDNF in the GDM group was significantly lower than that in the HP and control groups (P < 0.05), although the difference in this level between the HP and control groups was not significant (P > 0.05) (Figure 2 and Table 3). In the GDM group, the elevated DPP4 level significantly correlated with the elevated levels of IL-6 and 8-iso-PGF2α (P < 0.05). Moreover, the elevated IL-6 and 8-iso-PGF2α levels significantly correlated with decreased BDNF levels (P < 0.05) (Table 4). Cord blood DPP4 level in the GDM group was significantly higher than that in the HP group (P < 0.05) (Figure 2 and Table 3).
| DPP4 | DPP4 (UCB) | BDNF | IL-6 | 8-iso-PGF2α | |
| GDM (n = 81) | 8.94 ± 8.50 | 1.56 ± 0.18 | 29.72 ± 7.14 | 9.02 ± 1.50 | 31.24 ± 15.78 |
| HP (n = 85) | 2.87 ± 2.31 | 0.18 ± 0.16 | 51.72 ± 15.82 | 8.10 ± 1.08 | 24.81 ± 4.90 |
| CG (n = 51) | 2.85 ± 1.76 | / | 43.53 ± 8.93 | 8.11 ± 1.02 | 24.88 ± 5.07 |
| P value (GDM vs HP) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| P value (GDM vs CG) | < 0.001 | / | < 0.001 | < 0.001 | < 0.001 |
| P value (HP vs CG) | 0.98 | / | 0.18 | 0.96 | 0.97 |
| DPP4 | BDNF | |||
| Cor | P value | Cor | P value | |
| IL-6 | 0.617 | 0.006 | -0.749 | 0.001 |
| 8-iso-PGF2α | 0.547 | 0.019 | -0.349 | 0.029 |
Although the viewpoint that pregnant women experience deficits in memory is widespread, evidence supporting this supposition is limited in the literature, especially in humans[13]. Based on the results of this study, the following conclusions can be drawn. (1) Perinatal pregnant women with GDM experience cognitive decline, which mainly involves memory loss; (2) Elevated serum DPP4 level is involved in the cognitive decline of perinatal pregnant women with GDM, and they are significantly correlated. Possible mechanisms underlying these findings may include the activation of inflammatory mediators and oxidative stress pathways due to elevated DPP4 level; and (3) DPP4 levels are significantly higher in the cord blood of women with GDM than those without GDM.
Our demographic results showed that pregnant women with GDM had significantly different body weight as well as blood glucose and HbA1c levels than those in the HP group. Although the blood glucose levels in the GDM group were maintained within the normal range, most patients had blood glucose or HbA1c levels near the upper limit, thus indicating the persistence of insulin resistance and metabolic disorders. Some studies suggest that women with GDM have a 20%–70% chance of developing T2DM within 10 years of delivery[14]. The pathogenesis of GDM is complex, involving beta-cell dysfunction, abnormal neuroendocrine function, and abnormal lipid metabolism[15]. Current treatments for GDM are limited to dietary interventions and insulin use, which often has unsatisfactory therapeutic efficacy due to the presence of insulin resistance. Therefore, an in-depth analysis on the etiology, mechanisms, and treatment strategies of GDM is of crucial significance for reducing complications during pregnancy and neonatal morbidity.
In this study, the MoCA scale, which is the most sensitive instrument for detecting cognitive decline, was used to assess cognitive function in perinatal pregnant women with GDM. Our results showed that the GDM and HP groups were significantly different from the control group in terms of executive function and attention, but there was no difference between the GDM and HP groups for these parameters. In addition, there was a significant difference in language scores between the GDM group and the other two groups. It is worth noting that in terms of memory, there was a significant difference between the HP and control groups, as well as between the GDM and HP groups. This indicates that perinatal pregnant women with GDM experience a significant decline in memory. Since the memory task is worth 5 points in the MoCA, and accounts for the largest proportion of scores among all abilities, it may have contributed substantially to the differences in the total score between the groups. However, all groups were within the normal range. Thus, the results of the MoCA scale support our previous speculation that although GDM patients do not reach the threshold for MCI, they still experience some degree of cognitive decline.
The effect of childbirth on women’s cognitive ability is an important issue because it might affect the job opportunities of working women of childbearing age. As such, we discuss the influence of pregnancy on women’s cognitive function with caution. The average score of HP women was lower than that of healthy nonpregnant volunteer women of similar age with respect to the MoCA score. However, the degree of cognitive decline is smaller than that in pregnant women with GDM. In the late stages of pregnancy, most women are no longer in a working environment, and the brain moves into a state of excessive relaxation in terms of cognition, which might be one of the reasons for the mild cognitive decline in pregnant women[16,17]. The mild stress, anxiety, and depression surrounding childbirth during pregnancy could also affect the cognitive function of pregnant women to a certain extent[18,19]. However, most of these negative emotions during pregnancy would resolve following childbirth. Conversely, the levels of DPP4 and BDNF were closer to those of healthy women; therefore, we believe that the decline of cognitive function in pregnant women is minimal.
Our focus in this study was on memory loss in perinatal pregnant women with GDM, which was pronounced when compared with that in the HP group. This may be a transient phenomenon, and its long-term effects are still unclear; however, its mechanisms might be similar to that of cognitive impairment in patients with T2DM. Several recent studies have shown that DPP4 is strongly associated with cognitive impairment in T2DM patients[4], which prompted us to examine DPP4 levels. DPP4 (also known as CD26) is an exopeptidase that cleaves peptides after the second position from the N terminus (NH2-Xaa-Pro)[20]. DPP4 is a widely expressed mul
Systemic inflammation has been suggested to play an important pathogenic role in the late severe stages of cognitive decline, and inflammatory markers, such as IL-6, were only found to be elevated in patients with dementia but not in those with cognitive decline[31,32]. We propose that this discrepancy could be due in part to differences in the methods of detecting inflammatory cytokines and the diagnosis of cognitive dysfunction. The majority of the studies support the possibility that systemic inflammation could be a pathophysiological cascade response to cognitive decline, and do not explicitly exclude low-grade inflammatory diseases, such as T2DM, car
Besides systemic inflammation, oxidative stress has also been shown to cause cognitive decline. 8-iso-PGF2α is a biologically active prostaglandin-like substance and a specific product resulting from the oxygen free radical peroxidation of cellular membranes. This indicator can accurately reflect lipid peroxidation in patients with hypoxia–ischemia–reperfusion[33]. The production of 8-iso-PGF2α is not dependent on cyclooxygenase (COX), but on the damage caused by oxygen free radicals to polyunsaturated fatty acids (arachidonic acid) in lipid cell membranes. Thus, its expression levels in vivo are not affected by the use of nonsteroidal anti-inflammatory drugs, such as aspirin, which can alter COX activity. Therefore, 8-iso-PGF2α is an ideal biochemical indicator for the clinical determination of the degree of free radical oxidation in patients and the efficacy of antioxidant therapy. Furthermore, studies have shown that increased peripheral blood 8-iso-PGF2α levels in pregnant women positively correlate with disease severity[34]. One study confirmed that DPP4 increases the production of reactive oxygen species in endothelial cells in a dose-dependent manner[35]. Our data also suggest a positive correlation between 8-iso-PGF2α and DPP4 activity.
We also examined the serum levels of BDNF, which is a plasma marker associated with cognitive function. BDNF downregulation could lead to synaptic loss and neurodegeneration[36]. Our results indicate that patients in the GDM group had significantly lower expression levels. This suggests that patients in the GDM group not only experienced cognitive decline, but also showed changes in plasma marker levels, which is the greatest cause of our concern.
In this study, we found that DPP4, IL-6, and 8-iso-PGF2α levels were significantly higher and BDNF level was lower in pregnant women with GDM, and that these biomarkers were mutually correlated to a certain extent. Zheng et al[4] found that DPP4, IL-6 and 8-iso-PGF2α levels were elevated in patients with T2DM and associated with their cognitive dysfunction. Furthermore, the increased DPP4 levels correlated with increased IL-6 and 8-iso-PGF2α levels. In this study, we found that elevated serum DPP4 levels in perinatal pregnant women with GDM positively correlated with elevated serum IL-6 and 8-iso-PGF2α levels, while the latter was also correlated with decreased BDNF levels. Therefore, we speculate that elevated DPP4 levels might exacerbate the inflammatory response and oxidative stress in pregnant women with GDM. These inflammatory and oxidative stress factors can cross the blood–brain barrier and act on the nervous system to reduce BDNF levels, which will affect hippocampal function, thereby exacerbating memory loss. DPP4 might not only be involved in the process of cognitive dysfunction in pregnant women with GDM, but also in insulin resistance, lipid oxidative stress, and other pathophysiological processes. It may also serve as a potential target for GDM treatment, which is the next step in our research.
The limitations of this study were as follows: (1) the sample size was small; and (2) we observed the cognitive status of pregnant women with GDM and discussed the pathophysiological mechanisms associated with DPP4 from the perspective of inflammatory factors and oxidative stress. However, complex mechanisms may be involved in memory loss in pregnant women with GDM, not limited to the effects of DPP4 alone. Therefore, further elucidation of the underlying mechanisms is needed.
This study also found significantly elevated levels of DPP4 in the cord blood, suggesting that DPP4 could affect fetal development through the placental barrier. However, we did not examine the effect of this change on the fetus.
We believe that in addition to strict dietary control and glycemic control, it is also important to stabilize the moods of perinatal pregnant women with GDM to reduce inflammatory and stress responses. In particular, we should pay attention to methods of controlling intraoperative patient stress during anesthesia for painless natural delivery or caesarean delivery. This is not only crucial for the mother but also for the fetus.
Cognitive dysfunction in perinatal pregnant women with GDM mainly manifested as memory loss, which might be associated with elevated DPP4 levels.
Studies have confirmed that type 2 diabetes mellitus (DM) can cause cognitive impairment. The mechanism is not clear. Dipeptidyl peptidase-4 (DPP4) may be involved in this process.
The main problem in this study is whether cognitive impairment exists in pregnant women with gestational DM (GDM) and whether it is related to DPP4. This has a great impact on the physical and mental health of pregnant women with perinatal GDM and the health of the fetus.
Objective to study the cognitive function of pregnant women with GDM, and to find out whether the pathway is related to DPP4.
They were divided into three groups: GDM group, healthy pregnant group and control group. Women in the three groups were scored with Montreal Cognitive Assessment. Venous blood was collected from women in each group, serum was separated, and serum indexes such as DPP4, interleukin-6, and 8-iso-prostaglandin
Compared with the other two groups, the GDM group had cognitive impairment, especially memory impairment. DPP4 may induce the change of BDNF by promoting oxidative stress and inflammatory response.
GDM can lead to cognitive dysfunction in pregnant women, mainly manifested as memory loss. DPP4 may be involved in this process.
Perinatal cognitive decline is worth our attention, especially in GDM pregnant women. How to prevent and treat is the key. Whether DPP4 can be used as a therapeutic target needs further study.
Thanks for the support from the Department of Anesthesiology, the First Affiliated Hospital of Harbin Medical University. Thanks to the statistics teacher of Harbin Medical University for his guidance and statistics. Thanks for the help of the Department of Obstetrics and neurology, the First Affiliated Hospital of Harbin Medical University.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dąbrowski M S-Editor: Fan JR L-Editor: Kerr C P-Editor: Zhang YL
| 1. | Carpita B, Muti D, Dell'Osso L. Oxidative Stress, Maternal Diabetes, and Autism Spectrum Disorders. Oxid Med Cell Longev. 2018;2018:3717215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The Pathophysiology of Gestational Diabetes Mellitus. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 817] [Cited by in RCA: 1006] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 3. | Umegaki H, Hayashi T, Nomura H, Yanagawa M, Nonogaki Z, Nakshima H, Kuzuya M. Cognitive dysfunction: an emerging concept of a new diabetic complication in the elderly. Geriatr Gerontol Int. 2013;13:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Zheng T, Qin L, Chen B, Hu X, Zhang X, Liu Y, Liu H, Qin S, Li G, Li Q. Association of Plasma DPP4 Activity With Mild Cognitive Impairment in Elderly Patients With Type 2 Diabetes: Results From the GDMD Study in China. Diabetes Care. 2016;39:1594-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Glynn LM. Giving birth to a new brain: hormone exposures of pregnancy influence human memory. Psychoneuroendocrinology. 2010;35:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Janes C, Casey P, Huntsdale C, Angus G. Memory in pregnancy. I: Subjective experiences and objective assessment of implicit, explicit and working memory in primigravid and primiparous women. J Psychosom Obstet Gynaecol. 1999;20:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Casey P, Huntsdale C, Angus G, Janes C. Memory in pregnancy. II: Implicit, incidental, explicit, semantic, short-term, working and prospective memory in primigravid, multigravid and postpartum women. J Psychosom Obstet Gynaecol. 1999;20:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 8. | Keskin FE, Ozyazar M, Pala AS, Elmali AD, Yilmaz B, Uygunoglu U, Bozluolcay M, Tuten A, Bingöl A, Hatipoglu E. Evaluation of cognitive functions in gestational diabetes mellitus. Exp Clin Endocrinol Diabetes. 2015;123:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord. 2013;150:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 797] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 10. | O'Driscoll C, Shaikh M. Cross-Cultural Applicability of the Montreal Cognitive Assessment (MoCA): A Systematic Review. J Alzheimers Dis. 2017;58:789-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 11. | King LA, Peterson DS, Mancini M, Carlson-Kuhta P, Fling BW, Smulders K, Nutt JG, Dale M, Carter J, Winters-Stone KM, Horak FB. Do cognitive measures and brain circuitry predict outcomes of exercise in Parkinson Disease: a randomized clinical trial. BMC Neurol. 2015;15:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Silva-Batista C, Corcos DM, Kanegusuku H, Piemonte MEP, Gobbi LTB, de Lima-Pardini AC, de Mello MT, Forjaz CLM, Ugrinowitsch C. Balance and fear of falling in subjects with Parkinson's disease is improved after exercises with motor complexity. Gait Posture. 2018;61:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Brown E, Schaffir J. "Pregnancy Brain": A Review of Cognitive Changes in Pregnancy and Postpartum. Obstet Gynecol Surv. 2019;74:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62:905-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 578] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 15. | Radzicka S, Pietryga M, Iciek R, Brązert J. The role of visfatin in pathogenesis of gestational diabetes (GDM). Ginekol Pol. 2018;89:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 16. | Al-Thaqib A, Al-Sultan F, Al-Zahrani A, Al-Kahtani F, Al-Regaiey K, Iqbal M, Bashir S. Brain Training Games Enhance Cognitive Function in Healthy Subjects. Med Sci Monit Basic Res. 2018;24:63-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Nouchi R, Taki Y, Takeuchi H, Hashizume H, Nozawa T, Kambara T, Sekiguchi A, Miyauchi CM, Kotozaki Y, Nouchi H, Kawashima R. Brain training game boosts executive functions, working memory and processing speed in the young adults: a randomized controlled trial. PLoS One. 2013;8:e55518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Gawali NB, Bulani VD, Gursahani MS, Deshpande PS, Kothavade PS, Juvekar AR. Agmatine attenuates chronic unpredictable mild stress-induced anxiety, depression-like behaviours and cognitive impairment by modulating nitrergic signalling pathway. Brain Res. 2017;1663:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Glover V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Pract Res Clin Obstet Gynaecol. 2014;28:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 496] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 20. | De Meester I, Korom S, Van Damme J, Scharpé S. CD26, let it cut or cut it down. Immunol Today. 1999;20:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 352] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Matteucci E, Giampietro O. Dipeptidyl peptidase-4 (CD26): knowing the function before inhibiting the enzyme. Curr Med Chem. 2009;16:2943-2951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008;29:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 23. | Zheng T, Gao Y, Baskota A, Chen T, Ran X, Tian H. Increased plasma DPP4 activity is predictive of prediabetes and type 2 diabetes onset in Chinese over a four-year period: result from the China National Diabetes and Metabolic Disorders Study. J Clin Endocrinol Metab. 2014;99:E2330-E2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, Eckardt K, Kaufman JM, Ryden M, Müller S, Hanisch FG, Ruige J, Arner P, Sell H, Eckel J. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917-1925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 460] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 25. | Kosaraju J, Holsinger RMD, Guo L, Tam KY. Linagliptin, a Dipeptidyl Peptidase-4 Inhibitor, Mitigates Cognitive Deficits and Pathology in the 3xTg-AD Mouse Model of Alzheimer's Disease. Mol Neurobiol. 2017;54:6074-6084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Chen B, Zheng T, Qin L, Hu X, Zhang X, Liu Y, Liu H, Qin S, Li G, Li Q. Strong Association between Plasma Dipeptidyl Peptidase-4 Activity and Impaired Cognitive Function in Elderly Population with Normal Glucose Tolerance. Front Aging Neurosci. 2017;9:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Zheng T, Baskota A, Gao Y, Chen T, Tian H, Yang F. Increased plasma DPP4 activities predict new-onset hyperglycemia in Chinese over a four-year period: possible associations with inflammation. Metabolism. 2015;64:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Swomley AM, Butterfield DA. Oxidative stress in Alzheimer disease and mild cognitive impairment: evidence from human data provided by redox proteomics. Arch Toxicol. 2015;89:1669-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 29. | Gault VA, Lennox R, Flatt PR. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes Obes Metab. 2015;17:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 30. | Angelopoulou E, Piperi C. DPP-4 inhibitors: a promising therapeutic approach against Alzheimer's disease. Ann Transl Med. 2018;6:255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Stephan BC, Hunter S, Harris D, Llewellyn DJ, Siervo M, Matthews FE, Brayne C. The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry. 2012;17:1056-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Saleem M, Herrmann N, Swardfager W, Eisen R, Lanctôt KL. Inflammatory Markers in Mild Cognitive Impairment: A Meta-Analysis. J Alzheimers Dis. 2015;47:669-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Su G, Wang T, Zhang T, Yang HX, Yu SS, Dai WL, Mi SH. Urinary 8-iso-prostaglandin F2α as a risk marker for the vulnerability of culprit plaque in diabetic patients with stable coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 2019;140:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Turpin CA, Sakyi SA, Owiredu WK, Ephraim RK, Anto EO. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth. 2015;15:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | Ishibashi Y, Matsui T, Maeda S, Higashimoto Y, Yamagishi S. Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cardiovasc Diabetol. 2013;12:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 36. | Rosa E, Fahnestock M. CREB expression mediates amyloid β-induced basal BDNF downregulation. Neurobiol Aging. 2015;36:2406-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |