Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9970
Peer-review started: June 16, 2021
First decision: July 26, 2021
Revised: July 26, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: November 16, 2021
Processing time: 146 Days and 13.2 Hours
Resistant hypertension (RH) has always been a difficult problem in clinical diagnosis and treatment. At present, there is no recognized safe and effective minimally invasive treatment.
An 80-year-old woman was admitted to hospital due to trigeminal neuralgia (TN). The patient had a history of RH for more than 10 years and her blood pressure (BP) was not well-controlled. Before the treatment for TN, we decided to perform chemical renal sympathetic denervation with ethanol in the Pain Department of our hospital. One year after the operation, she stopped taking antihypertensive drugs, and her BP was satisfactorily controlled within 4 years after surgery.
Computed tomography-guided chemical renal sympathetic modulation may be a feasible method for the treatment of RH.
Core Tip: We report the use of computed tomography-guided renal sympathetic nerve modulation, for the first time, in the treatment of resistant hypertension in a patient with trigeminal neuralgia.
- Citation: Luo G, Zhu JJ, Yao M, Xie KY. Computed tomography-guided chemical renal sympathetic nerve modulation in the treatment of resistant hypertension: A case report. World J Clin Cases 2021; 9(32): 9970-9976
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9970.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9970
Hypertension is a common cardiovascular disease. Resistant hypertension (RH) is defined as failure to achieve target blood pressure (BP) when a patient adheres to the maximum tolerated doses of three antihypertensive drugs, including a diuretic[1]. A linear correlation has been established between BP and the risk of cardiovascular events[2]. Although the prevalence of RH has plateaued and decreased in recent years, RH is still common in the hypertensive population[3], and hence, treatment of RH is urgently required. Based on the continuous development of sympathetic modulation, we propose, for the first time, the use of computed tomography (CT)-guided chemical renal sympathetic nerve modulation in the treatment of RH. This provides a novel technique for renal sympathetic denervation (RSD) in the treatment of RH.
We report an 80-year-old female patient with paroxysmal pain in the right maxillary region for > 20 years, which was aggravated for six months.
Twenty years ago, without obvious inducement, the patient developed right facial pain, which was located in the maxillary region. The pain was paroxysmal, which could be induced when brushing her teeth and eating. During the past six months, the pain was aggravated and seriously affected her quality of life.
The patient had a history of RH for > 10 years, with the highest BP of 200/120 mmHg. We found that she did not take her medication according to the instructions and only took amlodipine besylate 5 mg/d, and the efficiency of this treatment was unsatisfactory. She also had a history of diabetes for more than 10 years.
No relevant personal or family history.
Physical examination was normal.
Laboratory examinations were normal.
Imaging examinations were normal.
The final diagnosis was trigeminal neuralgia (the second branch), RH, diabetes.
She was scheduled to undergo radiofrequency thermocoagulation of the trigeminal nerve. The BP of the right upper limb was measured for the first time as 210/108 mmHg. Hence, 10 mg nitroglycerin (0.2 mg/mL) was injected intravenously at a speed of 5 mL/h. BP was continuously monitored until and after it was stable.
After consultation with the cardiologists, we added valsartan hydrochlorothiazide (each containing valsartan 80 mg, hydrochlorothiazide 12.5 mg) and amlodipine besylate 10 mg/d. The mean blood pressure (MBP) within 24 h was 172/73 mmHg, and from 6 a.m.–10 p.m. was 176/77 mmHg, and from 10 p.m.–6 a.m. was 168/70 mmHg. In order to ensure the safety of the operation, we used RSD in the treatment of RH. Although CT-guided percutaneous puncture was easily performed, locating the renal artery under the guidance of CT was the key to success, and then anhydrous ethanol was used to modulate the renal sympathetic nerve in the adventitia of the renal artery to achieve renal denervation.
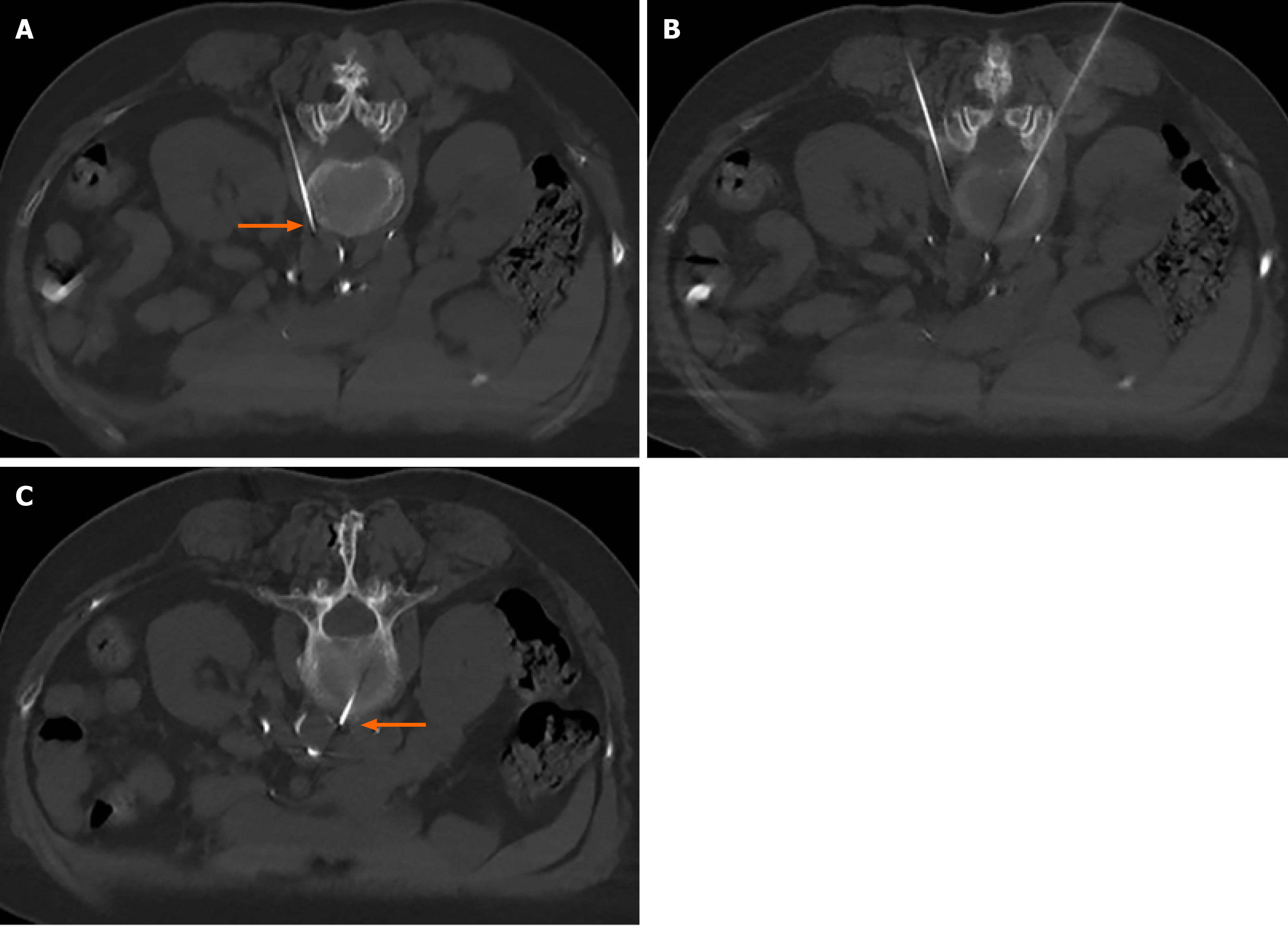
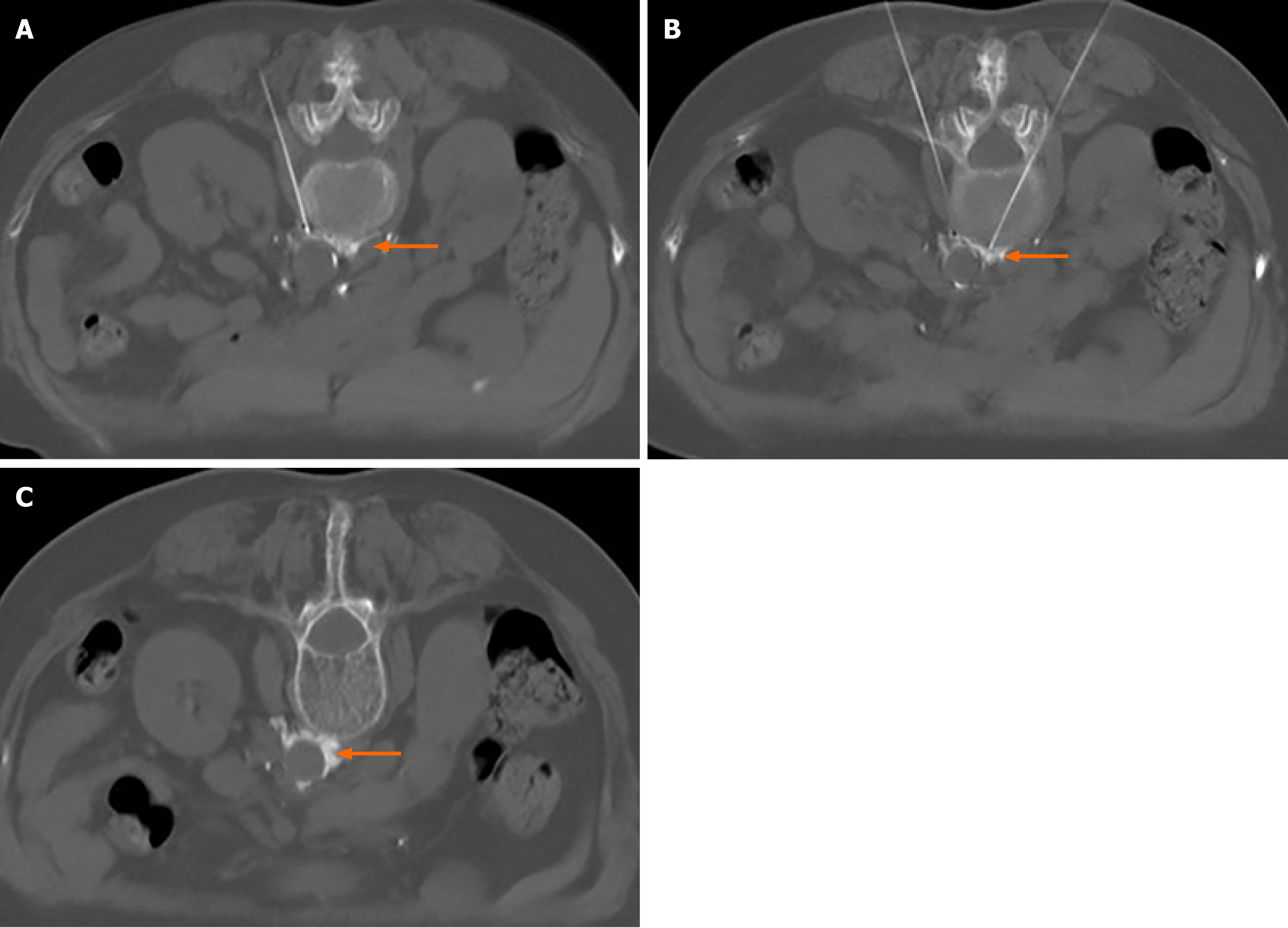
The patient was placed in the prone position and monitored. After a single CT scan, the depth and angle of the puncture needle were measured. When the puncture point was determined, the body surface was marked. After local infiltration anesthesia, the puncture was made according to the proposed path. After several adjustments, the puncture needle was finally close to the renal artery or adjacent to the abdominal aorta (Figure 1). When the return pump was determined to be free of blood, gas, and liquid, a mixture of 30% iohexanol (1 mL) and 1% lidocaine (4 mL) was injected, and the diffusion of anhydrous ethanol was observed after the CT scan confirmed the decrease in lidocaine and iohexanol. The diffusion of anhydrous ethanol was observed after the injection of ethanol (containing 0.9 mL ethanol and 30% iohexanol 0.1 mL) (Figure 2). All vital signs were stable after 20 min, and the patient was sent back to the ward.
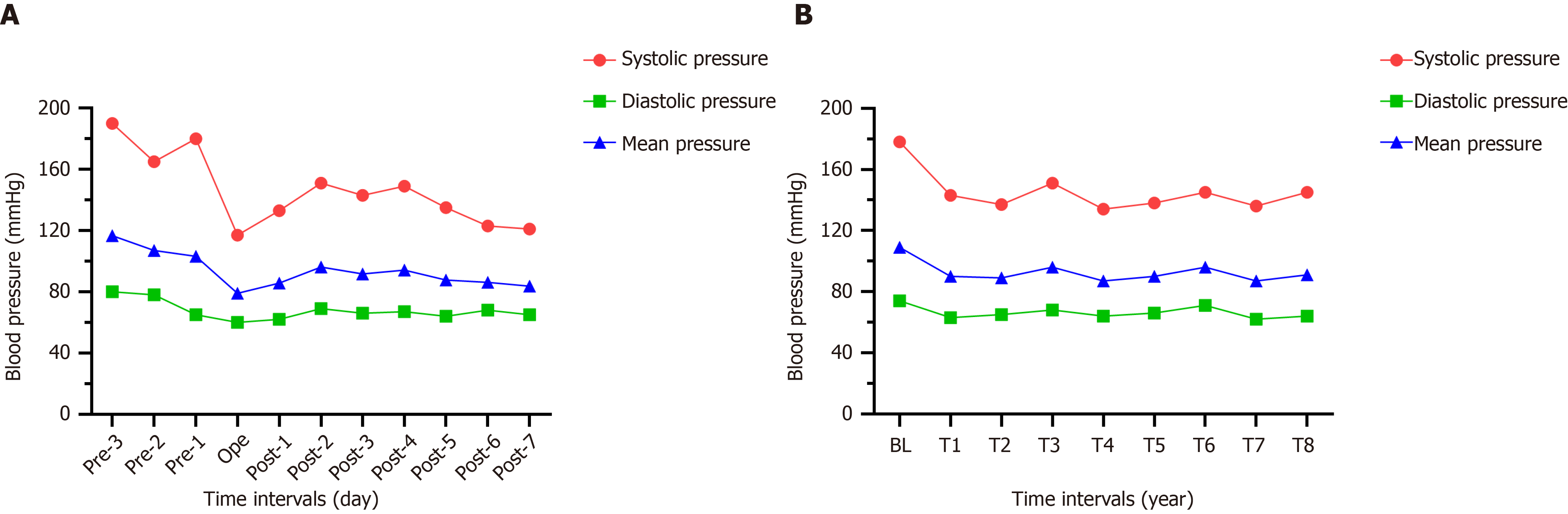
The patient’s BP before and after the operation was recorded for 11 d (Figure 3A). The MBP 3 d before the operation was 109 mmHg, which was defined as the baseline, and the MBP for 8 consecutive days after the operation was approximately 88 mmHg. Therefore, the short-term effect of chemical renal sympathetic modulation could be expected in the treatment of RH. In addition, we followed up this case for 4 years and the use of antihypertensive drugs is listed on Table 1. A telephone follow-up was conducted every 6 mo after discharge. Compared to the baseline, the decrease in systolic blood pressure at each interval within 4 years was -37 mmHg, and that in diastolic blood pressure and MBP was -9 mmHg and -19 mmHg, respectively. Figure 3B shows the changing trends in BP over 4 years.
| Time intervals | Blood pressure (mmHg) | Heart rate (bpm) | Antihypertensive drugs | Complications | ||
| SP | DP | MBP | ||||
| Pre-3 d1 | 178 | 74 | 109 | 87 | Valsartan 80 mg; hydrochlorothiazide; 12.5 mg; amlodipine besylate 10 mg | Headache |
| Operation | 117 | 60 | 79 | 65 | None | None |
| Post-1 d | 133 | 62 | 86 | 71 | Valsartan 80 mg; hydrochlorothiazide; 12.5 mg; amlodipine besylate 10 mg | None |
| Post-2 d | 151 | 69 | 96 | 73 | Valsartan 80 mg; hydrochlorothiazide; 12.5 mg; amlodipine besylate 10 mg | None |
| Post-3 d | 143 | 66 | 92 | 76 | valsartan 80 mg; hydrochlorothiazide; 12.5 mg; amlodipine besylate 10 mg | None |
| Post-1 yr | 137 | 65 | 89 | 69 | Amlodipine besylate 10 mg | None |
| Post-2 yr | 134 | 64 | 87 | 73 | Amlodipine besylate 10 mg | None |
| Post-3 yr | 145 | 71 | 96 | 77 | Amlodipine besylate 10 mg | None |
| Post-4 yr | 145 | 64 | 91 | 74 | None | None |
The sympathetic nervous system plays a major role in the regulation of BP and the formation of hypertension[4-6], which in turn, are regulated by changing cardiac output, vascular resistance, and the renin-angiotensin-aldosterone system. Activation of the renal sympathetic nerve significantly increases the absorption of sodium, renal vascular resistance and promotes the release of renin in the kidney[7,8]. Both animal experiments and clinical studies have proved that renal denervation reduces BP to a certain extent[9-12]. The treatments for RH include ultrasound therapy, neurotoxin injection, and radiofrequency ablation; of these, catheter-based radiofrequency renal-nerve ablation (CBRNA) is a mature and well-studied technique[13]. The catheter is withdrawn 1–2 cm and circumferentially rotated with further radiofrequency energy applications performed in this way, such that 4–6 applications on average are applied to the renal artery[14]. However, the heat produced during radiofrequency ablation might damage the renal artery and cause diffuse vasospasm and thrombosis[15,16], thereby necessitating the postoperative evaluation of renal arteriography. Sufficient evidence is lacking to support the long-term effect, safety, and other related complications of CBRNA.
Fischell et al[17] reported a method for renal denervation which involved injecting anhydrous ethanol into the adventitia. Later, the first human trial was carried out, and its safety and feasibility were proved[18]. However, like CBRNA, these methods were performed using a transcatheter puncture, and renal vascular injury is a common and severe risk in such surgery. In addition, if ethanol is inadvertently injected into a blood vessel, it can cause adverse events, such as embolism.
For several years, we have been exploring the treatment of sympathetically-driven diseases[19-21]. In this case, the application of CT-guided chemical renal sympathetic modulation for RH is reported for the first time. We attempted to transfer the therapeutic target from the lumbar sympathetic chain to the renal artery. During the perioperative period, the decrease in BP on the day of surgery was maximal, and the decrease in MAP was 24 mmHg; the lowest value was 134 mmHg for four years, and the average decrease in BP in the fourth year was -37.5/-11 mmHg.
We speculated that compared to classical transcatheter renal denervation, CT-guided chemical renal sympathetic nerve modulation has several advantages. First, the operation could be completed by percutaneous puncture without the need to insert a catheter, and hence the risks such as blood infection and thrombosis could be avoided. In addition, CBRNA often uses the method of multipoint radiofrequency ablation, but we do not think that the annular area formed by multiple radiofrequencies can cover all the branches of the renal sympathetic nerve around the renal artery, which could be due to the poor effect in some patients after CBRNA. However, due to diffusion, anhydrous ethanol is uniformly distributed around the adventitia of the renal artery after injection of anhydrous ethanol containing contrast agent (Figure 2). In addition, under the guidance of CT, the right puncture needle reaches the target through the erector muscle and intervertebral disc, while the other needle reaches between the lateral side of the abdominal aorta and the anterolateral edge of the lumbar vertebra and near the renal artery. Both sides of the puncture process avoid the kidney and celiac vessels. The incidence of puncture-related complications was low, and chemical modulation was achieved without causing vascular intimal injury. After the operation, we did not perform renal angiography to eliminate the risk of renal artery injury, which has good safety.
The difficulty of this technique lies in the puncture process. CT clarified the correlation between the puncture needle and adjacent important blood vessels or organs. Based on the determination of angle and depth, an ideal puncture path is designed to reduce the times of needle adjustment in the puncture process as much as possible. In addition, vascular damage may cause local hematoma or hemorrhagic shock during the puncture, and anhydrous ethanol should be injected cautiously. Repeated withdrawal should be carried out before injection to ensure that no blood, gas, and other liquids are drawn. Moreover, if, after injection of the mixture of local anesthetic and contrast medium, the contrast medium is not seen on CT scanning, it is necessary to readjust the position of the puncture needle. Also, it is necessary to focus on the possibility that anhydrous ethanol spreads along the interstitial space after injection and can cause adjacent nerve injury[22]. At this time, diluting ethanol with an appropriate amount of saline may be an emergency measure. Embolism caused by the injection of anhydrous ethanol into blood vessels may be the most severe complication. The introduction of CT provides an effective guarantee for the safety of the puncture, and the withdrawal before injection and controlling the amount of anhydrous ethanol are critical measures to reduce the incidence of complications. Overall, simple operation, less trauma, and low surgical risk are the unique advantages of this technique. We have followed up this case for a period of 4 years to provide evidence for the long-term effect of surgical treatment.
The purpose of this case report was to propose a novel, safe, and effective technique to provide a new minimally invasive treatment for the clinical therapy of RH. Thus, we speculate that the expansion of sample size and randomized controlled trials would provide convincing conclusions.
CT-guided chemical renal sympathetic modulation may be a feasible method for the treatment of RH. Randomized controlled trials may provide more reliable conclusions.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Nuclear Science and Technology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Prkacin I S-Editor: Wang JL L-Editor: Webster JR P-Editor: Xing YX
| 1. | Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol. 2008;52:1749-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Doroszko A, Janus A, Szahidewicz-Krupska E, Mazur G, Derkacz A. Resistant Hypertension. Adv Clin Exp Med. 2016;25:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Sinnott SJ, Smeeth L, Williamson E, Douglas IJ. Trends for prevalence and incidence of resistant hypertension: population based cohort study in the UK 1995-2015. BMJ. 2017;358:j3984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Thomas P, Dasgupta I. The role of the kidney and the sympathetic nervous system in hypertension. Pediatr Nephrol. 2015;30:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Komnenov D, Levanovich PE, Rossi NF. Hypertension Associated with Fructose and High Salt: Renal and Sympathetic Mechanisms. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | DeLalio LJ, Sved AF, Stocker SD. Sympathetic Nervous System Contributions to Hypertension: Updates and Therapeutic Relevance. Can J Cardiol. 2020;36:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation and systemic hypertension. Am J Cardiol. 2010;105:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol. 2011;1:731-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 206] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Li P, Huang PP, Yang Y, Liu C, Lu Y, Wang F, Sun W, Kong XQ. Renal sympathetic denervation attenuates hypertension and vascular remodeling in renovascular hypertensive rats. J Appl Physiol (1985). 2017;122:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Oosterhuis NR, Fernandes R, Maicas N, Bae SE, Pombo J, Gremmels H, Poston L, Joles JA, Samuelsson AM. Extravascular renal denervation ameliorates juvenile hypertension and renal damage resulting from experimental hyperleptinemia in rats. J Hypertens. 2017;35:2537-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1591] [Cited by in RCA: 1546] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 12. | Esler M. Renal denervation for hypertension: observations and predictions of a founder. Eur Heart J. 2014;35:1178-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Krum H, Sobotka P, Mahfoud F, Böhm M, Esler M, Schlaich M. Device-based antihypertensive therapy: therapeutic modulation of the autonomic nervous system. Circulation. 2011;123:209-215. [PubMed] [DOI] [Full Text] |
| 14. | Krum H, Schlaich M, Sobotka P. Renal sympathetic nerve ablation for treatment-resistant hypertension. Br J Clin Pharmacol. 2013;76:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Lobo MD, Sobotka PA, Pathak A. Interventional procedures and future drug therapy for hypertension. Eur Heart J. 2017;38:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Templin C, Jaguszewski M, Ghadri JR, Sudano I, Gaehwiler R, Hellermann JP, Schoenenberger-Berzins R, Landmesser U, Erne P, Noll G, Lüscher TF. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedural comparison with the Simplicity catheter system and the EnligHTN multi-electrode renal denervation catheter. Eur Heart J. 2013;34:2141-2148, 2148b. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Fischell TA, Vega F, Raju N, Johnson ET, Kent DJ, Ragland RR, Fischell DR, Almany SL, Ghazarossian VE. Ethanol-mediated perivascular renal sympathetic denervation: preclinical validation of safety and efficacy in a porcine model. EuroIntervention. 2013;9:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Fischell TA, Ebner A, Gallo S, Ikeno F, Minarsch L, Vega F, Haratani N, Ghazarossian VE. Transcatheter Alcohol-Mediated Perivascular Renal Denervation With the Peregrine System: First-in-Human Experience. JACC Cardiovasc Interv. 2016;9:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Guo JG, Fei Y, Huang B, Yao M. CT-guided thoracic sympathetic blockade for palmar hyperhidrosis: Immediate results and postoperative quality of life. J Clin Neurosci. 2016;34:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Liu M, Ni H, Tao J, Xie K. Lumbar Sympathetic Nerve Modulation Using Absolute Ethanol for the Treatment of Primary Lower-Extremity Hyperhidrosis: A Dose-Effect Pilot Study. Med Sci Monit. 2021;27:e928209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Huang B, Sun K, Zhu Z, Zhou C, Wu Y, Zhang F, Yan M. Oximetry-derived perfusion index as an early indicator of CT-guided thoracic sympathetic blockade in palmar hyperhidrosis. Clin Radiol. 2013;68:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Pennekamp W, Krumova EK, Feigl GP, Frombach E, Nicolas V, Schwarzer A, Maier C. Permanent lesion of the lateral femoral cutaneous nerve after low-volume ethanol 96%application on the lumbar sympathetic chain. Pain Physician. 2013;16:391-397. [PubMed] |