Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9960
Peer-review started: June 14, 2021
First decision: July 26, 2021
Revised: August 3, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: November 16, 2021
Processing time: 148 Days and 10.4 Hours
Sleep-disordered breathing, including hypoventilation and obstructive sleep apnea, is often observed in Prader-Willi syndrome (PWS). Particularly in adole
We present two cases of adolescent PWS with typical characteristics, including obesity, mental retardation, and scoliosis. Two boys, aged 12 and 13, diagnosed with PWS, both underwent scoliosis correction surgery. Before and immediately after surgery, arterial blood tests showed no abnormalities and no respiratory symptoms occurred. However, after 6-7 mo, both patients complained of daytime sleepiness, difficulty sleeping at night, dyspnea on exertion, and showed cyanosis. Hypercapnia and hypoxia were confirmed by polysomnography and transcu
Even after scoliosis surgery, “periodic” monitoring of respiratory failure with an “objective” test method is needed for timely respiratory support.
Core Tip: We describe two cases of adolescent Prader-Willi syndrome (PWS) with typical characteristics, such as obesity, mental retardation, and scoliosis, in which respiratory failure with severe desaturation and hypercapnia occurred after scoliosis correction surgery. Respiratory failure was confirmed and diagnosed by polysomno
- Citation: Yoon JY, Park SH, Won YH. Respiratory failure after scoliosis correction surgery in patients with Prader-Willi syndrome: Two case reports. World J Clin Cases 2021; 9(32): 9960-9969
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9960.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9960
Prader-Willi syndrome (PWS) is a genetic disorder arising from chromosome 15q11-q13, which causes hypotonia and delayed development in infancy, mental retardation, obesity, short stature, and behavioral and psychiatric disturbances with further growth[1,2].
Sleep disordered breathing (SDB) is known to be a common cardiopulmonary complication in PSW[3]. The most two representative causative factors are obstructive sleep apnea (OSA) due to airway narrowing caused by obesity and hypotonia[3,4], and hypoventilation due to progressive deterioration of the restrictive pattern of the lungs caused by scoliosis[3,5,6]. Respiratory symptoms appear from infancy, and OSA is known to be a major contributing factor after 2 years of age[7]. Growing up, adolescents with PWS are known to be in a period in which scoliosis progresses rapidly[8], and consequently, a restrictive pattern of the pulmonary system can progress. Therefore, scoliosis correction surgery can be performed to prevent and correct respiratory deterioration[3,8]. However, there has been no accurate report on changes in respiratory function before and after scoliosis surgery, and the prevalence of nocturnal hypoventilation and the rate of progression to cor pulmonale in PWS patients. Therefore, in order to establish criteria for prophylactic scoliosis surgery and to properly monitor respiratory function in PWS patients, reliable respiratory function tests are needed. To date, one of the methods of respiratory function evaluation for pediatric scoliosis, including children with PWS, is known as the patient effort-based test such as forced expiratory volume in the first second (FEV1)[4,9], total lung capacity (TLC)[5] and forced vital capacity (FVC)[6] in several studies. Even if reproducibility and reliability are improved through multiple test trials[10], results of patient effort-based tests such as FEV1, FVC, and TLC have a fundamental limitation that results might vary depending on patient participation[6]. In particular, multiple trials of tests will be clinically difficult due to poor cooperation for children who have neurologic deficits accompanied by mental retardation, such as PWS, and the efforts to participate are likely to be inconsistent by circumstance (parental attendance, evaluation room environment, psychological state, etc.), which would lower the reliability of the tests.
On the other hand, as a patient non-effort-based respiratory tests, end-tidal CO2 monitoring, transcutaneous CO2 monitoring[6,11] or polysomnography (PSG)[6] can be tried, but it is not easy when compared to the patient effort-based pulmonary function test because monitoring is required for several hours during sleep and hospitalization is required. In the case of neuromuscular disease in which respiratory muscle weakness is common, these tests are conventionally performed according to established guidelines[12,13]. Whereas for PWS, since scoliosis does not occur in all patients[3,8], and respiratory muscle weakness known to be not severe[3], there is no established evidence-based guideline for routine respiratory function monitoring, so somewhat burdensome non-effort-based respiratory test is not conventionally performed.
Taken together, there are two things to consider: (1) Whether scoliosis repair surgery is guaranteed to improve the respiratory system of the restrictive pattern of PWS; and (2) Search for reliable and objective measurement method of respiratory function of PWS.
We present cases of adolescent PWS in which hypercapnic respiratory failure with sleep apnea occurred after surgical treatment of scoliosis and treatment involved noninvasive ventilation (NIV). We aimed to highlight the need for clinical guidelines regarding the timely detection of respiratory failure through regular and objective respiratory monitoring in these patients, especially after scoliosis correction surgery.
Case 1: A 12-year-old male patient diagnosed with PWS complained of difficulty sleeping in the supine position, severe snoring, daytime sleepiness, sweating, and intermittent chest tightness at 6 mo after scoliosis correction surgery.
Case 2: A 13-year-old male patient diagnosed with PWS complained of shortness of breath even with light exertion, daytime sleepiness, and nighttime cyanosis and apnea at 7 mo after scoliosis correction surgery.
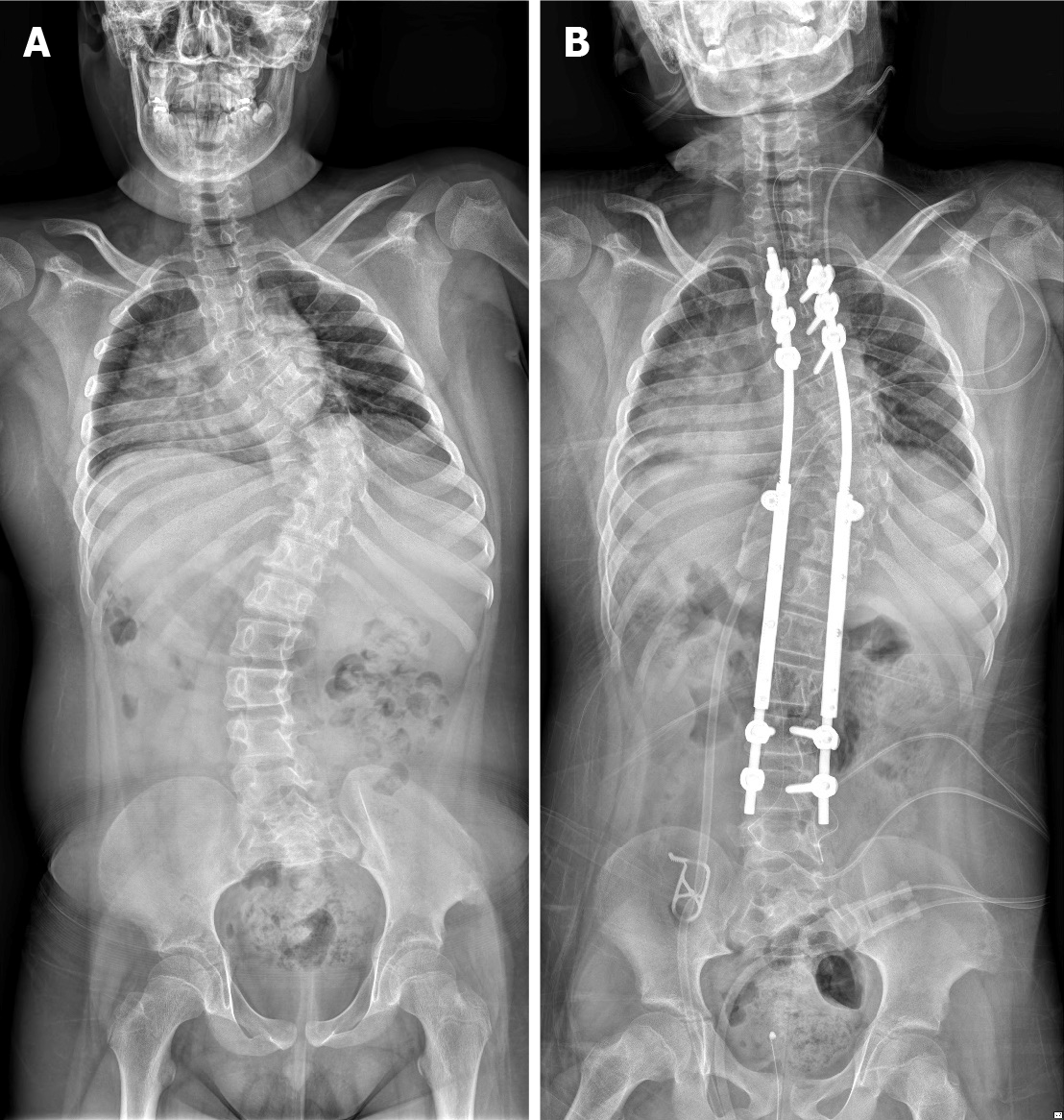
Case 1: The patient underwent surgery for scoliosis (thoracic Cobb’s angle of 66.1°, lumbar Cobb angle of 34.5°) at 12 years of age (Figure 1A, Table 1). Immediately after correction surgery, the patient was transferred to the Department of Rehabilitation Medicine and received treatment for 4 wk. The patient was discharged without side effects or sequelae and followed up as an outpatient. However, 6 mo postoperatively, the patient complained of the above-mentioned respiratory symptoms and was subsequently admitted for pulmonary evaluation at the Department of Rehabilitation Medicine in a tertiary hospital.
| Case 1 | Case 2 | |||
| Birth age (wk) | NSVD, 35 + 2 | NSVD, 41 + 1 | ||
| Weight at birth (g) | 2100 | 3100 | ||
| Age of PWS diagnosis | 5 mon | 2 yr | ||
| Sex | Male | Male | ||
| Growth hormone therapy | Ongoing for 4 yr | Not applied | ||
| Scoliosis surgery | Before OP | Post OP (At respiratory failure) | Before OP | Post OP (At respiratory failure) |
| Scoliosis Cobb’s angle (°) | 66.1 | 54 | 82.6 | 71.8 |
| Height (cm) | 134 | 137 | 141 | 143 |
| Weight (kg) | 53.5 | 57 | 79.5 | 87 |
| BMI (kg/m2) | 29.8 | 30.4 | 40.0 | 42.5 |
| Age (yr) | 12 | 12 | 13 | 13 |
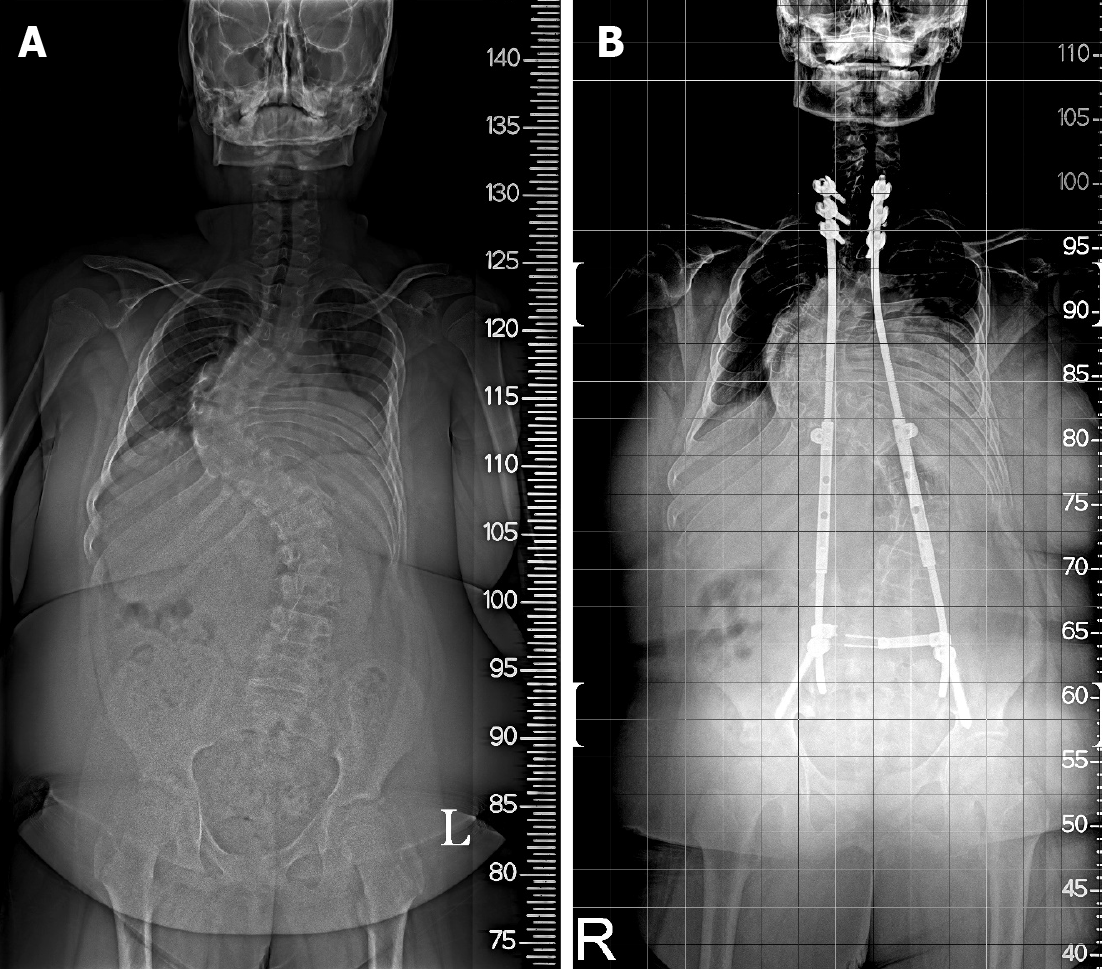
Case 2: The patient underwent surgery for scoliosis (thoracic Cobb’s angle of 82.6°, lumbar Cobb’s angle of 54.9°) at 13 years of age (Figure 2A, Table 1). Immediately after correction surgery, there were no prominent respiratory symptoms, and the patient was discharged without any side effects or sequelae and followed up as an outpatient. Seven months postoperatively, the patient complained of the above-mentioned respiratory symptoms and was subsequently admitted for pulmonary evaluation at the Department of Rehabilitation Medicine in a tertiary hospital.
Case 1: The patient underwent surgery for scoliosis (thoracic Cobb’s angle of 66.1°, lumbar Cobb angle of 34.5°) at 12 years of age (Figure 1A, Table 1). Immediately after correction surgery, the patient was transferred to the Department of Rehabilitation Medicine and received treatment for 4 wk. The patient was discharged without side effects or sequelae and followed up as an outpatient. However, 6 mo postoperatively, the patient complained of the above-mentioned respiratory symptoms and was subsequently admitted for pulmonary evaluation at the Department of Rehabilitation Medicine in a tertiary hospital.
Case 2: The patient underwent surgery for scoliosis (thoracic Cobb’s angle of 82.6°, lumbar Cobb’s angle of 54.9°) at 13 years of age (Figure 2A, Table 1). Immediately after correction surgery, there were no prominent respiratory symptoms, and the patient was discharged without any side effects or sequelae and followed up as an outpatient. Seven months postoperatively, the patient complained of the above-mentioned respiratory symptoms and was subsequently admitted for pulmonary evaluation at the Department of Rehabilitation Medicine in a tertiary hospital.
Case 1: The patient had no remarkable family history.
Case 2: The patient had no remarkable family history.
Case 1: In terms of cognitive function, the intelligence test could not be performed due to the patient's inability to comprehend.
Regarding respiratory function, the pulmonary function test showed a FVC of 0.96 L (48% of predicted) performed before scoliosis correction surgery. At the time of symptom onset (6 mo after surgery), the FVC was 0.68 L (31% of predicted) (Table 2).
| Case 1 | Case 2 | |||||||
| Post OP | Before NIV | After NIV | F/U (5 mo) | Post OP | Before NIV | After NIV | F/U (12 mo) | |
| ABG test1 | ||||||||
| pH | 7.55 | 7.3474 | 7.38 | 7.403 | 7.393 | 7.3754 | 7.3724 | 7.384 |
| PaO2 (mmHg) | 87 | 54.24 | 106.9 | 83.3 | 63.4 | 37.24 | 99.04 | 65.5 |
| PaCO2 (mmHg) | 42 | 55.24 | 47.7 | 36.0 | 56.3 | 53.44 | 48.94 | 48.9 |
| SaO2 (%) | 98 | 85.24 | 98.0 | 96.3 | 91.1 | 68.14 | 97.44 | 92.8 |
| Transcutaneous monitoring | ||||||||
| SaO2 min (%) | NT | 504 | 904 | 86 | NT | NT | 744 | 863 |
| SaO2 mean (%) | NT | 894 | 984 | 96 | NT | NT | 964 | 983 |
| TcCO2 mean (mmHg) | NT | 62.24 | 52.24 | 40.5 | NT | NT | 42.94 | 41.73 |
| TcCO2 max (mmHg) | NT | 71.64 | 61.14 | 48.1 | NT | NT | 53.14 | 433 |
| Pulmonary function test | ||||||||
| FVC (L) | 0.962 | 0.68 | NT | 0.42 | 1.092 | 0.89 | 0.84 | 0.98 |
| FVC (%) | 482 | 31 | NT | 18 | 472 | 35 | 34 | 39 |
| Respiratory muscle strength | ||||||||
| MIP (mmHg) | NT | 17 | NT | 17 | 452 | 59 | 62 | 53 |
| MIP (%) | NT | 19.5 | NT | 19.5 | 47.42 | 54.9 | 56.9 | 45.8 |
| MEP (mmHg) | NT | 14 | NT | 20 | 982 | 76 | 85 | 59 |
| MEP (%) | NT | 13.9 | NT | 19.8 | 102.62 | 71.4 | 79.8 | 52.7 |
With regard to body weight, the patient weighed 53.5 kg [body mass index (BMI): 29.8 kg/m2] at the time of operation. Six months after surgery, the patient’s weight was 57 kg (BMI: 30.4 kg/m2), which was a 3.5 kg weight gain compared to the preoperative weight (Table 1).
Case 2: In terms of cognitive function, the Wechsler Intelligence Scale for Children-III showed moderate intellectual disability (verbal scale IQ: 51, performance scale IQ: 58, full-scale IQ: 50).
Regarding respiratory function, the pulmonary function test showed a FVC of 1.09 L (47% of predicted) performed before scoliosis correction surgery. At the time of symptom onset (7 mo after surgery), the FVC was 0.89 L (35% of predicted) (Table 2).
With regard to body weight, the patient weighed 79.5 kg (BMI: 40.0 kg/m2) at the time of operation. Six months after surgery, the patient’s weight was 87 kg (BMI: 42.5 kg/m2), which was a 7.5 kg weight gain compared to the preoperative weight (Table 1).
Case 1: The early morning arterial blood gas (ABG) test result at postoperative day 2 was within normal range with a pH 7.55, pCO2 42 mmHg, pO2 87 mmHg, SaO2 98.0% (Table 2). After 1 mo of rehabilitation, the ABG test result was also normal with pH 7.465, pCO2 32.6 mmHg, pO2 126.3 mmHg, and SaO2 99.1%. At the time of symptom onset (6 mo after surgery), even with 2 L/min of O2 via nasal prong, the early morning ABG test revealed hypoxemia and hypercapnia with pH 7.347, pO2 54.2 mmHg, SaO2 85.2%, and pCO2 55.2 mmHg. Overnight transcutaneous CO2 and O2 monitoring also showed severe hypoxemia and hypercapnia (Table 2). In addition, daytime transcu
| Case 1 | Case 2 | |
| Polysomnography | 6 mon after surgery | 7 mon after surgery |
| CAI (events/h) | 0 | 0 |
| Obstructive apnea (events/h) | 10 | 1.4 |
| Hypopnea (events/h) | 82 | 32.5 |
| AHI (events/h) | 91.2 | 33.9 |
| ODI | 90.2 | 31.8 |
| Total arousal index (events/h) | 4 | 9.9 |
| Mean sleep SaO2% | 74.6 | 79.2 |
| Minimal SaO2% | 50 | 50 |
| Neck circumference (cm) | 39 | 39 |
Case 2: The preoperative ABG test showed normal findings: pH, 7.376; pCO2 43.9 mmHg; pO2 78.0 mmHg; and SaO2, 95.9%. Immediately after surgery, the ABG test exhibited the effect of atelectasis with pH 7.393, pCO2 56.3 mmHg, pO2 63.4 mmHg, and SaO2 91.1% (Table 2). At the time of symptom onset (7 mo after surgery), even with 5 L/min of O2 via nasal prong, the early morning ABG test showed findings of severe hypoxemia and moderate hypercapnia with pH 7.375, pO2 37.2 mmHg, SaO2 68.1%, and pCO2 53.4 mmHg (Table 2). Overnight transcutaneous CO2 and O2 monitoring was not performed prior to treatment because the patient's symptoms and abnormality were so severe that treatment was immediately performed at the time of admission to the hospital. PSG demonstrated severe OSA at AHI 33.9/h and mean SaO2 79.2% (Table 3). Echocardiography did not show any significant pulmonary hypertension.
Both patients were diagnosed with alveolar hypoventilation along with OSA.
Overnight NIV was initiated during admission. A nasal mask was used for ventilation, and the pressure support ventilation mode was used at an inspired positive airway pressure (IPAP) of 20 cm H2O, positive end-expiratory pressure (PEEP) of 5 cm H2O, and respiratory rate (RR) of 18 breaths/min without O2 supply.
The patient was initiated on overnight NIV on the day of admission. Pressure-synchronized intermittent mandatory ventilation at an IPAP of 10 cm H2O, PEEP of 5 cm H2O, pressure support of 6 cm H2O, and RR of 16 breaths/min were applied via an oronasal mask with 2 L of O2 supply.
The previously mentioned nighttime NIV application improved sleeping difficulties and shortness of breath, and overnight transcutaneous ventilation monitoring showed that hypoxia and hypercapnia were corrected (Table 2). In addition, daytime monitoring results without NIV application were within normal ranges with minimal SaO2 85%, mean SaO2 94%, maximal TcCO2 52.5 mmHg, and mean TcCO2 44.7 mmHg. Therefore, the patient was discharged while NIV was maintained overnight. Five months later, under identical settings, ABG and overnight transcutaneous CO2 and O2 monitoring demonstrated normal respiration. Therefore, the setting was maintained, and the patient was using NIV overnight (Table 2). Although FVC measured at 5 mo decreased to 0.42 L (18%) from initiation of NIV, it increased to 1.3 L (50%) at 1 year and 0.96 L (37%) at 2 years.
The previously mentioned nighttime NIV application improved nighttime cyanosis and apnea, and the patient was able to sleep well. In addition, as the shortness of breath with exertion decreased, daytime activity increased. Overnight transcutaneous monitoring showed improvements in ventilation with an O2 supply (Table 2). In addition, daytime monitoring results without NIV application were within normal ranges with minimal SaO2 83%, mean SaO2 95%, maximal TcCO2 47.9 mmHg, and mean TcCO2 31.5 mmHg. Therefore, the patient was discharged while NIV was maintained overnight. After 6 mo, the setting was changed to assist control ventilation mode at a tidal volume of 760 mL, PEEP of 5 cm H2O, and RR of 16 breaths/min for stable tidal volume. Transcutaneous CO2 and O2 monitoring demonstrated that respiration was well maintained. At follow-up (12 mo after NIV application), no significant respiratory gas abnormalities were observed compared to baseline in the ABG test.
This case series includes adolescent PWS patients with typical PWS characteristics, such as obesity, mental retardation, short stature, and scoliosis; who had a respiratory failure with severe desaturation 6-7 mo after scoliosis correction surgery; and were successfully treated with overnight NIV. Although both patients were regularly followed by a rehabilitation physician, respiratory failure progressed 6-7 mo after surgical treatment of scoliosis, without any particular preoperative symptoms. On PSG and TcCO2 monitoring, severe OSA and hypoventilation were observed in both cases. Although patient 1 had no nocturnal hypoventilation on the ABG test immediately after scoliosis surgery, the symptoms developed quickly, within 6 mo postoperatively. Patient 2 also had no respiratory symptoms or abnormalities on the ABG test before scoliosis surgery. However, 7 mo after surgery, daytime drowsiness, severe desaturation, and dyspnea on exertion began.
OSA, central sleep apnea, and nocturnal hypoventilation are the three main mechanisms that lead to respiratory failure in PWS. Each mechanism is interrelated and not completely independent[3]. Obesity with hypotonia and scoliosis are factors that commonly affect OSA and nocturnal hypoventilation, both of which increase respiratory loading[14].
Most research has focused on the co-occurrence of OSA and PWS. Approximately 80% of children with PWS have OSA, and more than 50% of them show mild severity[15]. The severity of OSA increases in younger patients and those with a higher BMI[15]. Although severe OSA is not common in PWS patients[15], nocturnal hypovent
Obesity in PWS patients shows abnormal hypercapnic and hypoxic ventilator responses, which are the pathophysiology of SDB[4]. It has been shown that weight loss improves OSA and nocturnal hypoventilation[16]. Both patients gained weight (3.5 kg and 7.5 kg, respectively) after surgery. Postoperative immobility and pain may have decreased the amount of activity, and the amount of food for children who want secondary compensation may not have been adequately controlled. However, since the degree of weight gain was not rapid in this case, weight gain would not have a decisive effect on respiratory failure. We believe that the correction of scoliosis is more likely to have made a greater contribution to respiratory failure in this case.
The incidence of PWS in scoliosis is reported to be 30%–70%[17], which is known to cause restrictive breathing patterns[10] and consequently, hypoventilation[18]. Considering its long-term effect, scoliosis repair surgery could be effective in relieving nocturnal hypoventilation and sleep apnea by reducing the restrictive pulmonary pattern[8]. However, the effect of scoliosis surgery on respiratory function remains controversial[5]. After surgery, atelectasis may occur, and since the thoracic spine is fixed (with thoracic ribs), it may cause immediate deterioration of the restrictive respiratory pattern[5]. In addition, factors such as respiratory depression due to pain during breathing after surgery[5] and maintenance of postoperative immobility posture for a certain period of time[5] could contribute to the worsening of breathing patterns.
Due to postoperative complications, multidisciplinary approaches associated with neuromuscular disease and PWS are required[17]. However, little research has been conducted on postoperative respiratory complications and the worsening of respiratory functions, especially in PWS.
In addition, regarding growth hormones, more attention should be given to the respiration of PWS. Research has focused on the relationship between scoliosis and growth hormone in cases of PWS, and recent consensus guidelines suggest that the incidence or progression of scoliosis is not influenced by growth hormone and that growth hormone therapy is not contraindicated[19]. However, there are studies on the possibility of sudden death from the use of growth hormones[1]. One of the possible leading causes of sudden death is respiratory issues due to abnormal arousal (insensitive to hypoxia) and cardiopulmonary response to hypoxia[3]. Therefore, respiratory monitoring of children with PWS using growth hormones is very important, which corresponds with patient 1.
In Duchenne muscular dystrophy or spinal muscular atrophy, which are typical pediatric neuromuscular disorders, there are age- and function-appropriate recom
As a patient effort-based test, FVC is known to reflect pulmonary function before and after repair surgery in patients with primary scoliosis rather than syndromic (secondary) scoliosis[5]. Moreover, since children with PWS typically show mental retardation, the reliability of patient effort-based pulmonary function tests is low. Therefore, it is necessary to perform “objective” tests such as TcCO2 monitoring and PSG, and timely ventilatory support through “periodic” tests, as in neuromuscular diseases[14]. Thus, irreversible deterioration findings, such as cor pulmonale, could be prevented[8]. Although a previous study reported that preoperative PSG did not predict prolonged postoperative ventilator use after scoliosis surgery[20], a recent study revealed that before severe scoliosis repair surgery, severely compromised respiratory function could be confirmed in advance through PSG and overnight TcCO2 monitoring, both of which measure hypoventilation. According to the PSG and TcCO2 results, there was an advantage of the timely use of a non-invasive ventilator that could avoid tracheostomy[6].
It is presumed that the correction surgery contributed to the cause of the respiratory failure of the patients in this case, but we are not sure whether it is purely due to correction surgery or other features of the underlying PWS (hypotonia, obesity, OSA) affects[3]. Whatever the cause, the dangerous situation due to hypoventilation, that could lead to irreversible complications such as cor pulmonale, seen in this case should be prevented.
In contrast to great deal of research conducted on OSA in PWS patients, there has been no accurate report on the prevalence of alveolar hypoventilation or the progression to cor pulmonale; but recent guidelines for pediatric patients with PWS only recommend the use of PSG to evaluate residual OSA after adenotonsillectomy[21] and prior to growth hormone therapy[22]. Therefore, guidelines on conducting PSG and recommending concurrent CO2 monitoring are required. Moreover, as is implied in the present case series, scoliosis surgery does not seem to guarantee improvement in respiratory function in PSW, and furthermore, respiratory-related monitoring must be performed for a certain period after surgery. Further PWS studies on the influence of the progression of surgical treatment for scoliosis on respiratory failure should be conducted in order to produce guidelines recommending a full PSG study, including CO2 monitoring within a certain period of time after surgical treatment for scoliosis.
Even after scoliosis surgery to improve respiratory function in PWS patients, it is necessary to “periodic” monitor respiratory failure with an “objective” test method such as PSG or EtCO2, and timely NIV support can prevent respiratory-related irreversible complications.
The authors thank all members of the Department of Physical Medicine & Rehabilitation and Translational Research and Clinical Trials Center for Medical Devices, Jeonbuk National University Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bersot CD, Lv Y S-Editor: Wang JJ L-Editor: A P-Editor: Wu RR
| 1. | Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14:10-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 916] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 2. | Irizarry KA, Miller M, Freemark M, Haqq AM. Prader Willi Syndrome: Genetics, Metabolomics, Hormonal Function, and New Approaches to Therapy. Adv Pediatr. 2016;63:47-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Tan HL, Urquhart DS. Respiratory Complications in Children with Prader Willi Syndrome. Paediatr Respir Rev. 2017;22:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Arens R, Gozal D, Omlin KJ, Livingston FR, Liu J, Keens TG, Ward SL. Hypoxic and hypercapnic ventilatory responses in Prader-Willi syndrome. J Appl Physiol (1985). 1994;77:2224-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Koumbourlis AC. Scoliosis and the respiratory system. Paediatr Respir Rev. 2006;7:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Al-Iede MM, Al-Zayadneh E, Bridge C, Alqutawneh B, Waters K. Risk factors for postoperative pulmonary complications in children with severely compromised pulmonary function secondary to severe scoliosis. Pediatr Pulmonol. 2020;55:2782-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Cohen M, Hamilton J, Narang I. Clinically important age-related differences in sleep related disordered breathing in infants and children with Prader-Willi Syndrome. PLoS One. 2014;9:e101012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | van Bosse HJP, Butler MG. Clinical Observations and Treatment Approaches for Scoliosis in Prader-Willi Syndrome. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Haqq AM, Stadler DD, Jackson RH, Rosenfeld RG, Purnell JQ, LaFranchi SH. Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88:2206-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Hákonarson H, Moskovitz J, Daigle KL, Cassidy SB, Cloutier MM. Pulmonary function abnormalities in Prader-Willi syndrome. J Pediatr. 1995;126:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Abel F, Tan HL, Negro V, Bridges N, Carlisle T, Chan E, Laverty A, Miligkos M, Samuels M, Kaditis AG. Hypoventilation disproportionate to OSAS severity in children with Prader-Willi syndrome. Arch Dis Child. 2019;104:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, Case LE, Cripe L, Hadjiyannakis S, Olson AK, Sheehan DW, Bolen J, Weber DR, Ward LM; DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17:347-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 685] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 13. | Finkel RS, Mercuri E, Meyer OH, Simonds AK, Schroth MK, Graham RJ, Kirschner J, Iannaccone ST, Crawford TO, Woods S, Muntoni F, Wirth B, Montes J, Main M, Mazzone ES, Vitale M, Snyder B, Quijano-Roy S, Bertini E, Davis RH, Qian Y, Sejersen T; SMA Care group. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018;28:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 394] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 14. | Mayer OH. Scoliosis and the impact in neuromuscular disease. Paediatr Respir Rev. 2015;16:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Sedky K, Bennett DS, Pumariega A. Prader Willi syndrome and obstructive sleep apnea: co-occurrence in the pediatric population. J Clin Sleep Med. 2014;10:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Vgontzas AN, Bixler EO, Kales A, Vela-Bueno A. Prader-Willi syndrome: effects of weight loss on sleep-disordered breathing, daytime sleepiness and REM sleep disturbance. Acta Paediatr. 1995;84:813-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M; speakers contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93:4183-4197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 322] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 18. | McPhail GL, Ehsan Z, Howells SA, Boesch RP, Fenchel MC, Szczesniak R, Jain V, Agabegi S, Sturm P, Wall E, Redding GJ. Obstructive lung disease in children with idiopathic scoliosis. J Pediatr. 2015;166:1018-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Deal CL, Tony M, Höybye C, Allen DB, Tauber M, Christiansen JS; 2011 Growth Hormone in Prader-Willi Syndrome Clinical Care Guidelines Workshop Participants. GrowthHormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. 2013;98:E1072-E1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 20. | Yuan N, Skaggs DL, Davidson Ward SL, Platzker AC, Keens TG. Preoperative polysomnograms and infant pulmonary function tests do not predict prolonged postoperative mechanical ventilation in children following scoliosis repair. Pediatr Pulmonol. 2004;38:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Aurora RN, Zak RS, Karippot A, Lamm CI, Morgenthaler TI, Auerbach SH, Bista SR, Casey KR, Chowdhuri S, Kristo DA, Ramar K; American Academy of Sleep Medicine. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 22. | McCandless SE; Committee on Genetics. Clinical report—health supervision for children with Prader-Willi syndrome. Pediatrics. 2011;127:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |