Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9942
Peer-review started: June 14, 2021
First decision: June 25, 2021
Revised: June 30, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: November 16, 2021
Processing time: 148 Days and 22 Hours
Intercostal arterial bleeding is unusual complication of percutaneous chest procedures. However, intercostal arterial bleeding is likely to result in critical complications such as abnormalities in vital signs, hypovolemic shock, and death due to massive bleeding. Therefore, it is very important to establish the diagnosis of intercostal arterial bleeding and to initiate treatment.
We report a case in which a 59-year-old woman who was hospitalized at intensive care unit with multiple trauma had a massive hemothorax after the removal of a percutaneous catheter. She sustained a refractory right pleural effusion due to biloma caused by a traumatic injury to the liver, despite persistent intraperitoneal drainage. As a result, atelectasis persisted in the dependent portion of the right lung. Therefore, we performed right percutaneous catheter drainage (8.5-F pigtail catheter) for pleural effusion drainage at the 7th intercostal space. After percuta
Massive hemothorax is a rare complication of percutaneous catheter removal. Clinicians should carefully examine and diagnose patients to improve prognosis. And interventional selective angiography may be a feasible and minimally invasive treatment for intercostal arterial bleeding control.
Core Tip: Intercostal arterial bleeding might cause massive hemothorax that is a rare complication after percutaneous catheter removal. However, if patients are early diagnosed and managed, prognosis can be highly improved. Interventional selective angiography may be a feasible and minimally invasive option for bleeding control.
- Citation: Park C, Lee J. Massive hemothorax due to intercostal arterial bleeding after percutaneous catheter removal in a multiple-trauma patient: A case report. World J Clin Cases 2021; 9(32): 9942-9947
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9942.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9942
Massive hemothorax is uncommon complication after thoracentesis or percutaneous chest procedures due to injury to the intercostal vessels. Intercostal arterial bleeding is likely to result in complications such as: Abnormalities in vital signs, hypovolemic shock, and death due to massive bleeding. Therefore, it is important to establish diagnosis of intercostal arterial bleeding and initiate treatment as soon as possible[1-3]. There have been many published reports of intercostal artery bleeding occurring during thoracentesis or percutaneous drainage procedures. However, there are a few reports of intercostal arterial bleeding occurring immediately after percutaneous catheter removal. Here, we present a case of intercostal arterial bleeding with massive hemothorax in a patient with multiple traumas. In this case, we describe how to quickly and properly treat intercostal arterial bleeding after catheter removal.
We described a 59-year-old woman, who was hospitalized with multiple trauma, experienced intercostal arterial bleeding occurring immediately after percutaneous catheter removal.
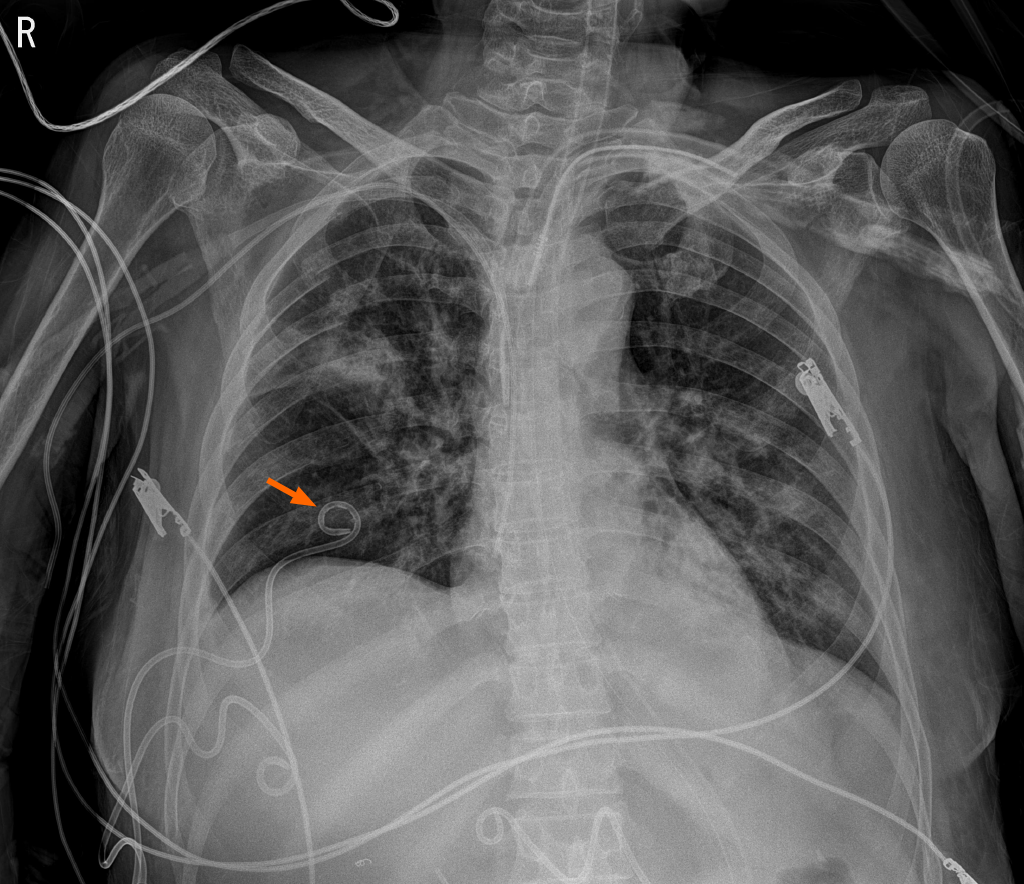
The patient underwent treatment in intensive care unit after multiple trauma caused by a pedestrian traffic accident. She had traumatic grade 4 liver injury, scanty right pneumothorax, lung contusion, and multiple rib fractures (Right 2nd-10th, left 6th-7th) due to pedestrian traffic accident with a bus. Active bleeding at the hepatic artery of segment 6 due to liver injury was embolized. Thereafter, no further procedures have been required due to bleeding. In addition, biloma due to duct injury, hematoma, was conservatively treated after percutaneous catheter drainage (PCD) insertion in the perihepatic space. Conservative treatment was also performed for ileus and ventilator-associated pneumonia. She sustained a refractory right pleural effusion due to biloma caused by a traumatic injury to the liver, despite persistent intraperitoneal drainage. As a result, atelectasis persisted in the dependent portion of the right lung. We performed right PCD (8.5-F pigtail catheter) for pleural effusion drainage at the 7th intercostal space in the interventional radiologic room by an interventionist (Figure 1). Immediately after the procedure, pleural fluid drainage was serous. On day 7 after PCD, the amount of drainage decreased to less than 50 cm3/d. Therefore, we removed the PCD (8.5-F pigtail catheter) that was inserted in the 7th intercostal space in the mid-clavicular line and compression dressing as usual. She had no coagulopathy or other precipitating factors, and bleeding was not identified immediately after PCD removal at the site of insertion.
The patient had a free previous medical and surgical history.
No special personal and family history was provided.
When we recognized the bleeding, the patient was pale and lacked skin turgor. The breath sound of the right chest was coarse, and the number of breaths decreased. Vital signs at that moment were temperature of 37.0 ºC, heart rate of 110 beats/min, blood pressure of 79/42 mmHg, respiratory rate of 21 breaths/min, and oxygen saturation of 97%.
The hemoglobin level was 7.9 g/dL at the time of recognition of bleeding, and there was no significant change when compared to 8.1 g/dL of morning routine blood test. Other laboratory data showed a platelet count of 236.0 × 103 cells/µL, prothrombin time of 12.9 s, and an activated partial thromboplastin time of 42.4 s. Arterial blood gas analysis (ABGA) showed a pH of 7.40, a PaO2 level of 71.4 mmHg, a PaCO2 level of 37.2 mmHg, and a HCO3- level of 23.5 mmol/L. However, despite the transfusion of eight packs of red blood cells (RBCs) before and after embolization, followed by a hemoglobin level was 9.3 g/dL. The next ABGA revealed a pH of 7.28, a PaO2 level of 84.9 mmHg, a PaCO2 level of 45.8 mmHg, and a HCO3- level of 21.7 mmol/L.
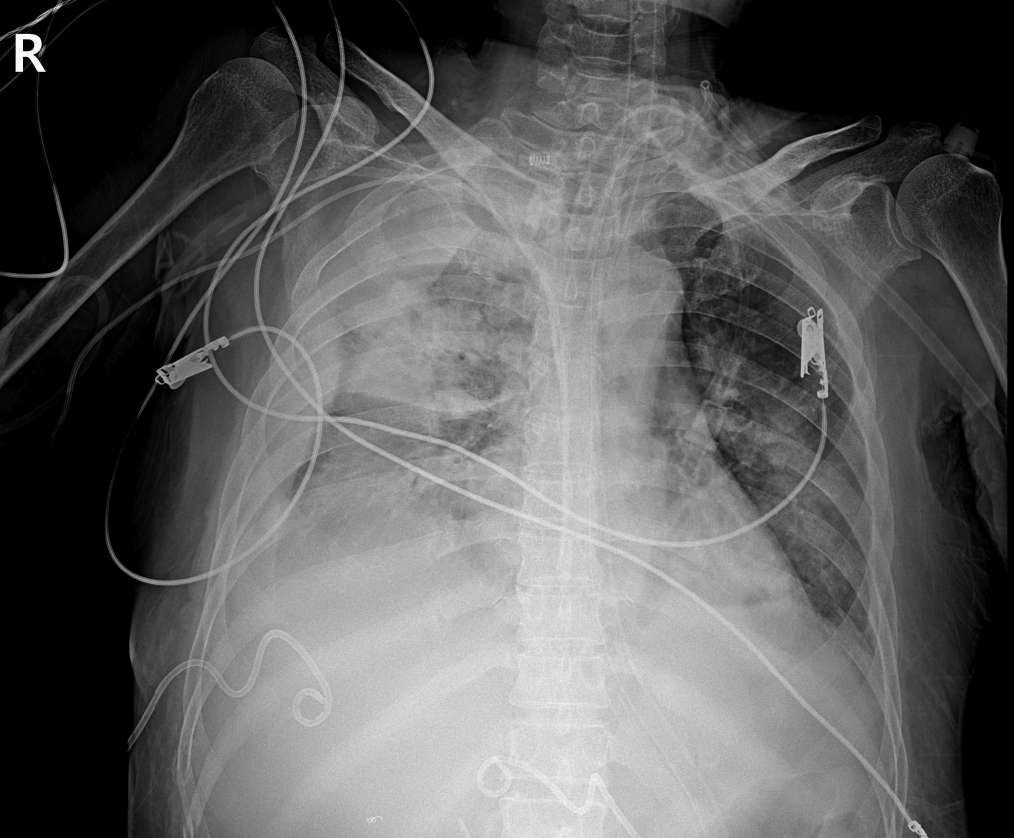
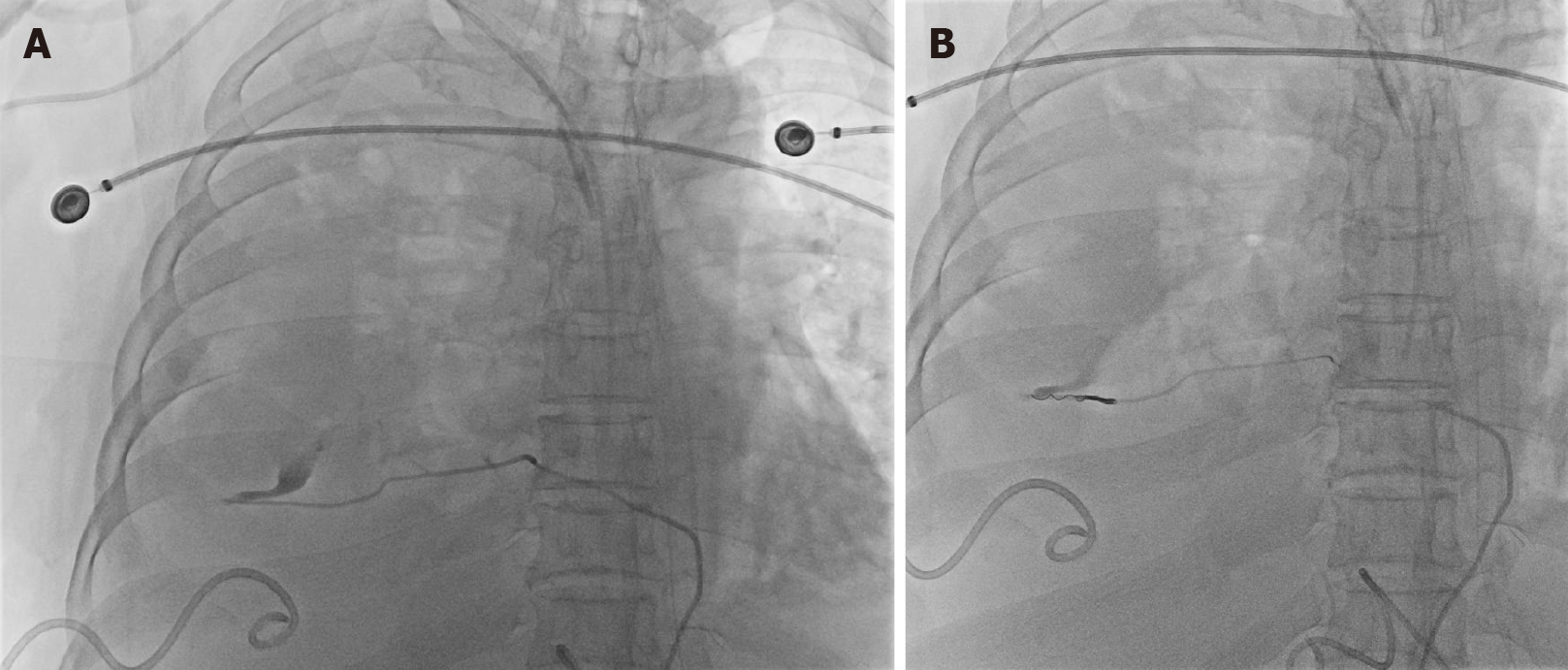
Chest radiography showed the complete opacification of the right hemithorax with diffuse peribronchial and parenchymal infiltration (Figure 2). Fluid was seen tracking up the lateral margin of the thorax. The indications confirmed that hemothorax had occurred. Thoracic aortography was performed, and we detected active bleeding in the 7th right intercostal artery.
Massive hemothorax due to bleeding from the 7th right intercostal artery.
The patient rapidly became distressed with tachycardia and hypotension (60/40 mmHg) 15 min after the drainage catheter was removed. Hemothorax was identified on chest radiography (Figure 2). However, there was no active bleeding at the removal site of skin. And hypotension and aggravated tachycardia persisted. Therefore, we immediately performed thoracic aortography and identified active bleeding from the 7th right intercostal artery. After super-selection of feeding arteries using a micro
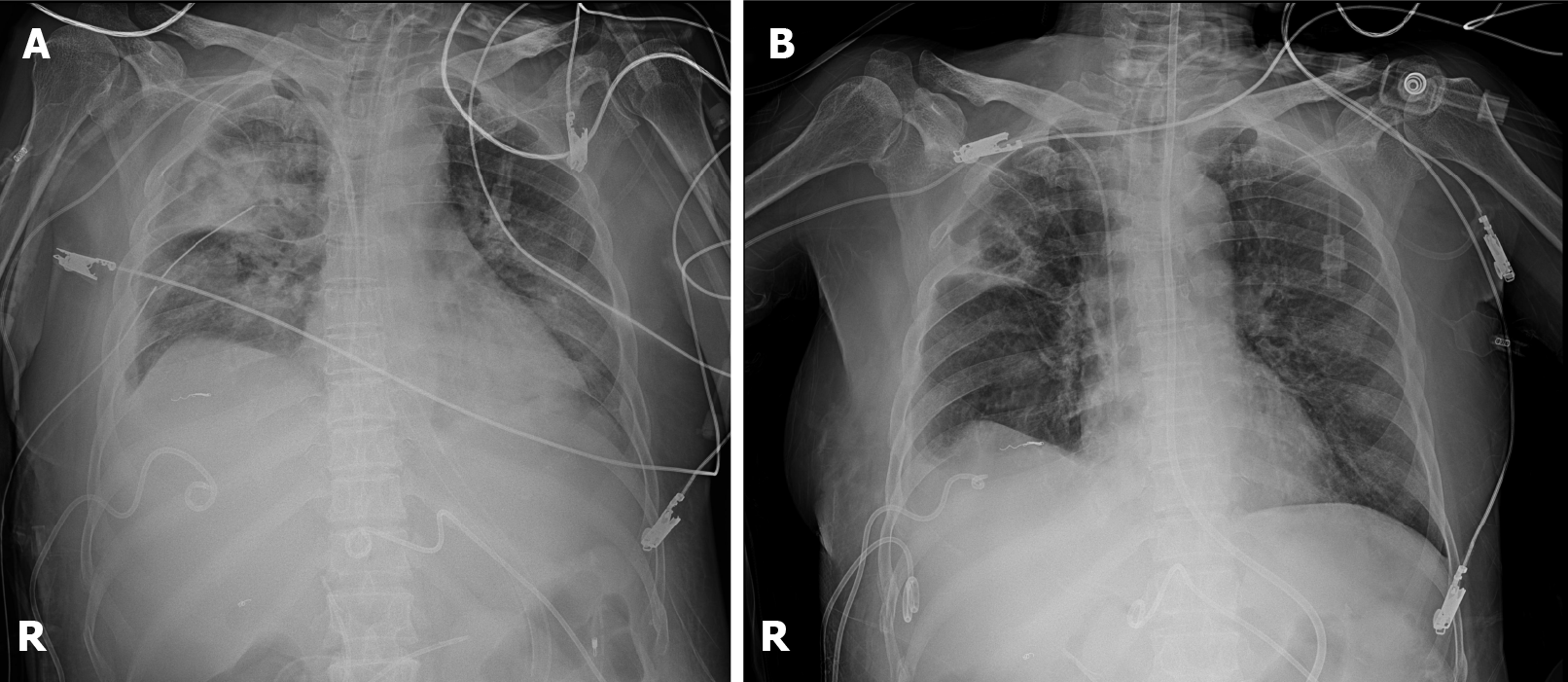
The patient’s general condition progressively improved, and no further intervention was required. During follow-up, no bleeding was observed on computed tomography in 30 d, and the hemothorax gradually decreased. The patient recovered relatively quickly due to rapid diagnosis and proper response at the intercostal arterial bleeding event. Similarly, the patient and her daughters were satisfied with the outcome. Since then, she has been receiving conservative treatment while undergoing rehabilitation in the intensive care unit.
Right-sided pleural effusion is accompanied by a large proportion of biloma and bleeding in patients with traumatic injury to the liver. When liver injury is healed and inflammatory response in the diaphragm decreases due to reductions in fluid in the abdominal cavity, pleural effusion disappears[4,5]. Percutaneous pleural effusion drainage is a common procedure to remove pleural effusion that exacerbates atelectasis and impedes breathing. Hemothorax that occurs during this process is also common.
In most cases, complications rarely occur during the procedure. However, bleeding events can occur due to hemorrhage at the insertion site leading to massive hemothorax. Previous studies indicated that the incidence of complications of thoracocentesis was 0.01%-0.1%[1,3]. It has been reported that patients with a tendency for bleeding with coagulopathy have a higher risk of bleeding. According to the consensus guidelines, it is recommended to avoid the procedure in cases of internationalized normalized ratio prolongation more than 1.5-2 and platelets count of less than 50000/µL. Clopidogrel should be stopped 5 d before the procedure. However, aspirin can be administered without interruption[3,6,7]. This patient had renal insufficiency without coagulopathy or anticoagulation agents.
The intercostal artery is composed of the posterior intercostal artery originating directly from the aorta, the anterior intercostal artery originating from the internal thoracic artery, and the collateral intercostal artery connecting them. The main bleeding site was not the anterior and posterior intercostal arteries. Bleeding occurred from the collateral intercostal artery. In general, placing a needle very close to the upper edge of the rib during intercostal puncture is widely used to avoid injury to the posterior intercostal artery. However, there is no clear way to avoid this because it cannot be predicted in the case of the collateral intercostal artery that crosses the intercostal space. Despite following the procedure principles, the risk of intercostal artery injury is mostly due to presence of anatomical variations. Recently, a method of using vascular ultrasound with color Doppler has also been introduced to assess this variation prior to thoracic puncture[2,8-11]. However, it has not been widely used in clinical practice.
Complications such as intercostal artery bleeding during pleural effusion drainage can be life-threatening. Bleeding during or immediately after drainage procedures is associated with inexperience in performing the procedure, unlike in catheter removal. Hemothorax developed in our patient. However, there was no coagulopathy or bleeding tendency. It might be difficult to directly note whether the intercostal artery was transected at the time of drain insertion procedure. Moreover, whether drainage catheter was compressing the intercostal artery and bleeding occurred after removal, or bleeding was caused by injury while removing the catheter could not be easily identified. However, it is also possible that the adjacent blood vessels were torn and bleeding occurred during removal of a pigtail catheter according to the "ripple-hook" mechanism.
In rare cases, complications can occur at any time. Therefore, clinicians should always consider hemothorax after thoracentesis or percutaneous drainage catheter removal. Vital signs unstable within a few hours after the procedure, dyspnea, decreased hemoglobin, and changes in pleural fluid color require additional examination and immediate treatment. In addition, using color Doppler flow imaging ultrasound to examine pseudoaneurysm or bleeding in the intercostal artery can similarly be useful for diagnosis[12].
Massive hemothorax due to intercostal arterial bleeding is a rare complication after percutaneous catheter removal. However, if clinicians carefully examine patients and initiate treatment after establishing an appropriate diagnosis and quick clinical judg
We would like to thank interventionist Dr. Kim for doing his best to treat this patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mu PY S-Editor: Wang JJ L-Editor: A P-Editor: Wu RR
| 1. | Cantey EP, Walter JM, Corbridge T, Barsuk JH. Complications of thoracentesis: incidence, risk factors, and strategies for prevention. Curr Opin Pulm Med. 2016;22:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Carney M, Ravin CE. Intercostal artery laceration during thoracocentesis: increased risk in elderly patients. Chest. 1979;75:520-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Begum S, Mukherjee S, Biswas D, Misra AK, Ghosh P, Bhanja P. A rare pleural effusion in a young male. Lung India. 2015;32:389-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 5. | Cooper AZ, Gupta A, Odom SR. Conservative management of a bilothorax resulting from blunt hepatic trauma. Ann Thorac Surg. 2012;93:2043-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Havelock T, Teoh R, Laws D, Gleeson F; BTS Pleural Disease Guideline Group. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-ii76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 477] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 7. | Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG, Saad WA; Standards of Practice Committee, with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 438] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 8. | Da Rocha RP, Vengjer A, Blanco A, de Carvalho PT, Mongon ML, Fernandes GJ. Size of the collateral intercostal artery in adults: anatomical considerations in relation to thoracocentesis and thoracoscopy. Surg Radiol Anat. 2002;24:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Dewhurst C, O'Neill S, O'Regan K, Maher M. Demonstration of the course of the posterior intercostal artery on CT angiography: relevance to interventional radiology procedures in the chest. Diagn Interv Radiol. 2012;18:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Kanai M, Sekiguchi H. Avoiding vessel laceration in thoracentesis: a role of vascular ultrasound with color Doppler. Chest. 2015;147:e5-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Salamonsen M, Dobeli K, McGrath D, Readdy C, Ware R, Steinke K, Fielding D. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18:942-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Casper KP, Sanchirico PJ, Pfeiffer DC. Intercostal artery pseudoaneurysm following thoracentesis: multi-modal imaging and treatment. BMC Med Imaging. 2019;19:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |