Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9926
Peer-review started: May 21, 2021
First decision: July 15, 2021
Revised: August 2, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: November 16, 2021
Processing time: 172 Days and 12.4 Hours
We report three patients with drug-induced gingiva overgrowth (DIGO) caused by nifedipine, a calcium channel blocker, who were treated and followed up for 1–3 years. We discussed their symptoms, treatment process, treatment prognosis, and follow-up results.
All the patients had a history of nifedipine treatment to control hypertension. Besides nifedipine, Patient 1 was prescribed immunosuppressant cyclosporine A to control nephritis, which is also implicated in GO. Thus, we assumed that a synergistic effect between the drugs contributed to the severity of Patient 1’s condition. This condition has been reported to be more pronounced in patients with periodontitis. In the course of treatment, Patients 1 and 2 did not stop or change drugs. After initial periodontal treatment, periodontal surgery, and later periodontal support and better plaque control, their gingival hyperplasia was well managed and controlled. Under the guidance of a physician, Patient 3 replaced her calcium-channel blocker drug with losartan potassium and hydrochloro
Patients’ compliance, self-plaque control, and professional periodontal therapy have a vital role in treating and preventing the recurrence of DIGO.
Core Tip: Our data suggest that initial periodontal therapy and replacing drugs may be useful for treating drug-induced gingival overgrowth (DIGO) in patients with heavy periodontitis and mild GO. For those patients who cannot replace medications, basic periodontal treatment, periodontal surgery, and timely and effective periodontal maintenance therapy can also be used to treat and prevent a recurrence. Patients’ compliance, self-plaque control, and professional periodontal therapy have a vital role in treating and preventing the recurrence of DIGO.
- Citation: Fang L, Tan BC. Clinical presentation and management of drug-induced gingival overgrowth: A case series. World J Clin Cases 2021; 9(32): 9926-9934
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9926.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9926
Drug-induced gingiva overgrowth (DIGO) is a gingival enlargement caused by some systemic medications, including anticonvulsants, immunosuppressants such as cyclosporine, and antihypertensives, specifically calcium-channel blockers[1,2]. The three important factors in the expression of gingival changes include drug variables, plaque-induced inflammation, and genetic factors. Careful clinical examination and thorough history are the basis for the diagnosis of DIGO. The primary treatment methods for DIGO include nonsurgical approaches followed by surgical treatment; both treatments are usually combined with maintenance therapy. Before establishing the treatment, drug discontinuation or changing medication is recommended, after which, plaque control should be performed (nonsurgical treatment). If GO persists and affects aesthetics or function, periodontal surgery is suggested. During treatment, periodontal maintenance and oral hygiene should be applied[3-6].
Some studies have hypothesized that bacterial plaque is the leading cause of DIGO. Maintaining good oral hygiene and frequent professional removal of plaque can decrease gingival enlargement and improve overall gingival health. Also, adequate plaque control may aid in preventing or retarding the recurrence of GO in surgically treated cases[3,7]. Here, we reported three patients with DIGO caused by nifedipine, a calcium channel blocker, who were treated and followed up for 1–3 years. We discussed their symptoms, treatment process, treatment prognosis, and follow-up results.
This report describes three patients with mild to severe GO in the maxilla and mandible who received calcium channel blocker nifedipine.
Case 1: A 58-year-old Chinese man complained of large painless GO that started ~10 years ago and progressively worsened.
Case 2: A 53-year-old Chinese woman presented to the periodontal department complaining of worsening gum bleeding while brushing her teeth and chewing solid food. She experienced mild but constant pain for 1 mo.
Case 3: A 59-year-old Chinese woman complained about gum growth and bleeding while brushing her teeth and chewing solid food lasting for several months.
Case 1: The patient had GO for nearly 10 years after he started taking antihypertensive drugs, with no obvious pain. Now the enlarged gingiva affected the diet and appearance. The patient had no history of periodontal treatment.
Case 2: The patient’s gums bled while brushing her teeth and chewing solid food. One month ago, the patient visited another hospital and panoramic films were taken (April 23, 2016), three teeth were extracted, but gum bleeding was not improved. For further treatment, she presented to our periodontal department (May 25, 2016) after supra
Case 3: The patient had GO for several months, no obvious pain, and bleeding while brushing her teeth and chewing solid food. It was getting worse. Ther was no history of periodontal treatment.
Case 1: A history of hypertension, diabetes, heart disease, and nephritis. He was prescribed nifedipine (10 mg QD orally), aspirin (81 mg QD orally), and cyclosporine A (500 mg QD orally). He started using nifedipine to control hypertension 10 years ago. He took medication regularly, and had stable control of systemic diseases. He stopped using aspirin for 4 d before his first visit (October 6, 2017).
Case 2: A history of hypertension. She had been taking nifedipine (10 mg QD orally) to control hypertension for > 10 years. She took medication regularly, and had stable control of systemic diseases.
Case 3: A history of hypertension lasting for ~8 years. She had been taking nifedipine (10 mg QD orally) to control hypertension for the past 6 years. She took medication regularly and her blood pressure control was steady.
Cases 1–3: The patients had no family history.
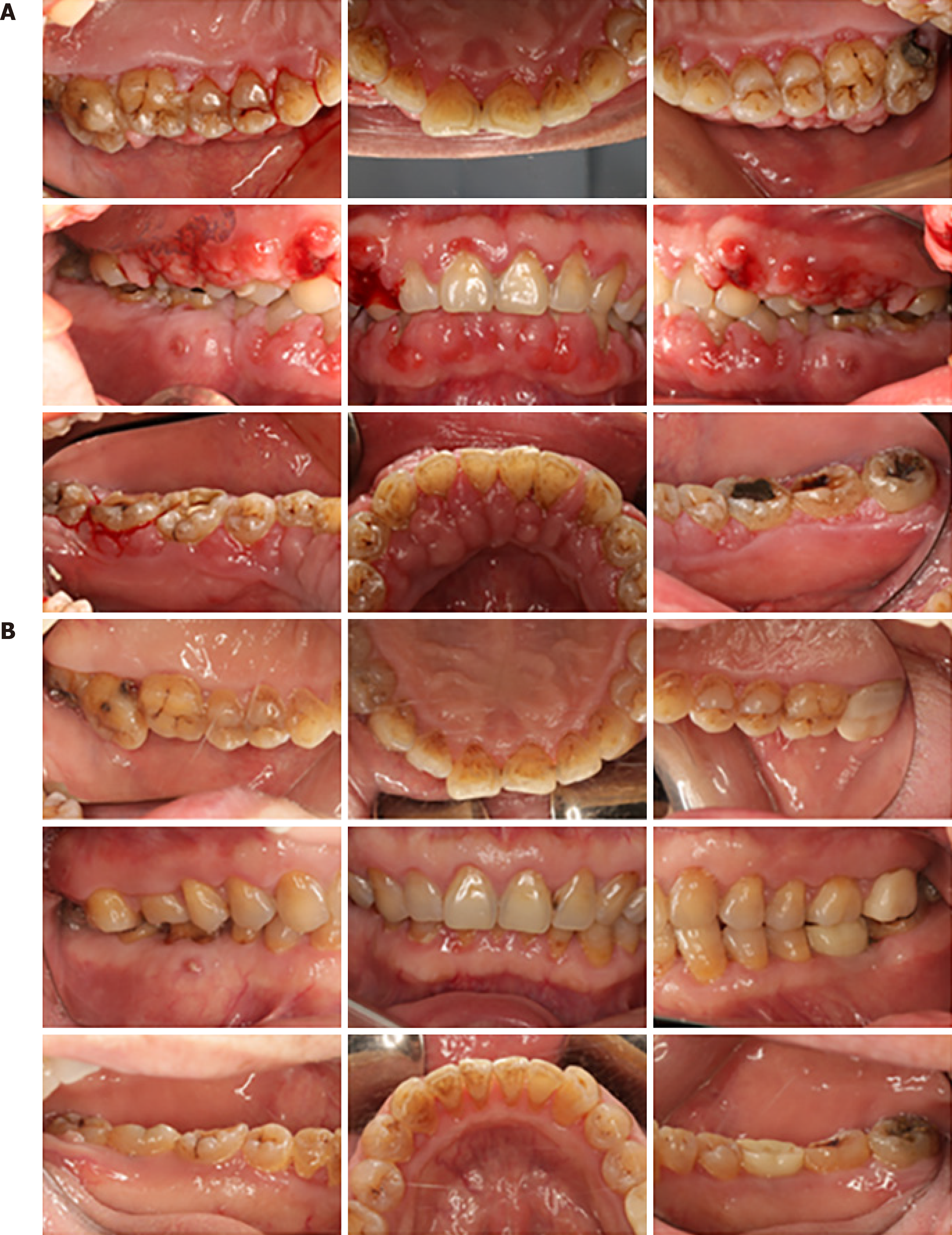
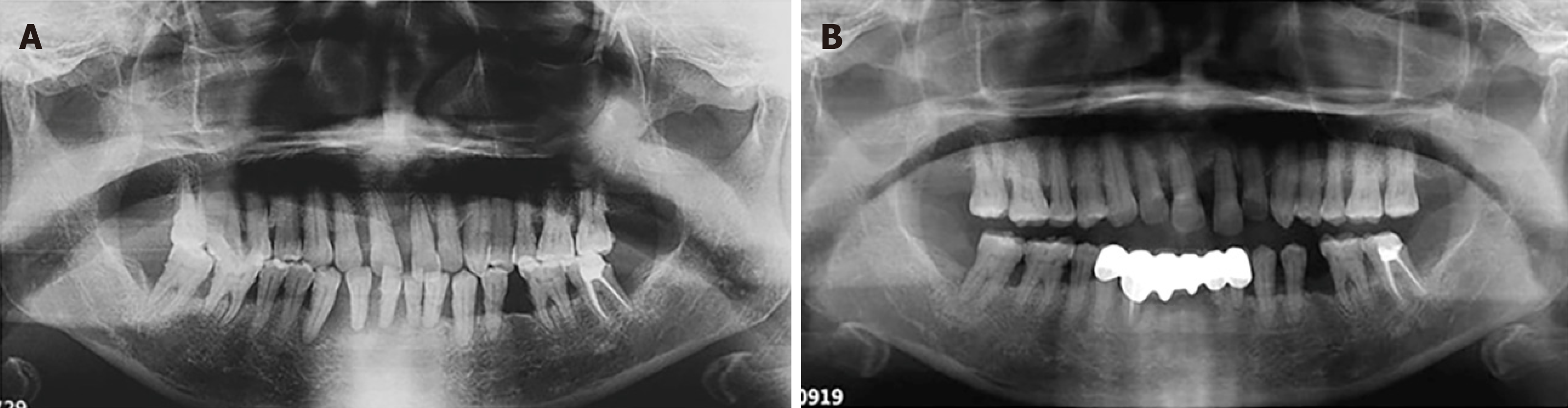
Case 1: Severe inflammatory and fibrotic GO with a spontaneous bleeding tendency were found. Except for periodontal pulp lesions in tooth #46, clinical examination revealed probing pocket depths of 2–5 mm, clinical attachment loss ranging from 0 to 3 mm, and generalized mobility ranging from Class I/II, generalized heavy sub- and supragingival calculus deposits. Tooth #27 had a large area of crown caries and no vital pulp. A large silver–mercury filling was observed in the crown of tooth #36. A complete charting of all the dentition was taken 2 wk after supragingival scaling (October 21, 2017) (Figure 1A).
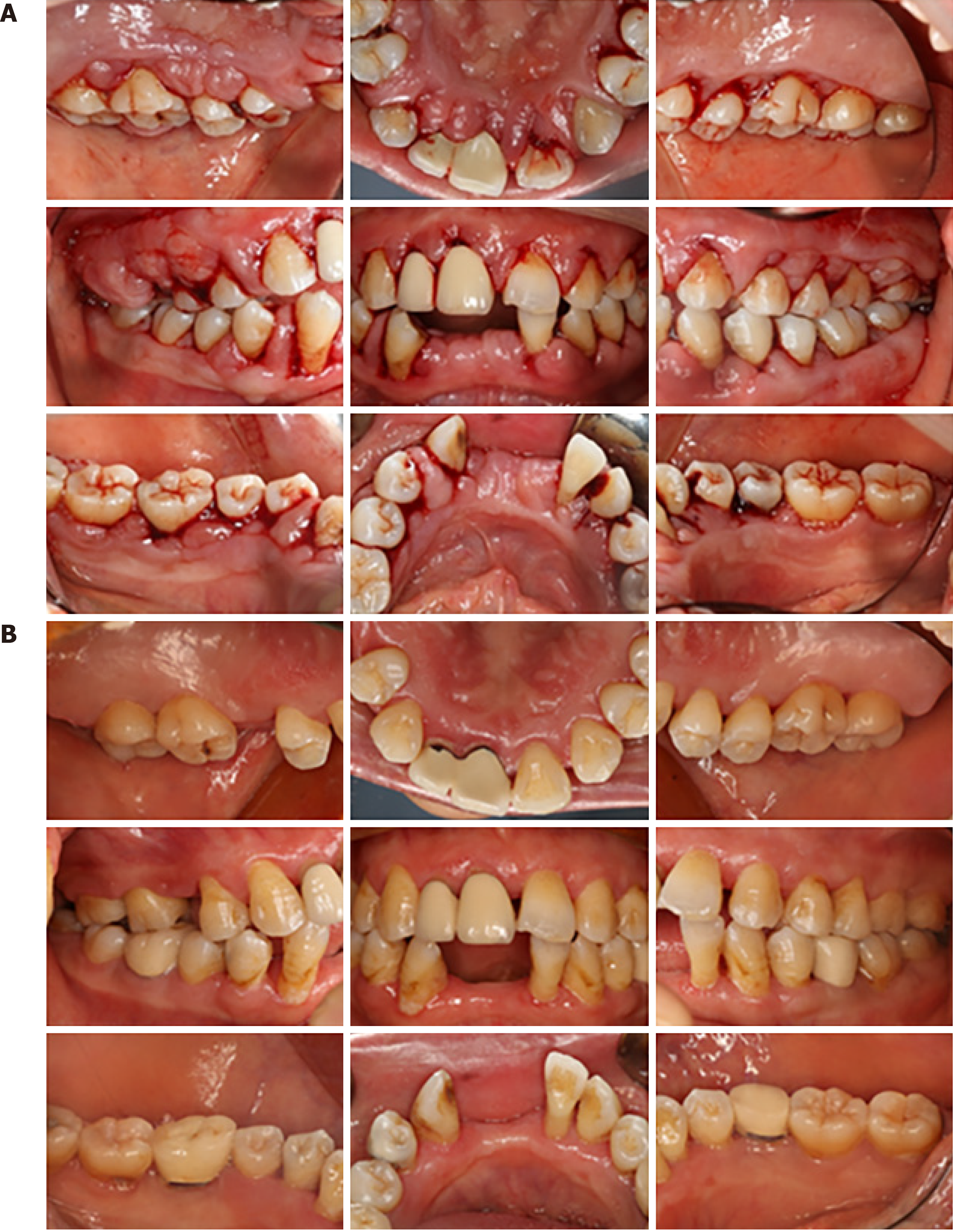
Case 2: Clinical examination showed probing pocket depths of 2–8 mm, with clinical attachment loss ranging from 0 to 12 mm, and generalized tooth mobility ranging from Class I to III. Moderately to serious inflammatory GO with spontaneous bleeding tendency were found on clinical examination. Compared to her previous panoramic radiograph, teeth #41, #42 and #31 were removed. A complete charting of all the dentition was taken at her first visit (Figure 2A).
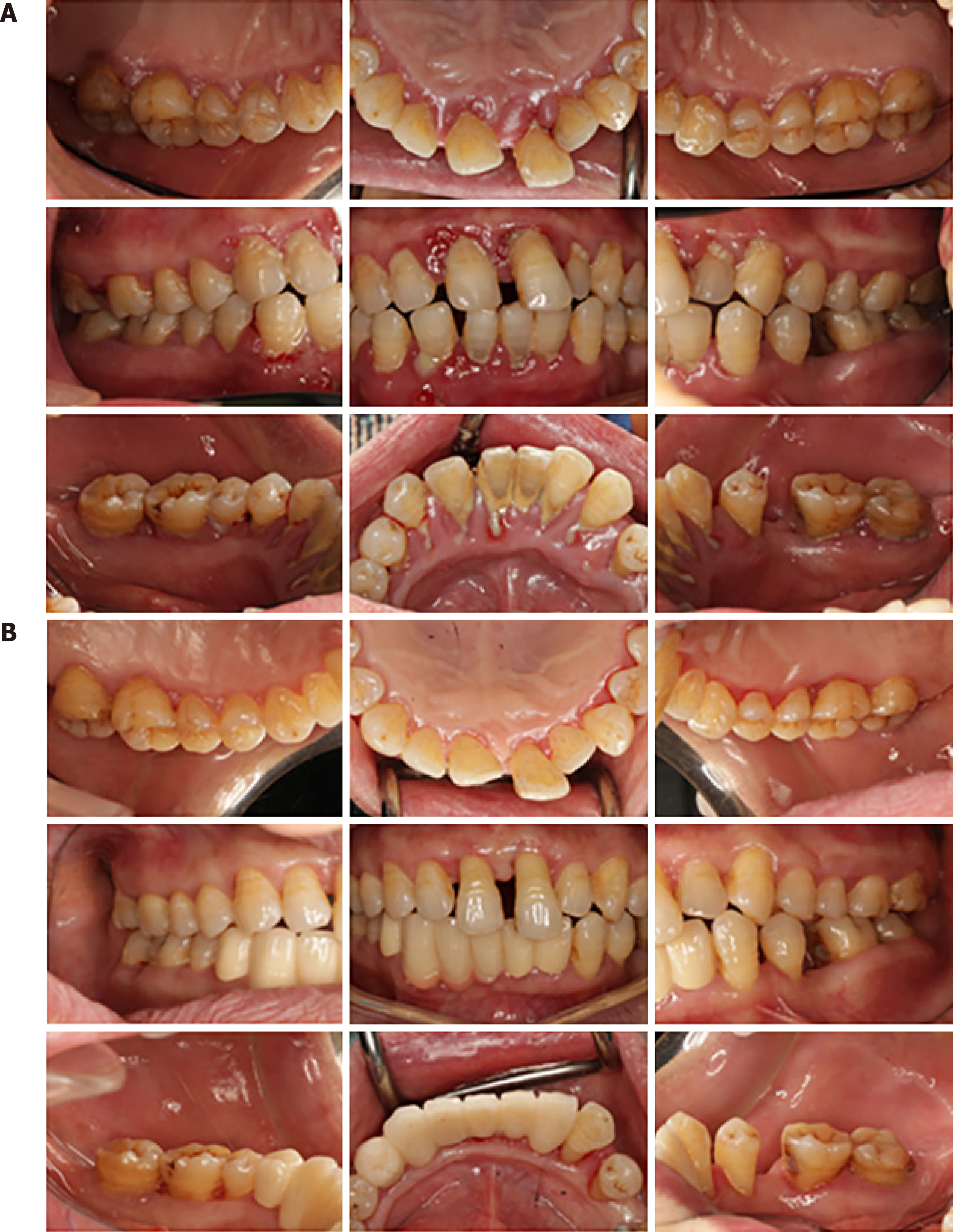
Case 3: Clinical front view of the patient at initial examination (July 29, 2019) showed intense inflammation and mild edema of the gingiva and generalized heavy sub- and supragingival calculus deposits. The upper and lower anterior teeth were drifting and purulent in the periodontal pocket. Clinical examination showed probing pocket depths of 2–15 mm, with clinical attachment loss ranging from 0 to 15 mm, and generalized tooth mobility ranging from Class I to III (Figure 3A).
Case 1: Conventional coagulation examinations and routine blood tests were measured before periodontal treatment. The results were normal.
Case 2: Routine blood tests, as well as conventional coagulation examinations were reportedly normal before the periodontal surgery.
Case 3: Routine blood examination was normal.
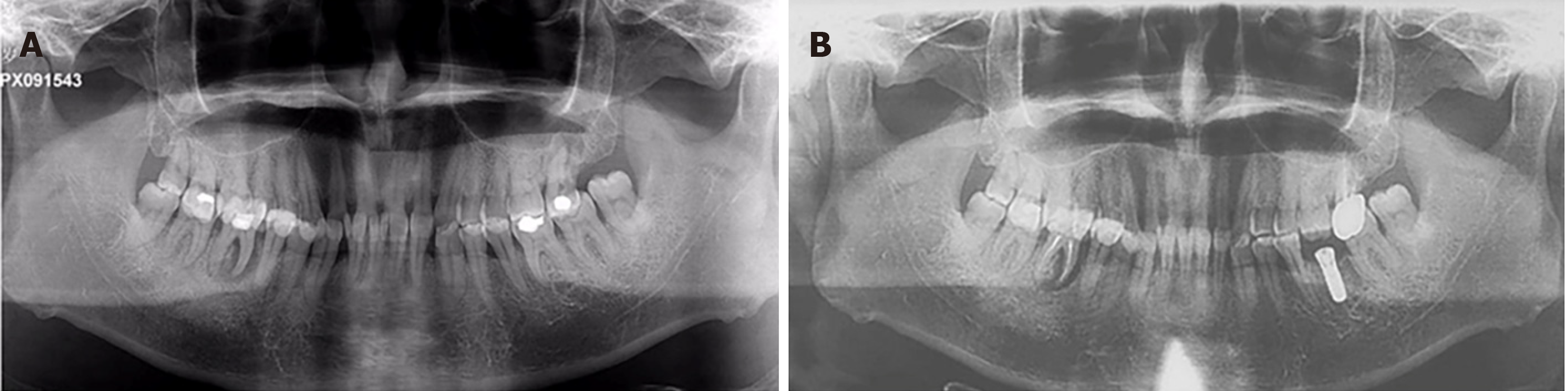
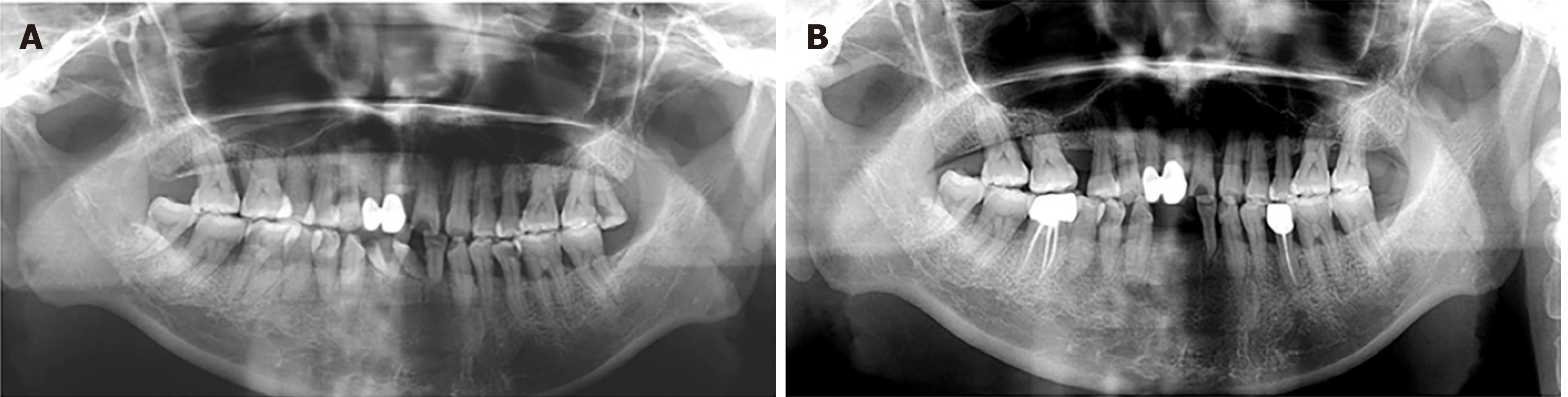
Case 1: Radiographic examination revealed mild-to-moderate bone loss, with a mesial root of tooth #46 that was completely exfoliated from the alveolar process (Figure 4A).
Case 2: The panoramic radiograph showed generalized horizontal bone loss of all teeth about half to two thirds of the root length and furcation involvement of all the maxillary molars and #36 (Figure 5A).
Case 3: The panoramic radiograph showed generalized horizontal bone loss of all teeth about half to all the root lengths and teeth #41 and #42 that were completely exfoliated from the alveolar process (Figure 6A).
Depending on clinical and radiological findings, Patient 1 was diagnosed with generalized chronic periodontitis (Stage III, Grade B), tooth #46 periodontitis associated with endodontic lesions, tooth #27 devitalized tooth, and severe gingival enlargement.
According to the radiographic and clinical examination, Patient 2 was diagnosed with severe gingival enlargement and generalized severe chronic periodontitis (Stage III, Grade C). Other diagnoses included teeth #35 and #46 chronic pulpitis, mandibular dentition defect.
Following the radiographic and clinical examination, Patient 3 was diagnosed with mild gingival enlargement and generalized severe chronic periodontitis (Stage IV, Grade C). Other diagnoses included mandibular dentition defect.
First of all, all patients were recommended to change medications after consulting their physician. Under a physician’s guidance, Patient 3 replaced her medication with losartan potassium and hydrochlorothiazide tablets, an angiotensin II receptor blocker (ARB)/thiazide combination. Patients 1 and 2 continued using the same drug for the maintenance of their disease, because other drugs did not work well for them.
All patients were given initial periodontal treatment. Initial therapy included oral health instruction, supragingival and subgingival scaling, and root planning. Additional treatment plans included initial therapy of periodontal diseases, surgical treatment if necessary, supportive periodontal therapy, and the final dental restorative treatment.
The treatment for Patient 1 also included the endodontic treatment of teeth #27 and #46 and subsequent crown repair.
For Patient 2, considering that the mobility of tooth #15 was categorized as Class III and tooth #28 was decayed down to the pulp and was elongated, the treatment included extraction of teeth #15 and #28. The endodontic treatment of teeth #35 and #46, and subsequent restorative treatment were performed.
The treatment for Patient 3 included extraction of teeth #41 and #42, and the final dental restorative treatment.
Patients requiring periodontal surgery opted for the periodontal flap and a small amount of gingival resection. Patient 1 received periodontal surgery, which is a simpler and faster technique to effectively reduce the enlarged gingival tissues of the mandibular anterior teeth area 2 mo later after basic treatment. Patient 2 also received a periodontal flap on her upper and lower anterior teeth because of persistent gum enlargement and severe periodontal inflammation after initial therapy. Since the initial periodontal treatment for Patient 3 was effective, she started supportive periodontal therapy immediately after initial periodontal treatment.
We recommend these patients be checked every 6–12 mo after periodontitis was controlled. For now, a favorable therapeutic effect was observed 2.5 years and 20 mo after surgery in Patients 1 and 2, respectively (Figures 1B and 2B, respectively). For Patient 3, positive effects and treatment outcome were observed 14 mo after initial periodontal treatment (Figure 3B).
Among all calcium-channel blockers, nifedipine has been associated with the highest incidence of gingival hyperplasia. Nifedipine is a commonly used medication in China. All the patients mentioned in this paper had a history of nifedipine treatment used to control their hypertension. Besides nifedipine, Patient 1 was prescribed immunosuppressant cyclosporine A to control nephritis, which is also implicated in GO. Thus, we assumed that a synergistic effect between the drugs contributed to the severity of Patient 1’s condition. This condition has been reported to be more pronounced in patients with periodontitis[8].
DIGO mainly affects the patients’ aesthetics and function, especially in the anterior tooth area. These patients usually do not benefit from basic treatment and need to undergo surgical treatment when periodontal inflammation is controlled. In this study, all patients first underwent simple periodontal basic treatment. Nonetheless, this treatment did not improve the degree of DIGO in the anterior tooth area, which may be related to the Chinese population’s brushing style. In China, the mandibular anterior tooth area is more prone to accumulate plaque, especially on the lingual face of mandibular anterior teeth. After good plaque control and drug substitution attempts, GO may persist. In these cases, periodontal surgery, including gingivectomy or periodontal flaps, is required. Gingivectomy is a rapid and straightforward approach. Moreover, the periodontal flap can be used to reconstruct alveolar bone contours and reduce keratinized tissue loss[9]. These two methods are often combined in order to preserve keratinized gingiva as much as possible.
Compared with Patients 2 and 3, Patient 1 had the smallest amount of periodontal inflammation and alveolar bone absorption but also the most severe DIGO, which suggests that DIGO is not only caused by plaque and inflammation, but may also be related to the type and dose of drugs and duration of treatment.
In the course of treatment, Patients 1 and 2 did not stop or change drugs. After initial periodontal treatment, periodontal surgery, and later periodontal support and better plaque control, their GO was well managed and controlled. Under the guidance of a physician, Patient 3 replaced her calcium-channel blocker drug with losartan potassium and hydrochlorothiazide tablets, which can be considered replacement drugs for DIGO caused by calcium-channel blockers. She received initial treatment without surgery, obtaining a good curative effect, which may suggest that the degree of DIGO was mild. The degree of periodontal inflammation in Patient 3 was more serious, but GO was relatively mild, which may be related to the poor plaque control and the short duration of treatment with antihypertensive drugs. Therefore, we believe that basal periodontal therapy is more effective for DIGO with heavy periodontitis and relatively mild GO.
For Patients 1–3, professional plaque control and oral hygiene guidance were conducted through follow-up at different times, and the efficacy was stable. This suggests that good oral hygiene and frequent professional plaque removal can control the extent of GO, improve the overall health of the gingivae, and reduce the recurrence of drug-induced gingival hyperplasia.
There are reasons to believe that adequate plaque control has an important role in preventing or retarding the recurrence of gingival enlargement in surgically treated cases. DIGO is associated with pseudopocket formation and may cause plaque accumulation, thus aggravating or developing periodontitis[9]. Frequent, careful and professional plaque removal is beneficial for periodontal regeneration and reducing alveolar bone loss[10,11] (Figures 4B, 5B, 6B). At the beginning of the DIGO treatment, it is suggested to change or stop the drugs under the guidance of physicians, which may cause gingival hyperplasia.
Our data suggest that initial periodontal therapy and replacing drugs may be useful for treating DIGO in patients with heavy periodontitis and relatively mild GO. For those patients who cannot replace medications, basic periodontal treatment, periodontal surgery, and timely and effective periodontal maintenance therapy can also be used to treat and prevent recurrence. Patients’ compliance, self-plaque control, and professional periodontal therapy have a vital role in treating and preventing the recurrence of DIGO.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish ME S-Editor: Wang LL L-Editor: Kerr C P-Editor: Li JH
| 1. | Dongari-Bagtzoglou A; Research, Science and Therapy Committee, American Academy of Periodontology. Drug-associated gingival enlargement. J Periodontol. 2004;75:1424-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Luciani F, Paolantonio G, Calabrese C, Calabrese L. Cytology and molecular mechanisms of drug-induced gingival hypertrophy: a rewiew. Oral Implantol (Rome). 2017;10:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Bán Á, Pintér E, Kun J. [Proper oral health can protect from developing gingival hyperplasia induced by calcium channel blockers]. Orv Hetil. 2018;159:1183-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 4. | Chang CC, Lin TM, Chan CP, Pan WL. Nonsurgical periodontal treatment and prosthetic rehabilitation of a renal transplant patient with gingival enlargement: a case report with 2-year follow-up. BMC Oral Health. 2018;18:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Singh P, Singh J. Prevalence of drug induced gingival enlargement among patients attending OPD of Department of Periodontia, Patna Dental College & Hospital. Int J Community Health & Medical Research. 2019;5:31-33. |
| 6. | Samudrala P, Chava VK, Chandana TS, Suresh R. Drug-induced gingival overgrowth: A critical insight into case reports from over two decades. J Indian Soc Periodontol. 2016;20:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Pundir AJ, Pundir S, Yeltiwar RK, Farista S, Gopinath V, Srinivas TS. Treatment of drug-induced gingival overgrowth by full-mouth disinfection: A non-surgical approach. J Indian Soc Periodontol. 2014;18:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Deeb JG, Lyons DJ, Laskin DM, Deeb GR. Severe drug-induced gingival enlargement and periodontitis: A case series with clinical presentation and management. J Oral Maxil Surg Cases. 2020;6:100143-100148. |
| 9. | Camargo PM, Melnick PR, Pirih FQ, Lagos R, Takei HH. Treatment of drug-induced gingival enlargement: aesthetic and functional considerations. Periodontol 2000. 2001;27:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Fu E, Nieh S, Wikesjö UM, Lin FG, Shen EC. Gingival overgrowth and dental alveolar alterations: possible mechanisms of cyclosporin-induced tooth migration. An experimental study in the rat. J Periodontol. 1997;68:1231-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Mavrogiannis M, Ellis JS, Thomason JM, Seymour RA. The management of drug-induced gingival overgrowth. J Clin Periodontol. 2006;33:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |